Abstract
Introduction:
Small cell lung cancer (SCLC) is characterized by rapid progression after platinum resistance. Circulating tumor (ctDNA) dynamics early in treatment may help determine platinum-sensitivity.
Methods:
Serial plasma samples were collected from patients receiving platinum-based chemotherapy for SCLC on the first three days of cycle 1 and on the first days of subsequent cycles with paired samples collected both before and again after infusions. Tumor-informed plasma analysis was carried out using CAncer Personalized Profiling by deep Sequencing (CAPP-Seq). The mean variant allele frequency (VAF) of all pre-treatment mutations was tracked in subsequent blood draws and correlated with radiologic response.
Results:
ctDNA kinetics were assessed in 122 samples from 21 patients. Pre-treatment VAF did not differ significantly between patients who did and did not respond to chemotherapy (mean 22.5% versus 4.6%, p=0.17). A slight increase in ctDNA on cycle 1, day 1 immediately post-treatment was seen in 6 of the 7 patients with available draws (fold change from baseline: 1.01–1.44), half of whom achieved a response. All patients who responded had a >2-fold decrease in mean VAF on cycle 2 day 1 (C2D1). Progression-free survival (PFS) and overall survival (OS) were significantly longer in patients with a >2-fold decrease in mean VAF after one treatment cycle (6.8 versus 2.6 months, log-rank p=0.0004 and 21.7 versus 6.4 months, log rank p=0.04, respectively).
Conclusions:
A >2-fold decrease in ctDNA concentration was observed by C2D1 in all patients who were sensitive to platinum-based therapy and was associated with longer PFS and OS.
Keywords: small cell lung cancer, circulating tumor DNA (ctDNA), ctDNA kinetics, ctDNA dynamics, liquid biopsy, CAPP-Seq
Introduction
Small cell lung cancer (SCLC) is an aggressive disease,1–3 characterized by frequent mutations in TP53 and RB1.4–7 SCLC is prone to hematogenous spread and most patients have extensive stage (ES) disease at presentation,3,8 with a median survival on the order of months.2,9,10
Given the rapidly progressive nature of SCLC, early therapeutic optimization remains crucial. Most patients receive platinum-based regimens as first line therapy,2,8,11 with more recent evidence supporting addition of checkpoint inhibitors to chemotherapy in ES disease.9,10,12 Although most cases are initially platinum sensitive, the development of eventual platinum resistance is inevitable and is typically followed by rapid progression. Early identification of platinum-resistance is crucial.
Liquid biopsies have shown utility in tracking therapeutic response in a variety of tumors.13–22 However, there have been few studies on SCLC.23–25 26–28 Moreover, there is limited information on the kinetics of changes in circulating tumor DNA (ctDNA) levels following chemotherapy administration. While it has been hypothesized, largely based on animal studies, that tumor apoptosis in the hours after chemotherapy administration may lead to an increase or “spike” in ctDNA in the plasma of responders,29 little is known about changes in ctDNA concentration immediately after chemotherapy infusions for SCLC. Therefore, data on ctDNA dynamics in response to therapy are needed to determine at what points increases or decreases in ctDNA can reliably predict radiologic response.
To characterize the dynamics of changes in ctDNA in patients with SCLC, we assessed changes in mean variant allele frequency (VAF) with twice daily plasma collection before and after receipt of chemotherapy for the first three days of the first treatment cycle with additional pre-and post-chemotherapy draws in subsequent cycles.
Materials & Methods
Patient enrollment:
This study was approved by both the institutional review boards (IRBs) of Memorial Sloan Kettering Cancer Center (MSK) and Stanford University School of Medicine. Twenty-one patients with treatment-naïve SCLC who were scheduled to receive platinum-based therapy at MSK were enrolled.
Plasma was collected both before and after receipt of chemotherapy on cycle 1 day 1 (C1D1), cycle 1 day 2 (C1D2), cycle 1 day 3 (C1D3), and at the start of subsequent cycles. Pre-treatment blood draws were denoted as “draw A”, whereas post-treatment draws were denoted as “draw B”; for example, the pre-treatment draw from C1D1 was denoted C1D1A, while the same-day post-treatment draw was C1D1B.
Patients underwent scans after completion of two chemotherapy cycles or as clinically indicated, with first scans performed a median of 40 days post-treatment initiation. Best overall response (BOR) was determined using RECIST version 1.1. Patients were labelled responders if at any point their BOR was either partial (PR) or complete response (CR), as opposed to stable (SD) or progressive disease (PD).
Progression-free survival (PFS) was defined from the date of first pre-treatment blood draw to radiographic progression. For patients who died or otherwise experienced clear clinical progression without available scans, the date of progression was determined in accordance with the treating physicians’ evaluation.
Blood collection and processing
Peripheral blood was collected in two Streck tubes (Streck, Omaha, Nebraska) and processed within 4 hours to enable plasma and peripheral blood mononuclear cell (PBMC) extraction using a refrigerated centrifuge at 4oC at 1600 rpm for 10 minutes. The plasma was pooled and re-spun at 1500 rpm for 10 minutes then stored in 1.8 ml aliquots at −80oC. The PBMCs were separated from the remaining cell fraction after plasma pooling by gentle pipetting and stored in 1–2 aliquots at −80oC.
