Abstract
Background:
To inform clinical guidance, public health efforts, and research directions, probiotic use in U.S. health care needs to be better understood. This work aimed to assess the prevalence of inpatient probiotic use in a sample of U.S. hospitals.
Methods:
Probiotic use among patients discharged in 2012 was estimated using the MarketScan Hospital Drug Database. In addition, the annual trend in probiotic use (2006-2012) was assessed among a subset of hospitals.
Results:
Among 145 hospitals with 1,976,167 discharges in 2012, probiotics were used in 51,723 (2.6%) of hospitalizations occurring in 139 (96%) hospitals. Patients receiving probiotics were 9 times more likely to receive antimicrobials (P < .0001) and 21 times more likely to have a Clostridium difficile infection diagnosis (P < .0001). The most common probiotic formulations were Saccharomyces boulardii (32% of patients receiving probiotics), Lactobacillus acidophilus and Lactobacillus bulgaricus (30%), L acidophilus (28%), and Lactobacillus rhamnosus (11%). Probiotic use increased from 1.0% of 1,090,373 discharges in 2006 to 2.9% of 1,006,051 discharges in 2012 (P < .0001).
Conclusions:
In this sample of U.S. hospitals, a sizable and growing number of inpatients received probiotics as part of their care despite inadequate evidence to support their use in this population. Additional research is needed to guide probiotic use in the hospital setting.
Keywords: Probiotic, hospital, prevalence, microbial, Lactobacillus, Saccharomyces, Bifidobacterium, Dietary supplement
Probiotics, commonly defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” 1 are used among the general population for health maintenance purposes. Use of probiotics for prevention and treatment of antibiotic-associated diarrhea (AAD) and Clostridium difficile infection (CDI) is receiving increasing attention2 as patients, clinicians, and researchers search for ways to mitigate the effects of antibiotic use.3 However, the evidence supporting their efficacy and safety when used for this purpose is inconclusive.
Pooled analyses of data from randomized controlled trials of probiotics used for prophylaxis suggest reduced risk of AAD4-6 and C difficile–associated diarrhea5,7 in adults and children receiving antibiotics. In the meta-analyses for prevention of AAD, however, moderate to substantial statistical heterogeneity between the trials was observed.4-6 In addition, a recent, well-powered study of hospitalized adults ≥65 years of age that used a high-dose multistrain probiotic (1 Bifidobacterium bifidum, 1 Bifidobacterium lactis, and 2 Lactobacillus acidophilus strains) highlights the need to tease out this heterogeneity. Despite the strengths in the design, risk of AAD and C difficile–associated diarrhea was equivalent between the probiotic and placebo arms.8 Such findings indicate the need for focused evidence using specific strains, antimicrobials, timing, dosing, and patient populations evaluated in studies of sufficient power to better understand under what circumstances probiotics are effective.
Probiotics can be marketed as dietary supplements, which require compliance with Good Manufacturing Practices and premarketing notification to the U.S. Food and Drug Administration for a new dietary supplement ingredient documenting a reasonable expectation for safety. Premarketing demonstration of product efficacy and obtaining Food and Drug Administration approval based on evidence of product efficacy and safety, which are required for New Drug Applications, however, are not required for the marketing of dietary supplements.9 A recent survey of U.S. academic medical centers found 87% of 114 respondents stocked or used at least 1 probiotic, with a total of 10 probiotic products among the centers.10 In a separate study in an academic medical center, 0.4% of patients were prescribed a probiotic in 2007-2008, with 96% of these patients receiving a combination product (L acidophilus–Lactobacillus bulgaricus) and 4% receiving a product containing Saccharomyces boulardii.11 Prevention and treatment of CDI, treatment for unspecified diarrhea, and prevention of AAD comprised 78.3% of the justifications for probiotic use in this center.
