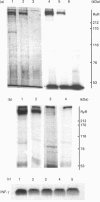
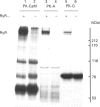
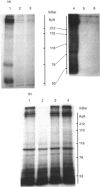
Abstract
The ryanodine receptor is the main Ca(2+)-release structure in skeletal and cardiac sarcoplasmic reticulum. In both tissues, phosphorylation of the ryanodine receptor has been proposed to be involved in the regulation of Ca2+ release. In the present study, we have examined the ability of the purified cardiac ryanodine receptor to serve as a substrate for phosphorylation by exogenously added catalytic subunit of the cyclic AMP (cAMP)-dependent protein kinase (PK-A), cyclic GMP (cGMP)-dependent protein kinase (PK-G), or calmodulin-dependent protein kinase (PK-CaM). A large amount of phosphate incorporation was observed for PK-CaM (938 +/- 48 pmol of Pi/mg of purified channel protein), whereas the level of phosphorylation was considerably lower with PK-A or PK-G (345 +/- 139 and 96 +/- 6 pmol/mg respectively). In addition, endogenous PK-CaM activity co-migrates with the ryanodine receptor through several steps of purification, suggesting a strong association of the two proteins. This endogenous PK-CaM activity is abolished by a PK-CaM-specific synthetic peptide inhibitor. Endogenous cAMP- and cGMP-dependent phosphorylation was not observed in the purified ryanodine-receptor preparation. Taken together, these observations imply that PK-CaM is the physiologically relevant protein kinase, capable of phosphorylating the channel protein to a minimum stoichiometry of 2 mol of Pi per mol of tetramer.
Full text
PDF

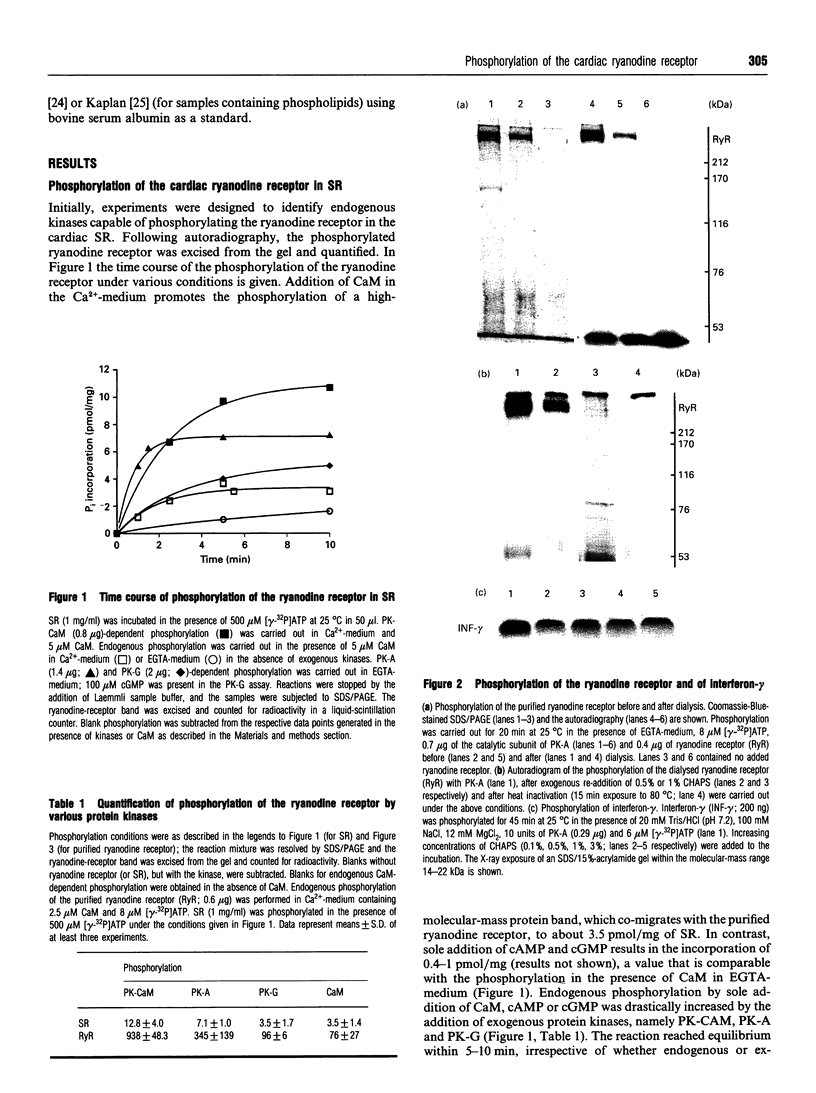

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Lai F. A., Liu Q. Y., Rousseau E., Erickson H. P., Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989 Jan 15;264(2):1329–1335. [PubMed] [Google Scholar]
- Chu A., Sumbilla C., Inesi G., Jay S. D., Campbell K. P. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry. 1990 Jun 26;29(25):5899–5905. doi: 10.1021/bi00477a003. [DOI] [PubMed] [Google Scholar]
- Flores I., Mariano T. M., Pestka S. Human interferon omega (omega) binds to the alpha/beta receptor. J Biol Chem. 1991 Oct 25;266(30):19875–19877. [PubMed] [Google Scholar]
- Heinemann S. H., Terlau H., Stühmer W., Imoto K., Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992 Apr 2;356(6368):441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Flockerzi V. Characterization of phosphorylated and native cGMP-dependent protein kinase. Eur J Biochem. 1983 Feb 15;130(3):599–603. doi: 10.1111/j.1432-1033.1983.tb07191.x. [DOI] [PubMed] [Google Scholar]
- Hymel L., Schindler H., Inui M., Fleischer S. Reconstitution of purified cardiac muscle calcium release channel (ryanodine receptor) in planar bilayers. Biochem Biophys Res Commun. 1988 Apr 15;152(1):308–314. doi: 10.1016/s0006-291x(88)80715-0. [DOI] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987 Nov 15;262(32):15637–15642. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B. Anal Biochem. 1985 Oct;150(1):97–104. doi: 10.1016/0003-2697(85)90445-2. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kim D. H., Ikemoto N. Involvement of 60-kilodalton phosphoprotein in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1986 Sep 5;261(25):11674–11679. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Anderson K., Rousseau E., Liu Q. Y., Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1988 Feb 29;151(1):441–449. doi: 10.1016/0006-291x(88)90613-4. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Meissner G. The muscle ryanodine receptor and its intrinsic Ca2+ channel activity. J Bioenerg Biomembr. 1989 Apr;21(2):227–246. doi: 10.1007/BF00812070. [DOI] [PubMed] [Google Scholar]