Stored plasma aliquots were thawed at room temperature and centrifuged at 13,000 rpm for 15 minutes to remove residual cellular components. Cell free DNA (cfDNA) was extracted using the QiaAmp Circulating Nucleic Acid Kit (Qiagen). Genomic DNA from PBMCs was isolated with DNeasy Blood & Tissue Kit (Qiagen). DNA was quantified using the Qubit dsDNA High Sensitivity Kit (Thermo Fisher Scientific) and High Sensitivity NGS Fragment Analyzer (Agilent). PBMC isolated DNA was fragmented to smaller sizes using Covaris S2 sonicator and purified with QIAquick PCR Purification Kit as described previously (Qiagen).30
CAPP-Seq Analysis
Targeted capture and sequencing analysis of all plasma samples and their corresponding PBMC samples was performed using the CAncer Personalized Profiling by deep Sequencing (CAPP-Seq) ctDNA assay, as previously described.30–33 Briefly, changes in mean VAF of all mutations detected in tumors and at baseline were tracked before and after chemotherapy. We used a focused custom-designed 355 kb panel targeting 255 recurrently mutated lung cancer and 11 clonal hematopoiesis-associated genes.32 A maximum of 32 ng of DNA was inputted into sequencing library preparation. For samples with less than 32 ng isolated, all extracted cfDNA was used for library preparation. The KAPA LTP Library Prep Kit was used for library preparation according to the manufacturer’s protocol with minor modifications (Kapa Biosystems). DNA was subjected to end repair and A-tailing, followed by ligation with custom made adapters.32 This was followed by a hybridization-based enrichment of specific sequences using a custom designed pool of biotinylated DNA oligonucleotides (Roche NimbleGen).32 Samples were sequenced using 2×150-bp paired-end reads on Illumina HiSeq4000. Sequencing data were processed using a custom bioinformatics pipeline and SNV was genotyped in all samples as previously described.32
Mutation calling
Next generation sequencing (NGS) was conducted on tumor samples from most patients via the MSK-IMPACT assay.34,35 Patient LUP640 solely had tumor AmpliSeq Cancer Hotspot Panel data. We aligned the chromosomal locations of MSK-IMPACT or AmpliSeq reported mutations with the CAPP-Seq lung cancer selector to identify overlapping positions. We also conducted tumor-naïve variant calling on all pre-treatment plasma samples. Tumor specific mutations were identified through comparison with corresponding leukocyte samples using previously described methods.36
Monitoring the kinetics of ctDNA
Mutations from MSK-IMPACT tumor sequencing and tumor-naïve calling of pretreatment plasma were combined to generate a tumor variant list to track ctDNA changes in patients’ serial plasma samples following treatment. Monitoring of tumor mutations in subsequent samples was performed using a previously described Monte-Carlo based statistical approach.30,32,36
Statistical analysis
Analysis of continuous variables, including the number of haploid genome equivalents (hGE) per mL of plasma and the number of detected mutations in responders and non-responders, was carried out using two-tailed Mann-Whitney tests, while analysis of categorical variables was performed using Fisher exact testing. All survival analyses were carried out using the Kaplan-Meier method with Log-rank (Mantel-Cox) analysis. Statistical analyses were done with GraphPad Prism version 8 (San Diego, California), and significance was defined as p <0.05.
Results
Patient and sample characteristics
We analyzed 122 samples from 21 patients with SCLC using CAPP-Seq, including 101 plasma and 21 germline samples. Clinical data are shown in Supplementary Table S1. Pre-treatment plasma was available in 19 patients (Figure 1a). All patients were treated with platinum-based chemotherapy, mostly in conjunction with etoposide. Four patients received a concurrent immune checkpoint inhibitor. Eighteen of the patients had ES disease, while three had limited stage disease. Eleven patients received thoracic radiation, which was completed after the first cycle of chemotherapy in all cases. One patient had a history of thoracic radiation more than ten years prior. Eight patients were female (38.1%) and the median age across all patients was 62. All patients had a history of current or former tobacco use, with a median of 31.5 pack-years among patients with available data (Supplementary Table S2).
Figure 1. Summary of patient characteristics and tumor variants detected in SCLC pretreatment plasma samples.

a. Clinical and molecular features of patients with SCLC included in this study. Each column represents an individual patient. Boxes are color coded for stage, treatment, RECIST, smoking status, tumor genotyping approach, and mutation type as indicated. Nonsynonymous mutations and indels in genes recurrently mutated in the cohort are shown in descending order of prevalence. Mutation recurrence rate in the cohort is depicted by bar graphs to the right. Carbo = carboplatin; etop = etoposide; pembro = pembrolizumab; atezo = atezolizumab. Best overall response to therapy was determined using RECIST PR = partial response; SD = stable disease; PD = progression of disease. b. Mean variant allele frequency (VAF%) of all mutations in responders vs. non-responders. c. Number of mutations detected in responders vs. non-responders. No differences were observed in either the mean VAF% (p=0.105) or number of mutations (p=0.11) detected in the baseline plasma sample between the two groups of patients.
Pre-treatment ctDNA analysis
Using tumor-naive CAPP-Seq analysis, involving sequencing of cfDNA and corresponding leukocyte DNA from plasma depleted whole blood to remove clonal hematopoiesis mutations, we detected ctDNA in 15 of the 19 patients (78.9%) with baseline plasma samples (Supplementary Table S3). The two patients with limited stage disease and baseline plasma available both had detectable ctDNA. The number of mutations detected ranged from 1 −18 (median 8). The most frequently mutated genes were TP53 (13/15, 86.7%), RB1 (4/15, 26.7%) and SLITRK3 (4/15, 26.7%).
We compared ctDNA detection using tumor-naïve versus tumor-informed ctDNA analysis. Tumor sequencing data was available for twelve of the 19 patients, including 11 using the MSK-IMPACT NGS assay, a tumor-normal targeted sequencing test, and for one patient using the AmpliSeq Cancer Hotspot Panel (Supplementary Figure S1a).30 Supplementary Table S-4 illustrates tumor mutations covered in the CAPP-Seq selector. For the eleven patients with baseline plasma, ten had at least one mutation identified by tumor sequencing that was also covered by the CAPP-Seq selector. (Supplementary Figure S1a). 468 regions consisting mainly of exons were covered by both the CAPP-Seq and MSK-IMPACT panels (Supplementary Figure S1b).