Although these studies provide a useful starting point in the description of probiotic utilization in the inpatient setting, the former did not quantify inpatient prescribing practices, and the latter reported the experience of only 1 medical center. A study with a larger sample of hospitals that quantifies inpatient probiotic utilization and provides clinical context is needed to inform clinical guidance, public health efforts, and research directions. The primary objective of this study, therefore, was to assess and characterize the prevalence of probiotic use from a sample of 145 U.S. hospitals.
METHODS
Study design
An observational study was conducted to describe the prevalence of probiotic use in the inpatient setting. The study was divided into 2 parts: a cross-sectional study of prevalence of probiotic use in 2012 and a longitudinal study of probiotic use among the subset of hospitals reporting yearly from 2006-2012, inclusive. Because the data were deidentified at the patient and hospital levels, this work was determined not to involve human subjects and therefore was exempt from the regulations governing the protection of human subjects in research under 45 CFR 46.101(b)(5).12 This work was conducted under the provisions of the Centers for Disease Control and Prevention–MarketScan Data Use Agreement.
Data source
The Truven Health MarketScan Hospital Drug Database (HDD) from the years 2006-2012, inclusive, was used to estimate probiotic use in the inpatient setting. The HDD is a relational database developed from hospital charge detail master data, containing all charges accumulated during the hospitalization, including room and board, supplies, procedures, laboratory testing, and pharmacy products. The drug data are derived from free-form text fields, which are then mapped to a drug classification system by a clinical coder. Codes of interest are obtained through text string searches of the generic drug name in the description field of the corresponding drug reference table. The database also includes standard administrative elements, such as patient demographics, hospitalization diagnosis and procedure codes, and facility characteristics.
To facilitate an informal comparison with a nationally representative sample, the Healthcare Utilization Project’s National Inpatient Sample (NIS) estimates from 2012 were compared with study sample estimates whenever possible (Supplemental Tables S1 and S2).
Population
Data were restricted to those of hospitals reporting directly to Truven Health. Within these data, the study population consisted of all discharges, unless otherwise noted. Individual patients may have been present multiple times in the data as a result of multiple hospitalizations. Prior to database release, any discharges identified as having critical errors were removed. Critical errors include patient age <0 or >124 years, missing or invalid primary diagnosis or procedure codes, and diagnoses or procedures not corresponding to age or sex of the patient.
Identification of probiotics
To identify probiotic use, text strings were searched in the HDD reference tables consisting of terms at the genus, species, and strain level and terms indicative of probiotics that were identified from several sources.13-16 These terms included the following: probiotic, Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, rhamnosus, plantarum, acidophilus, casei, johnsonii, boulardii, helveticus, bulgaricus, infantis, and reuteri (see Supplemental Table S3 for a longer list). In addition to these root terms, different spelling permutations were searched using the Perl Regular Expression function PRXMATCH (SAS Institute, Cary, NC).17 Identified codes and corresponding descriptions were reviewed by hand. Codes with terms or phrases in the descriptions inconsistent with a probiotic were removed (examples are shown in Supplemental Table S4). To ensure identification of all possible codes specific to probiotics, sections of the reference tables were also hand checked. Ultimately, 8 unique generic probiotic formulations consisting of ≥1 species were identified in the HDD drug reference tables (see Supplemental Table S5 for additional details). Dose was not considered in the identification process because relevant information (eg, number of colony forming units per dose) was not available.
Identification of antimicrobials
The process for identifying antimicrobial use was conducted in a similar manner to that for probiotics. A previously developed list of term18 was used, which included antibacterial, antifungal, antiviral, and antiparasitic agents. Route of administration was restricted to inhalation, oral, and parenteral.
Analytic and statistical methods
Prevalence of probiotic use was defined as the number of patients receiving a probiotic during hospitalization divided by the total number of patients discharged in 2012. Distributions of patient-, facility-, and hospitalization-level characteristics were tallied by probiotic group. For categorical variables, the denominator consisted of the number of discharges unless otherwise noted. For continuous variables, the mean, 95% confidence interval (CI), and median were presented. Unadjusted comparisons of patient- and hospital-level characteristics between patients with and without probiotic use were conducted using the independent samples t test for continuous variables and χ2 test for categorical variables.