- Meissner G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986 Jan 14;25(1):244–251. doi: 10.1021/bi00349a034. [DOI] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Morii H., Takisawa H., Yamamoto T. A possible role of protein phosphorylation in the inactivation of a Ca2+-induced Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biochem. 1987 Aug;102(2):263–271. doi: 10.1093/oxfordjournals.jbchem.a122050. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Penner R., Neher E., Takeshima H., Nishimura S., Numa S. Functional expression of the calcium release channel from skeletal muscle ryanodine receptor cDNA. FEBS Lett. 1989 Dec 18;259(1):217–221. doi: 10.1016/0014-5793(89)81532-7. [DOI] [PubMed] [Google Scholar]
- Suko J., Hasselbach W. Characterization of cardiac sarcoplasmic reticulum ATP-ADP phosphate exchange and phosphorylation of the calcium transport adenosine triphosphatase. Eur J Biochem. 1976 Apr 15;64(1):123–130. doi: 10.1111/j.1432-1033.1976.tb10280.x. [DOI] [PubMed] [Google Scholar]
- Suko J., Maurer-Fogy I., Plank B., Bertel O., Wyskovsky W., Hohenegger M., Hellmann G. Phosphorylation of serine 2843 in ryanodine receptor-calcium release channel of skeletal muscle by cAMP-, cGMP- and CaM-dependent protein kinase. Biochim Biophys Acta. 1993 Jan 17;1175(2):193–206. doi: 10.1016/0167-4889(93)90023-i. [DOI] [PubMed] [Google Scholar]
- Suko J., Wyskovsky W., Pidlich J., Hauptner R., Plank B., Hellmann G. Calcium release from calmodulin and its C-terminal or N-terminal halves in the presence of the calmodulin antagonists phenoxybenzamine and melittin measured by stopped-flow fluorescence with Quin 2 and intrinsic tyrosine. Inhibition of calmodulin-dependent protein kinase of cardiac sarcoplasmic reticulum. Eur J Biochem. 1986 Sep 15;159(3):425–434. doi: 10.1111/j.1432-1033.1986.tb09904.x. [DOI] [PubMed] [Google Scholar]
- Takasago T., Imagawa T., Furukawa K., Ogurusu T., Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991 Jan;109(1):163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Takasago T., Imagawa T., Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. J Biochem. 1989 Nov;106(5):872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- Tuana B. S., MacLennan D. H. Isolation of the calmodulin-dependent protein kinase system from rabbit skeletal muscle sarcoplasmic reticulum. FEBS Lett. 1988 Aug 1;235(1-2):219–223. doi: 10.1016/0014-5793(88)81266-3. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Williams A. J. Ion conduction and discrimination in the sarcoplasmic reticulum ryanodine receptor/calcium-release channel. J Muscle Res Cell Motil. 1992 Feb;13(1):7–26. doi: 10.1007/BF01738423. [DOI] [PubMed] [Google Scholar]
- Witcher D. R., Kovacs R. J., Schulman H., Cefali D. C., Jones L. R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991 Jun 15;266(17):11144–11152. [PubMed] [Google Scholar]
- Wyskovsky W., Hohenegger M., Plank B., Hellmann G., Klein S., Suko J. Activation and inhibition of the calcium-release channel of isolated skeletal muscle heavy sarcoplasmic reticulum. Models of the calcium-release channel. Eur J Biochem. 1990 Dec 12;194(2):549–559. doi: 10.1111/j.1432-1033.1990.tb15651.x. [DOI] [PubMed] [Google Scholar]