For patients with tumor sequencing, we examined differences in detection of tumor mutations by CAPP-Seq using tumor-informed versus tumor-naïve analysis. Patient LUP701 was excluded from this analysis as MSK-IMPACT only detected 2 indels that were not covered by CAPP-Seq. With the more sensitive tumor-informed approach, ctDNA was detected in the plasma in 9 out of the 9 (100%) patients who had SNVs detected in tumor tissue by MSK-IMPACT (Figure 2, Supplementary Table S5). Tumor-naïve ctDNA detection identified mutations in 5 out of the 9 (55.6%; Figure 2a), consistent with this approach having lower sensitivity.31,37 Among the mutations detected by tumor-naïve calling of pre-treatment plasma, 5 mutations were located at genomic positions also covered by MSK-IMPACT. All five were also reported on tissue testing (Figure 2b), indicating high positive predictive value.
Figure 2. Comparison of tumor-informed and tumor-naïve ctDNA detection.

a. Percent of patients with tumor variants covered by both MSK-IMPACT and CAPP-Seq panels in whom ctDNA was detected in baseline plasma samples using either the tumor-informed or tumor-naïve ctDNA detection modes of CAPP-Seq. b. Comparisons of VAFs in tumor tissue and pre-treatment plasma for five patients in whom tumor-naïve CAPP-Seq identified mutations in plasma that were also present on MSK-IMPACT tumor analysis.
To generate a comprehensive list of baseline mutations to track in subsequent plasma samples, we combined detection of MSK-IMPACT mutations by tumor-informed analysis and those detected by tumor-naïve calling by CAPP-Seq analysis of pre-treatment plasma samples (Supplementary Table S6). The median number of mutations detected in the 19 baseline plasma samples with the combined approach was 6 (range: 0–18). The most commonly mutated gene was TP53, found in 15 of the 19 patients (78.9%). Alterations in RB1 were detected in 6 patients (31.6%) (Figure 1a). The average absolute concentration of ctDNA in pre-treatment plasma samples was 14,168 hGE/mL (range: 0–196,094 hGE/mL; median: 1,767) and the average relative concentration was 19.1% (range: 0–54.2%; median: 13.1%) (Table S-7). No differences were observed in mean VAF (p=0.105) or number of mutations (p=0.11) detected in the baseline plasma sample between patients who went on to respond to therapy versus those who did not (Figures 1b–1c).
Changes in ctDNA concentration during treatment
In order to investigate ctDNA kinetics during therapy, we collected plasma samples at multiple time points during chemotherapy cycles(Figure 3a). There were no statistically significant differences in pre-treatment ctDNA concentrations between responders on first scans after two cycles of therapy versus non-responders (22.5% versus 4.6%, p=0.17; Figure 3b). Patients with eventual radiologic responses demonstrated significant decreases in ctDNA concentration at C2D1 (21 days after starting treatment) while those who never responded did not (Figure 3c). ctDNA concentration prior to treatment on C2D1, i.e. after a single cycle of chemotherapy, was significantly lower than baseline in eventual radiologic responders (p=0.0002) but not in non-responders (p=0.91). Median C2D1A VAF in responders was 0.4% compared to 12.2% in non-responders (Figure 3b).
Figure 3: ctDNA fold change after one cycle of chemotherapy stratifies responders and non-responders.

a. Event charts for patients in the present study. Each row represents a patient with circles depicting the time of blood collections relative to scheduled chemotherapy cycles (as annotated at the top of the figure). Arrow heads at the end of bars indicate that the patient has not progressed. Circles are color coded by mean VAF of detected alterations. Stars denote progression of disease by scans. PR = partial response, SD = stable disease; POD = progression of disease. b. Mean VAF in pre-treatment and cycle 2 day 1 plasma samples in responders and non-responders with available plasma at these time points. c. Time courses of ctDNA responses measured by mean VAF in radiologic responders and non-responders. d. Mean VAFs in C1D1A and C2D1A plasma samples and ctDNA fold change normalized to C1D1A VAFs in responders and non-responders with available plasma at these time points.
To further explore the ability of ctDNA changes after one cycle to predict radiologic response, we defined a threshold of a 2-fold decrease in ctDNA concentration from baseline to the start of cycle 2 based on a prior study of intra-day ctDNA variation.38 Fourteen patients had both pre-treatment C1D1A and C2D1A plasma. As shown in Figure 3d, all patients who ultimately developed radiologic responses had a decrease in mean VAF of at least 2-fold by the start of cycle 2, after just 21 days on treatment. The C2D1A mean VAF% of 4 patients dropped to below 0.05%. For the other seven patients, the C2D1A VAF fold drop ranged from 9.4 to 122 (median 46.4). Patients who did not respond had no decrease in VAF or a decrease of less than 2-fold (range: 1–1.2-fold drop; median: 1.1).
Changes in mean VAF suggestive of response preceded changes observed on scans. Figure 4a shows an example of ctDNA changes in a responder. The patient was a 61-year-old male former smoker (80 pack-years) who had ES disease, and was treated with carboplatin/etoposide. The patient had a 62-fold drop in mean VAF by C2D1A (Supplementary Figure S2a). Scans on day 40 showed a 44.7% decrease in tumor volume. By contrast, no significant drop in mean VAF was seen by C2D1 in a non-responder (Figure 4b; Supplementary Figure S2b). The patient was a 70-year-old female former smoker (25–30 pack-years) with ES disease, treated with carboplatin/etoposide. The patient’s day 37 scans showed SD, with progression on day 77 scans.
Figure 4. Patient vignettes for a representative responder and non-responder.