To describe trends in the prevalence of probiotic use over time, annual prevalence of probiotic use from 2006-2012 was calculated among the subset of hospitals reporting data during each of these years. The need to adjust for facility-level effects was confirmed using the covtest option in SAS’s PROC GLIMMIX (SAS Institute). The final model was adjusted for within-facility residual correlations using PROC GENMOD with a first-order autoregressive correlation structure and assuming a gamma distribution. Robust SEMs were used to safeguard against misspecification of correlation structure. The annual and overall change in prevalence was estimated using this model.
RESULTS
Cross-sectional study
In 2012, 145 hospitals with 1,976,167 pediatric and adult hospital discharges submitted data directly to Truven Health. Probiotics were used in 51,723 (2.6%) of the hospitalizations and in 139 (96%) hospitals. At the hospital level, probiotic use ranged from 0.3%-8.5% (5th-95th percentile, 139 hospitals) of discharges.
Patients with any probiotic use were older (median age, 70 vs 57 years; P < .0001), had a longer mean length of stay (8.8 vs 4.4 days, P < .0001), and incurred higher charges ($63,732 vs $34,130, P < .0001) than patients without probiotic use. The probiotic group was also 21 times more likely to have a discharge diagnosis of C difficile infection (95% CI, 20.4-21.7; P < .0001), almost 9 times as likely to have used antimicrobials (odds ratio [OR], 8.6; 95% CI, 8.3-8.8; P < .0001), more likely to be admitted from another inpatient health care facility (OR, 1.4; 95% CI, 1.4-1.5; P < .0001), and more likely to be transferred to another health care facility at discharge (OR, 2.9; 95% CI, 2.8-2.9; P < .0001) than the nonprobiotic group (Table 1).
Table 1.
Characteristics of discharges with and without probiotic use during hospitalization, HDD 2012
| Variable | Value | Probiotic | No probiotic | P value |
|---|---|---|---|---|
| Discharges, n | 51,723 | 1,924,444 | ||
| Hospitals, n | 139 | 145 | ||
| Patient characteristics | ||||
| Age category, % | 0 y | 1.1 | 10.3 | <.0001 |
| 1-17 y | 2.4 | 2.7 | ||
| 18-64 y | 34.4 | 48.4 | ||
| 65-84 y | 44.5 | 29.6 | ||
| ≥85 y | 17.7 | 9.1 | ||
| Sex, % | Male | 42.2 | 42.9 | .0021 |
| Female | 57.8 | 57.1 | ||
| Principal payer, % | Medicare | 67.4 | 42.4 | <.0001 |
| Medicaid | 9.7 | 20.0 | ||
| Private | 15.9 | 26.2 | ||
| Other | 2.8 | 3.3 | ||
| Self-pay | 4.1 | 7.7 | ||
| No charge | <0.1 | 0.1 | ||
| Unknown | 0.1 | 0.4 | ||
| Hospital characteristics | ||||
| Census region, % | Northeast | 0.7 | 0.3 | <.0001 |
| Midwest | 12.6 | 19.2 | ||
| South | 76.5 | 65.5 | ||
| West | 10.1 | 14.9 | ||
| Urban-rural, % | Rural | 14.6 | 11.3 | <.0001 |
| Urban | 85.4 | 88.7 | ||
| Bed category, % | 1-199 beds | 23.6 | 21.9 | <.0001 |
| 200-299 beds | 21.0 | 19.1 | ||
| 300-499 beds | 33.3 | 32.2 | ||
| ≥500 beds | 22.1 | 26.8 | ||
| Teaching | 4.3 | 9.6 | <.0001 | |
| Hospitalization characteristics | ||||
| Admission type, % | Elective, routine | 12.2 | 21.4 | <.0001 |
| Emergency admit | 67.5 | 50.3 | ||
| Newborn delivery | 0.5 | 9.6 | ||
| Other | 0.4 | 0.6 | ||
| Urgent | 19.4 | 18.1 | ||
| Admission source, % | Inpatient health care facility | 9.0 | 6.5 | <.0001 |
| Referral | 90.0 | 83.0 | ||
| Newborn | 0.5 | 9.6 | ||
| Other | 1.0 | 1.0 | ||
| Discharge disposition, % | Transferred to another health care facility | 32.7 | 14.5 | <.0001 |
| Discharged to home | 63.9 | 82.4 | ||
| Left against medical advice | 0.5 | 1.0 | ||
| Died | 2.8 | 1.9 | ||
| Missing | 0.1 | 0.2 | ||
| Length of stay, d | Mean | 8.8 | 4.4 | <.0001 |
| Median | 6.0 | 3.0 | ||
| Charge, $ | Mean | 63,732 | 34,130 | <.0001 |
| Median | 35,570 | 20,459 | ||
| Any stay in ICU, % | 37.2 | 26.2 | <.0001 | |
| CDI diagnosis, % | Primary code (ICD-9-CM code: 008.45) | 5.3 | 0.2 | <.0001 |
| Secondary code | 7.2 | 0.4 | <.0001 | |
| Any code | 12.5 | 0.7 | <.0001 | |
| Antimicrobial use, % | 91.9 | 57.1 | <.0001 |
CDI, Clostridium difficile infection; HDD, MarketScan Hospital Drug Database; ICU, intensive care unit.
A total of 54,242 probiotic courses were used; 95% of patients in the probiotic group received just 1 probiotic formulation during their hospitalization, and the remaining 5% received 2-3 formulations. Each formulation contained between 1 and 4 organisms identified at the species level. As detailed in Table 2, the most commonly used probiotic formulations were S boulardii (32% of discharges in the probiotic group), L acidophilus and L bulgaricus (30%), L acidophilus (28%), and Lactobacillus rhamnosus (11%), not mutually exclusive.
Table 2.
Utilization of specific probiotic formulations by age among discharges with any probiotic use, HDD, 2012
| Binary name (genus, species) | No. of hospitals | All ages | 0 y* | 1-17 y | 18-64 y | 65-84 y | ≥85 y | ||
|---|---|---|---|---|---|---|---|---|---|
| Any probiotic, N | 139 | 51,723 discharges | 575 | 1217 | 17,772 | 23,025 | 9,134 | ||
| Lactobacillus acidophilus, Lactobacillus bulgaricus | 77 | 55% | 15,483† | 30%‡ | 52% | 28% | 28% | 31% | 31% |
| Saccharomyces boulardii | 74 | 53% | 16,358 | 32% | 5% | 18% | 29% | 33% | 36% |
| Lactobacillus acidophilus | 71 | 51% | 14,645 | 28% | 12% | 15% | 32% | 27% | 26% |
| Lactobacillus rhamnosus | 41 | 29% | 5,789 | 11% | 29% | 37% | 11% | 10% | 9% |
| Lactobacillus acidophilus, Streptococcus thermophilus, Lactobacillus paracasei, Bifidobacterium animalis§ | 17 | 12% | 831 | 2% | 0% | 0% | 2% | 2% | 1% |
| Lactobacillus reuteri | 7 | 5% | 510 | 1% | 3% | 3% | 1% | 1% | 1% |
| Bifidobacterium infantis | 3 | 2% | 629 | 1% | – | 1% | 2% | 1% | 1% |
NOTE. Only 7 of the 8 formulations identified from the HDD reference tables were actually used.
HDD, MarketScan Hospital Drug Database.
Age <1 year or those missing age.
Summation of probiotic courses across formulations will add up to 54,242 total probiotic courses.