a. Changes in ctDNA mean VAF (left y-axis, indicated by green line) in a patient who achieved radiologic response. The black dotted line shows percent change in tumor burden by RECIST sum of lesion diameters (SLD; right y-axis) at days 85 and 138 days post treatment. Scans done on day 40 showed a 44.7% decrease in tumor burden by RECIST. B: Changes in ctDNA mean VAF (pink line) in a non-responder, with black dotted line showing no change in tumor burden by RECIST SLD on days 37 and 77 post treatment. Persistence of tumors was shown on scans done on day 37. The patient was classified as having RECIST progression on scans performed on day 77.
We explored whether ctDNA changes earlier than C2D1 were associated with radiologic response. Seven patients had plasma collected before the first chemotherapy infusion (C1D1A) as well as immediately after (C1D1B). The median fold change from pre- to post-infusion was 1.05 (range 0.89–1.44) and did not significantly differ between radiologic responders and non-responders (p=0.63; Figure 5a–5b). Six of the 7 patients had an increase in ctDNA on the first day of chemotherapy (fold-change from baseline 1.01–1.44). Three of these had a spike in ctDNA levels of ≥10%, one of whom responded and two of whom had SD.
Figure 5. ctDNA fold changes during the days of the first or second cycles of chemotherapy are not associated with radiologic response.
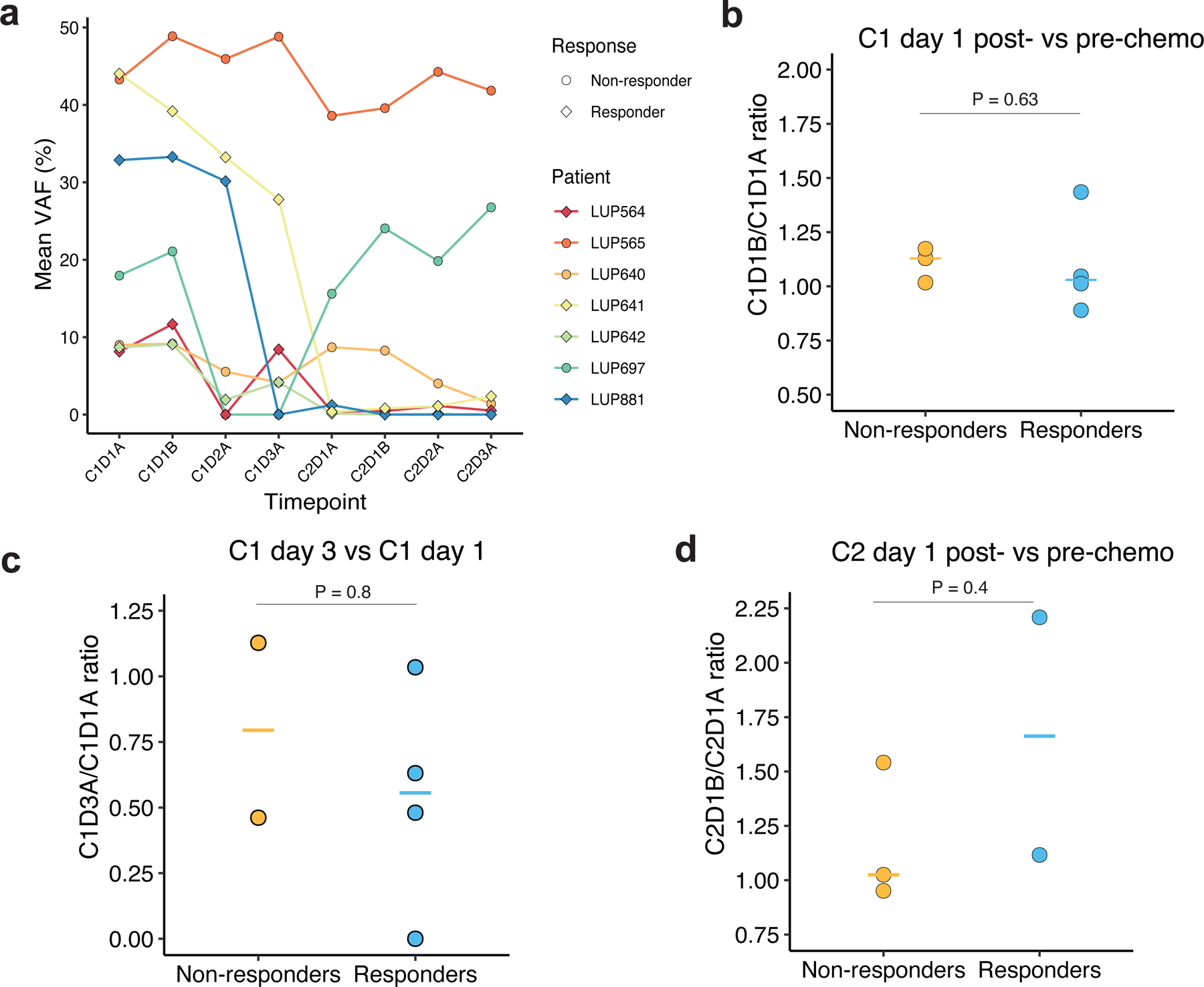
a. Time courses of ctDNA responses measured by mean VAF in patients with multiple same day plasma samples collected during the first two cycles of chemotherapy. b. Ratio of ctDNA concentration as measured by mean VAF in C1D1B (post-chemotherapy on cycle 1, day 1) compared to baseline plasma samples C1D1A (pre-chemotherapy on cycle 1, day 1). c: Ratio of ctDNA concentration as measured by mean VAF before chemotherapy on the third day of treatment in cycle 1 (C1D3A) compared to baseline, prior to start of cycle 1. d: Ratio of ctDNA concentration as measured by mean VAF in C2D1B (post-chemotherapy on cycle 2, day 1) compared to C2D1A plasma samples (pre-chemotherapy on cycle 2, day 1).