Percent = (No. of courses) ÷ (51,723 patient discharges with probiotic use). Summation of this value (%) across formulations will exceed 100% because 5% of discharges received 2-3 formulations.
Actual text description in HDD reference table: LA, LA-PCAS, STREP, BIF ANIMALIS. Potential identity: Lactobacillus acidophilus, Streptococcus thermophilus, L. paracasei, Bifidobacterium animalis.
Infectious, inflammatory, and gastrointestinal conditions comprised the bulk of the most common primary diagnostic categories in the probiotic group (Table 3). Obstetric and cardiovascular conditions were more common in both the nonprobiotic group (Table 3), the overall HDD sample, and the NIS (Supplemental Table S6).
Table 3.
Ten most frequent diagnostic categories based on primary ICD-9-CM diagnosis codes among discharges from 145 U.S. hospitals with and without probiotic use, HDD, 2012
| Probiotic* |
No probiotic† |
Comparison |
||||
|---|---|---|---|---|---|---|
| CCS‡ | CCS category description | Rank | % | Rank | % | P value§ |
| 2 | Septicemia (except in labor) | 1 | 10.5 | 3 | 2.9 | <.0001 |
| 122 | Pneumonia (except that caused by tuberculosis or sexually transmitted disease) | 2 | 6.6 | 4 | 2.7 | |
| 135 | Intestinal infection | 3 | 6.5 | 54 | 0.5 | |
| 197 | Skin and subcutaneous tissue infections | 4 | 4.3 | 19 | 1.6 | |
| 159 | Urinary tract infections | 5 | 3.7 | 17 | 1.6 | |
| 237 | Complication of device, implant, or graft | 6 | 3.2 | 13 | 1.7 | |
| 127 | Chronic obstructive pulmonary disease and bronchiectasis | 7 | 2.9 | 9 | 1.9 | |
| 108 | Congestive heart failure, nonhypertensive | 8 | 2.8 | 5 | 2.6 | |
| 238 | Complications of surgical procedures or medical care | 9 | 2.6 | 24 | 1.3 | |
| 254 | Rehabilitation care, fitting of prostheses, and adjustment of devices | 10 | 2.6 | 35 | 0.7 | |
| 218 | Live-born | 48 | 0.5 | 1 | 9.6 | |
| 203 | Osteoarthritis | 16 | 1.7 | 2 | 2.9 | |
| 106 | Cardiac dysrhythmias | 17 | 1.5 | 6 | 2.4 | |
| 657 | Mood disorders | 52 | 0.4 | 7 | 2.2 | |
| 100 | Acute myocardial infarction | 20 | 1.2 | 8 | 2.0 | |
| 109 | Acute cerebrovascular disease | 23 | 1.0 | 10 | 1.8 | |
CCS, Clinical Classifications Software; HDD, MarketScan Hospital Drug Database.
Probiotic: 51,723 discharges.
No probiotic: 1,924,444 discharges.
Clinical Classifications Software. Healthcare Cost and Utilization Project (HCUP). June 2015. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
Comparison: probiotic versus no probiotic by 11 categories (top 10 CCS categories and all others): χ210 = 52,087, P < .0001.
Longitudinal study
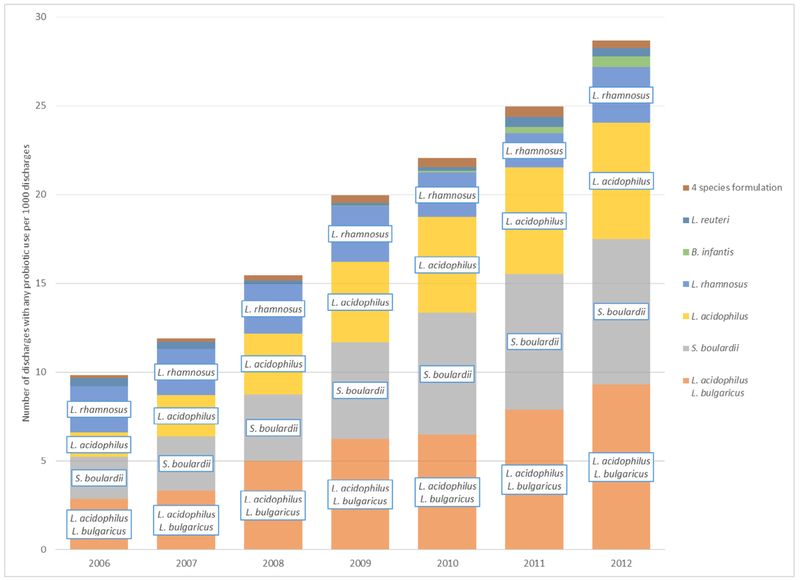
Sixty hospitals submitted annually from 2006-2012 and therefore were included in the trend analysis. In 2006, 1.0% of discharges received at least 1 course of probiotics. Usage increased annually 1.2-fold (95% CI, 1.1-1.3; P < .0001) through 2012, in which 2.9% of discharges received at least 1 course of probiotics for an overall 2.9-fold (95% CI, 1.8-4.5; P < .0001) increase between 2006 and 2012 (Fig 1, Supplemental Table S7).
Fig 1.
Trend in probiotic use among 60 U.S. hospitals reporting annually, HDD, 2006-2012. Corresponding data table is displayed in Supplemental Table S7. HDD, MarketScan Hospital Drug Database; 4 species formulation consists of Lactobacillus acidophilus, Streptococcus thermophilus,Lactobacillus paracasei, and Bifidobacterium animalis.
DISCUSSION
Summary
In this sample of 145 U.S. hospitals, a sizable number of patients received probiotics as part of their care. Although the proportion of probiotic use among discharges was small, the magnitude was considerable because almost 52,000 hospitalizations included probiotic use as part of prescribed medical care in 2012. Projected nationally, probiotics may have been utilized in the care of approximately 850,000 patients discharged from US hospitals (based on weights provided by Truven Health; additional details in Supplemental Appendix). In addition, the consistent increase in probiotic use from year to year between 2006 and 2012 suggests probiotic use is continuing to increase. The most common categories of diagnosis codes among the patients with probiotic use were associated with infectious diseases, antibiotic use, increased severity of illness, or exposure to health care.
Comparability with other studies
These findings are similar to survey results on the increasing use of probiotic use among nonhospitalized persons. A recent study of probiotic use among a sample of noninstitutionalized adults in the United States also showed an increase in probiotic use over time. Recorded probiotic use in the prior 30 days grew from 0.4% (projected national estimate of 865,000) of adults in 2007 to 1.6% (3,857,000) in 2012.19 In the same time span, use among children was 0.3% (number not estimated) in 2007 and 0.5% (294,000) in 2012.20
We identified 7 probiotic formulations in use across 96% of the 145 hospitals, with 71% of hospitals using 1-2 formulations (mean, 2.1; range, 1-6) per hospital in 2012. This finding is comparable with the aforementioned survey of probiotic use among U.S. academic medical centers in which 10 products were identified in stock or in use across 87% of the 114 responding centers, with 79% of the centers reported stocking or using only 1 product.10 At the species level, the top 4 probiotic products most frequently reported across the centers10 were also similar to those used in the current study, consisting of L rhamnosus, Lactobacillus gasseri and L bulgaricus, Lactobacillus acidophilus, and S boulardii.