We also examined changes in ctDNA over the three consecutive infusion days of cycle 1. The median fold change between the third day of the first cycle (C1D3) and baseline was 0.56 in responders versus 0.79 in non-responders (p=0.8) and therefore did not correlate with BOR (Figure 5c). Five patients had plasma collected pre- and post-infusion on C2D1. Comparison of these time points revealed a median fold change of 1.11 (range 0.95–2.21) which also did not differ based on radiologic response (p=0.40) (Figure 5d). Thus, ctDNA changes predictive of radiographic response do not appear to occur immediately after chemotherapy infusion in SCLC.
Association of ctDNA concentration and kinetics with outcomes
With a median follow-up of 12.7 months, no significant differences were seen in overall or progression free survival between patients with a mean VAF at baseline above or below the median for the study. There was a trend toward better overall survival (OS) with lower pre-treatment ctDNA (Supplementary Fig S-3a). No survival differences were seen when the analysis was repeated using absolute pretreatment ctDNA concentration (Supplementary Figure S3b). By contrast, patients with a >2-fold decrease in mean VAF after one cycle had significantly longer PFS compared to those who did not (6.8versus 2.6 months, log-rank p=0.0004). OS among patients with baseline and C2D1 plasma available was also significantly longer among patients with a >2-fold drop in mean VAF (21.7 versus 6.4 months, log rank p=0.039) (Figure 6a–6b). Results were similar when analyzed using absolute ctDNA concentration, with a cut-off of >5-fold predicting survival (Supplementary Figure S4). LUP564 was excluded in fold change analyses relative to baseline since C1D1A cfDNA was extracted with a different method with lower cfDNA yield.
Figure 6. Fold change in relative ctDNA concentration after one cycle of chemotherapy is associated with patient outcomes.

a. Progression free survival of patients grouped according to whether they achieved a ≥2 fold-decrease in mean VAF comparing C2D1A to baseline plasma samples. The lower graph shows follow-up time, with the grey line representing median PFS (6.8 months versus 2.6 months, p=0.0004). b. Overall survival of patients grouped according to whether they achieved a ≥2 fold-decrease in mean VAF comparing C2D1A to baseline plasma samples. The lower graph shows follow-up time, with the grey line representing median OS (21.7 months versus 6.4 months, p=0.039).
In univariate analysis, the C2/C1 ratio of mean VAF%, calculated as a continuous variable, was associated with both OS and PFS, but only met statistical significance for PFS (OS: HR 4.99, p=0.057; PFS: HR 13.94, p=0.030). Other factors, such as type of treatment received, age, sex, and smoking pack-years, were not associated with either OS or PFS (Supplementary Figure S5).
We explored whether patient outcomes could be predicted using C2D1 samples alone. We observed a difference in mean VAF at C2D1A of radiologic responders and non-responders at a threshold of 5%, with all responders having VAFs below and all non-responders having VAFs above this threshold (Supplementary Figure S6a). Significant differences in both OS and PFS were observed between patients with C2D1 ctDNA concentration above and below this threshold (p=0.0082 and 0.0075, respectively; Supplementary Figure S6b–6c). Thus, a single ctDNA measurement at C2D1 may allow early identification of response to chemotherapy in SCLC.
Discussion
This study examined changes in ctDNA concentration early in the course of chemotherapy for SCLC. We found that changes in ctDNA concentration after a single cycle correlate with platinum sensitivity, but changes during the first 3 days of chemotherapy (i.e. during cycle 1) do not. To the best of our knowledge, our study is the first to examine changes in ctDNA concentrations in multiple ctDNA draws performed on the same day, before and after receipt of chemotherapy.
Our most significant finding was that all patients who eventually responded to platinum-based therapy by RECIST criteria had a >2-fold decrease in mean VAF by the start of cycle 2, just 21 days after starting therapy. This is earlier than has been reported by three studies on ctDNA in SCLC. Almodovar, et al., showed that tumor-specific copy number alterations in the plasma were able to detect relapse before imaging, at a median of 42 days post-treatment (Supplementary Figure S-7).24 Studies by Mohan, et al., and Nong., et al. demonstrated that changes in ctDNA levels measured on average 53 and 90 days post-baseline were indicative of radiologic response.23,25 In our study, a >2-fold decrease in mean VAF after just one cycle correlated with increased PFS and OS. Thus, patients with SCLC who do not show early signs of platinum-sensitivity based on ctDNA analysis may benefit from early initiation of alternative therapeutic strategies.
Through dense blood sampling during the course of treatment, we were able to show that ctDNA changes during days 1–3 of treatment within cycle 1 were not associated with radiologic outcomes. Prior animal research pointed to a spike in ctDNA in the hours following initiation of chemotherapy, which was hypothesized to occur secondary to apoptotic release of tumor DNA into the plasma.29,39 A handful of reports from human studies have also observed an increase in ctDNA occurring prior to radiologic response in selected patients, although the treatments and patient populations in these studies differed from ours. For example, a spike of ctDNA was seen within 2 weeks following the infusion of tumor infiltrating lymphocytes in patients treated for BRAF V600E mutant melanoma,40 and in two patients with non-small cell lung cancer (NSCLC) within one week of EGFR tyrosine kinase inhibitor initiation.41 A spike of total cfDNA was observed following the first cycle of neoadjuvant epirubicin treatment for breast cancer,39 and in select additional patients treated for NSCLC.42 In one study of first-line chemotherapy for colorectal cancer, including with oxaliplatin-based treatment, levels of ctDNA rose three days after initiation of chemotherapy in four patients, of whom three were responders. As in our study, this relatively early rise in ctDNA was not as strongly correlated with radiologic response as a drop in ctDNA prior to cycle 2.43
Thus, C2D1 appears to be the optimal time point for molecular response assessment in SCLC. It is theoretically possible that a time point between 1 day and 3 weeks after first infusion could be equally informative, but testing this would require future studies. Given that SCLC is an aggressive disease with the potential for rapid spread, early identification of non-responders could potentially guide therapy. Based on our study, one could envision a clinical trial that tests changing therapy for patients who do not have a drop in mean VAF% of ≥2-fold by the start of cycle 2.
Our study is limited in that patients were treated at a single institution and that it was retrospective. Moreover, only a small percentage of patients in our study were treated with concurrent chemoimmunotherapy. While the kinetics of changes in VAF in these patients appeared similar to the kinetics in patients treated with chemotherapy alone, we are currently carrying out further dedicated investigations on immunotherapy. Finally, while our study represents the first investigation analyzing ctDNA dynamics both pre- and post-chemotherapy at multiple time points, our cohort size resulted in limited power to detect small differences. We did not see a significant difference between ctDNA concentrations at baseline in responders versus non-responders (22.5% versus 4.6%, p=0.17). However, we cannot exclude the possibility that a larger study would detect a difference between pre-treatment ctDNA concentrations.
In summary, using dense, serial ctDNA collections across multiple time points, we found that changes in ctDNA after a single cycle of chemotherapy can determine platinum sensitivity in SCLC. Liquid biopsies may therefore enable early identification of patients who could benefit from alternative therapies and/or timely intensification of treatment.
Supplementary Material
Figure S1: Comparison of CAPP-Seq and MSK-IMPACT. a. Number of patients for whom somatic mutations to track were identified in pre-treatment plasma (by CAPP-Seq), tumor tissue (by MSK-IMPACT), or both. b. Overlap of genomic regions, determined by comparison of contiguous spaces of chromosomal positions for genes covered by the CAPP-Seq and MSK-IMPACT panels.
Figure S2: VAFs of individual mutations for patients included in Figure 4.
Figure S3: Lack of association of overall survival or progression free survival with baseline ctDNA levels. Overall and progression free survival stratified by baseline plasma sample a. mean variant allele fraction (median VAF = 13.1%) or b. absolute concentration (median hGE/mL=1767.2).
Figure S4: Fold change in absolute ctDNA concentration after one cycle of chemotherapy is associated with patient outcomes. a. Progression-free survival of patients grouped according to whether the patient achieved a >5 fold-decrease in absolute ctDNA concentration comparing C2D1A to pretreatment plasma samples. b. Overall survival of patients grouped according to whether the patient achieved a >5 fold-decrease in absolute ctDNA concentration comparing C2D1A to pretreatment plasma samples.
Figure S5: Clinical and ctDNA factors associated with overall and progression free survival. Results from the univariable Cox proportional hazards models including C2/C1 VAF ratio, platinum-based treatment regimen received, age, sex, and pack-years of smoking history.
Figure S6: ctDNA concentration prior to cycle 2 of chemotherapy is associated with response. a. Mean VAF in C2D1A samples of responders and non-responders. b. Overall survival and c. progression free survival of patients grouped according to C2D1A mean VAF%. All panels include patients LUP880 and LUP884 for whom no baseline plasma was available but who had C2D1A samples.
Figure S7: Results of current study compared to prior studies examining ctDNA in SCLC. L = limited stage SCLC; E = extensive stage SCLC.
Context Statement.
Key objective:
Tracking circulating tumor DNA (ctDNA) kinetics early during treatment for small cell lung cancer (SCLC) has the potential identify patients who are not responding and could allow for early treatment adaptation or intensification. We aimed to investigate early ctDNA kinetics by intensively tracking ctDNA dynamics in patients receiving platinum-based chemotherapy.
Knowledge generated:
We demonstrate that all patients who went on to have a radiologic response to treatment had a >2-fold decrease in ctDNA concentration after one cycle of treatment and that such a drop was associated with improved progression free and overall survival.
Relevance:
These results suggest that ctDNA may be able to guide early intensification of therapy and/or changes in treatment for patients with SCLC. They also contribute to characterizing the timeline of dynamic changes in ctDNA levels in response to platinum-based therapy.
Acknowledgments:
Y.R.M.G. gratefully acknowledges the support of the Andrew Sabin Family Foundation. She acknowledges support from the Druckenmiller Center for Lung Cancer Research and from the Society for MSK. She has received training through an institutional K30 grant from the NIH (CTSA UL1TR00457). This work was supported by the National Cancer Institute (R01CA188298, R01CA254179, and R01CA244526 to A.A.A. and M.D.), the Tobacco Related Disease Research Program (M.D.), the Virginia and D.K. Ludwig Fund for Cancer Research (A.A.A. and M.D.), the Bakewell Foundation (A.A.A. and M.D.), the CRK Faculty Scholar Fund (M.D.). This work benefitted from the NIH’s funding of the MSK Support Grant- Core Grant Program to Memorial Sloan Kettering Cancer Center (P30 CA008748) as well as funding from the Druckenmiller Center for Lung Cancer Research. The work was also funded by NIH R35 CA263816 and U24 CA213274 (C.M.R.) The authors gratefully acknowledge administrative support from Clare Wilhelm and Reeja Thomas.