Limitations
A limitation of this study is the utilization of a nonprobability (convenience) sample of hospitals, which increases the potential for selection bias,21 The Northeast region was under-represented at 0.3% of discharges in the HDD sample compared with 19.1% in the Healthcare Utilization Project’s NIS, a nationally representative sample of hospital discharge data. Despite this regional difference, more clinically relevant measures, including top hospital discharge diagnoses, average length of stay, and discharge disposition, were similar to national estimates (Supplemental Table S6). Therefore, our findings may reflect an estimate of U.S. inpatient hospital probiotic use, although this extrapolation must be interpreted with caution. A second limitation is that this study relied on administrative data collected primarily for billing purposes. Administrative data lack clinical detail beyond what is needed for the purposes of tracking costs and charges in particular. Third, although the probiotic formulations were identified at the species level, strain identity (the unit at which probiotic activity is determined), product potency (eg, number of colony forming units at the time of expiration), and product source (eg, name of brand or manufacturer) were generally absent. These details would have been helpful in verifying the product identities. Fourth, because estimation of probiotic use was based on drug charge data, the prevalence may be underestimated. Unaccounted for sources of probiotics include foods containing probiotics (eg, yogurt) provided by the hospital and dietary supplements and foods containing probiotics brought to the hospital by the patient or patient’s family.
Need for additional research
Manipulation or remediation of the microbiome may be an important strategy for prevention of health care–associated infections in the future. Whether probiotics are effective in preserving or restoring a healthy microbiome remains unknown, but the high prevalence of probiotic use among hospitalized patients may indicate a growing belief among clinicians that these agents may be an effective strategy for doing so. Currently, there is not enough evidence to support use of currently available probiotics in this clinical setting. Correspondingly, several professional societies directly state that they do not yet recommend probiotics for the prevention of CDI because of the need for further research in the areas of efficacy and safety.22-25
Recently, the Agency for Health Research and Quality sponsored the development of an evidence report to compile safety assessments of probiotic agents containing specific microorganisms used in research studies for risk reduction, prevention, and treatment of disease. As a result of issues, including lack of safety assessments, inconsistent reporting, and poor documentation of interventions, one of the major conclusions of the report was that “the current literature is not well equipped to answer questions on the safety of probiotic interventions with confidence.”13 Adverse events among probiotic users that have been reported in case reports and clinical trials include bacteremia, fungemia, endocarditis, functional ileus, bowel distension, bowel ischemia, diarrhea, and increased risk of death.26,27 Risk may be increased among patients with central venous catheterization, bacterial translocation, immunosuppression, critical illness, pancreatitis, and organ transplants.26 Patients with these conditions are likely to be prescribed antibiotics, which places them at increased risk for microbiome disruption. Because the patients most likely to benefit are also most at risk for an adverse event, preclinical research focused on the selection of likely probiotics and carefully designed clinical trials with systematic assessment of safety is particularly important.
In the planning process of trials of probiotic efficacy and safety, issues to be mindful of include product labeling inaccuracies at the species and even genus level for reasons such as difficulty in identification, desire for consumer recognition,28 and cross-contamination during manufacturing29; varying susceptibility of strains to antibiotics30; and product viability at the time of ingestion. The general questions needing to be answered include the following: which strain-specific organisms, which patient populations, at what doses, and in what time frames (related to antibiotic use in particular) are both safe and effective in the prevention or treatment of which diseases?
CONCLUSIONS
This study found a sizable and growing number of hospitalized patients who receive probiotics as part of their care. These findings, given the lack of sufficient evidence for the efficacy and safety of probiotic use in hospitalized patients, suggest that research is critically needed to guide the use of these agents in the hospital setting.
Supplementary Material
Acknowledgements
We thank Dan Budnitz, MD, MPH, and Nadine Shehab, PharmD, MPH, of Division of Healthcare Quality Promotion for review of the manuscript.
Funding/Support: Supported through salary funds from the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
APPENDIX: SUPPLEMENTARY MATERIAL
Supplementary data related this article can be found at doi:10.1016/j.ajic.2015.12.001.
Conflicts of Interest: None to report.