Footnotes
Data from this paper was previously presented as a poster at the American Society for Clinical Oncology (ASCO) meeting, associated with an abstract found at: Journal of Clinical Oncology 2020;38:no15_suppl, abstract 9067. https://doi.org/10.1200/JCO.2020.38.15_suppl.9067
Conflict of interest statement:
Y.R.M.-G. reports travel, accommodation, and expenses from AstraZeneca and Loxo Oncology/ Eli Lilly. She acknowledges honoraria from Virology Education and Projects in Knowledge (for a CME program funded by an educational grant from Amgen). She has been on an advisory board for Revolution Medicines. She acknowledges associated research funding to the institution from Mirati Therapeutics, Bristol Myers Squibb, Loxo Oncology at Eli Lilly, Elucida Oncology, Taiho Oncology, Hengrui USA, Ltd/ Jiangsu Hengrui Pharmaceuticals, Luzsana Biotechnology, Endeavor Biomedicines, and AbbVie. She is an employee of Memorial Sloan Kettering Cancer Center, which has an institutional interest in Elucida. She acknowledges royalties from Rutgers University Press and Wolters Kluwer. She acknowledges food/beverages from Endeavor Biomedicines. Y.R. Murciano-Goroff acknowledges receipt of training through an institutional K30 grant from the NIH (CTSA UL1TR00457). She has received funding from a Kristina M. Day Young Investigator Award from Conquer Cancer, the ASCO Foundation, endowed by Dr. Charles M. Baum and Carol A. Baum. She is also funded by the Fiona and Stanley Druckenmiller Center for Lung Cancer Research, the Andrew Sabin Family Foundation, the Society for MSK, the Squeri Grant for Drug Development, and a Paul Calabresi Career Development Award for Clinical Oncology (NIH/NCI K12 CA184746) as well as through NIH/NCI R01 CA279264.
A.B-Y.H. reports no competing interests.
J.A.A.F. reports no competing interests.
E.G.H. reports no competing interests.
J.J.C. is an employee of Foresight Diagnostics, reports patent filings related to cancer biomarkers, and ownership interest in Foresight Diagnostics.
E.J.M. has served as a paid consultant for DeciBio.
R.F.B. reports no competing interests.
E.S.L. is an equity owner at Oncia Technologies; has a fiduciary role/position in Oncia Technologies.
D.G. reports research agreements with Varian, AstraZeneca, and Merck; consulting/honoraria with Medtronic, Johnson and Johnson, MedLearning Group, and Varian; advisory board member participation for GRAIL, Olympus, Medtronic, and AstraZeneca.
A.R. reports grants to the intuition for investigator-initiated trials from Varian Medical Systems, AstraZeneca, Merck, Pfizer, and Boehringer Ingelheim; consulting fees from Boehringer Ingelheim, AstraZeneca, Merck, and MoreHealth; Scientific Advisory Board Member for KEYLYNK-012 and KEYLYNK-013 studies at Merck; Vice President of the International Thymic Malignancies Interest Group; Board Member of the International Mesothelioma Interest Group; Lung Track Chair of the ASTRO Annual Meeting; and is a Member of the Board of Examiners of the American Board of Radiology.
M.S.G. reports no competing interests.
M.O. reports personal fees from PharmaMar, Novartis, Targeted Oncology, Bristol-Myers Squibb, Merck Sharp & Dohme, Jazz Pharmaceuticals, Pfizer, and the American Society for Radiation Oncology.
R.K. reports no competing interests.
V.A. reports no competing interests.
L.N. reports no competing interests.
J.S.R-F. reports receiving personal/consultancy fees from Goldman Sachs, REPARE Therapeutics, Paige.AI and Eli Lilly; membership on the scientific advisory boards of VolitionRx, REPARE Therapeutics, Paige.AI and Personalis; membership on the Board of Directors of Grupo Oncoclinicas, and ad hoc membership of the scientific advisory boards of Roche Tissue Diagnostics, Ventana Medical Systems, Novartis, Genentech and InVicro.
P.R. received institutional grant/funding: Grail, Illumina, Novartis, AstraZeneca, Epic Sciences, Invitae/ArcherDx, Biothernostics, Tempus, Inivata, Biovica, Guardant and consulting/Ad board/ddvisorry for Novartis, AstraZeneca, Pfizer, Lilly/Loxo, Prelude Therapeutics, Epic Sciences, Foundation Medicine, Inivata, Natera, Tempus, SAGA Diagnostics, Paige.ai, Guardant and is co-founder and board member for Odyssey Biosciences.
A.D. reports honoraria: 14ner/Elevation Oncology, Amgen, Abbvie, ArcherDX, AstraZeneca, Beigene, BergenBio, Blueprint Medicines, Bristol Myers Squibb, Chugai Pharmaceutical, EcoR1, EMD Serono, Entos, Exelixis, Helsinn, Hengrui Therapeutics, Ignyta/Genentech/Roche, Janssen, Loxo/Bayer/Lilly, Merus, Monopteros, MonteRosa, Novartis, Nuvalent, Pfizer, Prelude, Regeneron, Repare RX, Springer Healthcare, Takeda/Ariad/Millenium, Treeline Bio, TP Therapeutics, Tyra Biosciences, Verastem. Advisory Boards: Bayer, MonteRosa, Abbvie, EcoR1 Capital, LLC, Amgen, Helsinn, Novartis, Loxo/ Lilly, AnHeart Therapeutics. Consulting: MonteRosa, Innocare, Boundless Bio, Treeline Bio, Nuvalent, 14ner/Elevation Oncology, Entos, Prelude, Zymeworks. Associated Research Paid to Institution: Foundatin Medicine, GlaxoSmithKlein, Teva, Taiho, PharmaMar. Equity: mBrace. Copyright: Selpercatinib-Osimertinib (filed/pending). Royalties: Wolters Kluwer; Other (Food/Beverage): Merck, Puma, Merus, Boehringer Ingelheim; CME Honoraria: Answers in CME, Applied Pharmaceutical Science, Inc, AXIS, Clinical Care Options, Doc Congress, EPG Health, Harborside Nexus, , I3 Health, Imedex, Liberum, Medendi, Medscape, Med Learning, MedTalks, MJH Life Sciences, MORE Health, Ology, OncLive, Paradigm, Peerview Institute, PeerVoice, Physicians Education, Projects in Knowledge, Resources, Remedica Ltd , Research to Practice, RV More, Targeted Oncology, TouchIME, WebMD.