References
- 1.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers B, Kirley K, Mounsey A. PURLs: prescribing an antibiotic? Pair it with probiotics. J Fam Pract 2013;62:148–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown K, Valenta K, Fisman D, Simor A, Daneman N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med 2015;175:626–33. [DOI] [PubMed] [Google Scholar]
- 4.Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012;307:1959–69. [DOI] [PubMed] [Google Scholar]
- 5.Pattani R, Palda VA, Hwang SW, Shah PS. Probiotics for the prevention of antibiotic-associated diarrhea and Clostridium difficile infection among hospitalized patients: systematic review and meta-analysis. Open Med 2013;7:e56–67. [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 2010;16:2202–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenberg JZ, Ma S, Saxton JD, Martzen MR, Vandvik PO, Thorlund K, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD009329. [DOI] [PubMed] [Google Scholar]
- 8.Allen SJ, Wareham K, Wang DL, Bradley C, Hutchings H, Harris W, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013;382:1249–57. [DOI] [PubMed] [Google Scholar]
- 9.Venugopalan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use. Emerg Infect Dis 2010;16:1661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe AM, Gregory PJ, Hein DJ, Cochrane ZR, Wilson AF. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm 2013;48:834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simkins J, Kaltsas A, Currie BP. Investigation of inpatient probiotic use at an academic medical center. Int J Infect Dis 2013;17:e321–4. [DOI] [PubMed] [Google Scholar]
- 12.Office for Human Research Protections. 45 CFR part 46 Protection of Human Subjects. http://www.hhs.gov/ohrp/policy/ohrpregulations.pdf. Accessed December 30, 2015.
- 13.Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep) 2011;200:1–645. [PMC free article] [PubMed] [Google Scholar]
- 14.Fijan S Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health 2014;11:4745–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr 2001;73(Suppl):365S–373S. [DOI] [PubMed] [Google Scholar]
- 16.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis in’t Veld JH. Overview of gut flora and probiotics. Int J Food Microbiol 1998;41:85–101. [DOI] [PubMed] [Google Scholar]
- 17.Cassell DL. The Basics of the PRX Functions. Paper presented at: SAS Global Forum; April 16–19, 2007; Orlando, FL. [Google Scholar]
- 18.Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014;63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report 2015;79:1–16. [PMC free article] [PubMed] [Google Scholar]
- 20.Black LI, Clarke TC, Barnes PM, Stussman BJ, Nahin RL. Use of complementary health approaches among children aged 4-17 years in the United States: national health interview survey, 2007-2012. Natl Health Stat Report 2015;78:1–19. [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman DA. Sampling. In: Lewis-Beck MS, Bryman A, Liao TF, editors. Encyclopedia of Social Science Research Methods, Vol. 3. Thousand Oaks (CA): Sage Publications; 2004. p.986–90. [Google Scholar]
- 22.Dubberke ER, Carling P, Carrico R, Donskey CJ, Loo VG, McDonald LC, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:628–45. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431–55. [DOI] [PubMed] [Google Scholar]
- 24.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013;108:478–98. [DOI] [PubMed] [Google Scholar]
- 25.Sartelli M, Malangoni MA, Abu-Zidan FM, Griffiths EA, Di Bella S, McFarland LV, et al. WSES guidelines for management of Clostridium difficile infection in surgical patients. World J Emerg Surg 2015;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelan K, Myers CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr 2010;91:687–703. [DOI] [PubMed] [Google Scholar]
- 27.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:651–9. [DOI] [PubMed] [Google Scholar]
- 28.Yeung PS, Sanders ME, Kitts CL, Cano R, Tong PS. Species-specific identification of commercial probiotic strains.J Dairy Sci 2002;85:1039–51. [DOI] [PubMed] [Google Scholar]
- 29.Drisko J, Bischoff B, Giles C, Adelson M, Rao RV, McCallum R. Evaluation of five probiotic products for label claims by DNA extraction and polymerase chain reaction analysis. Dig Dis Sci 2005;50:1113–7. [PubMed] [Google Scholar]
- 30.Goldstein EJ, Tyrrell KL, Citron DM. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clin Infect Dis 2015;60(Suppl):S98–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.