D.J. is a consultant with Merck, and Genentech; is on the Advisory Board at AstraZeneca.
J.M.I. receives institutional research support from Invitae, Guardant Health and Foresight Diagnostics; has received travel and research support from Intuitive Surgical; has served in an advisory role for AstraZeneca and Merck; has an equity ownership with LumaCyte, LLC.
W.V.L. reports no competing interests. Subsequent to the completion of the work, W.V.L. became an employee at AstraZeneca.
C.R. consulted regarding oncology drug development with AbbVie, Amgen, Astra Zeneca, Epizyme, Genentech/Roche, Ipsen, Jazz, Lilly, and Syros; serves on the Scientific Advisory Boards of Bridge Medicines, Earli, and Harpoon Therapeutics.
A.A.A. reports ownership interest in CiberMed and Foresight Diagnostics, patent filings related to cancer biomarkers, and paid consultancy from Genentech, Roche, Chugai, Gilead, and Celgene.
B.T.L. has served as an uncompensated advisor and consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Genentech, and Lilly. He has received research grants to his institution from Amgen, AstraZeneca, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, Hengrui USA, and Lilly. He has received academic travel support from Jiangsu Hengrui Medicine and MORE Health. He is an inventor on two institutional patents at MSK (US62/685,057, US62/514,661) and has intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press. He is supported by the Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748 and Research Project Grant R01CA249666 from the National Institutes of Health.
M.D. reports grants or contracts from AstraZeneca and Varian Medical Systems; royalties or licenses from Roche and Foresight Diagnostics; consulting fees from AstraZeneca, Boehringer Ingelheim, Genentech, Illumina, Gritstone Bio, and Regeneron; payments or honoraria from Bristol Myers Squibb, Japan and Novartis; support for attending meetings and/or travel from Foresight Diagnostics and Regeneron; has patents planned, issued or pending with Roche, Foresight Diagnostics, and Celgene; holds leadership or fiduciary role at Foresight Diagnostics; and holds stock or stock options in CiberMed Inc, Foresight Diagnostics, and Gritstone Bio.
Ethics approval statement: This study was approved by both the institutional review boards (IRBs) of MSK and the Stanford University School of Medicine.
Patient consent statement: Patients provided informed consent in accordance with the IRB policies of the MSK IRB.
Data availability statement:
Sequencing data will be deposited at the European Genome-Phenoma Archive (EGA), which is hosted by The European Bioinformatics Institute (EBI) and the Centre for Genomic Regulation (CRG), upon acceptance. Further information about EGA can be found on https://ega-archive.org.
Citations:
- 1.Govindan R, Page N, Morgensztern D, et al. : Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539–44, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Poirier JT, George J, Owonikoko TK, et al. : New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J Thorac Oncol 15:520–540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers LA, Rudin CM: Small cell lung cancer: where do we go from here? Cancer 121:664–72, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabari JK, Lok BH, Laird JH, et al. : Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 14:549–561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George J, Lim JS, Jang SJ, et al. : Comprehensive genomic profiles of small cell lung cancer. Nature 524:47–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J, Wang Y, Zhang Y, et al. : Comprehensive genomic profiling of small cell lung cancer in Chinese patients and the implications for therapeutic potential. Cancer Med 8:4338–4347, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudin CM, Durinck S, Stawiski EW, et al. : Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 44:1111–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalemkerian GP, Loo BW, Akerley W, et al. : NCCN Guidelines Insights: Small Cell Lung Cancer, Version 2.2018. J Natl Compr Canc Netw 16:1171–1182, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Dvorkin M, Chen Y, et al. : Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394:1929–1939, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Horn L, Mansfield AS, Szczęsna A, et al. : First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 379:2220–2229, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Rossi A, Di Maio M, Chiodini P, et al. : Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 30:1692–8, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Rudin CM, Awad MM, Navarro A, et al. : Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 38:2369–2379, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz DM, Scherer F, Jin MC, et al. : Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J Clin Oncol 36:2845–2853, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Park S, Kim WS, et al. : Correlation between progression-free survival, tumor burden, and circulating tumor DNA in the initial diagnosis of advanced-stage EGFR-mutated non-small cell lung cancer. Thoracic Cancer 9:1104–1110, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok T, Wu YL, Lee JS, et al. : Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res 21:3196–203, 2015 [DOI] [PubMed] [Google Scholar]
- 16.O’Leary B, Hrebien S, Morden JP, et al. : Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nature communications 9:896–896, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal C, Wang X, Ranganathan A, et al. : Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer 112:118–125, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Carter L, Rothwell DG, Mesquita B, et al. : Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nature Medicine 23:114–119, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Hou JM, Greystoke A, Lancashire L, et al. : Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 175:808–16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foy V, Fernandez-Gutierrez F, Faivre-Finn C, et al. : The clinical utility of circulating tumour cells in patients with small cell lung cancer. Transl Lung Cancer Res 6:409–417, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiltermann TJN, Pore MM, van den Berg A, et al. : Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 23:2937–2942, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Blackhall F, Frese KK, Simpson K, et al. : Will liquid biopsies improve outcomes for patients with small-cell lung cancer? Lancet Oncol 19:e470–e481, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Mohan S, Foy V, Ayub M, et al. : Profiling of Circulating Free DNA Using Targeted and Genome-wide Sequencing in Patients with SCLC. Journal of Thoracic Oncology 15:216–230, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almodovar K, Iams WT, Meador CB, et al. : Longitudinal Cell-Free DNA Analysis in Patients with Small Cell Lung Cancer Reveals Dynamic Insights into Treatment Efficacy and Disease Relapse. J Thorac Oncol 13:112–123, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nong J, Gong Y, Guan Y, et al. : Circulating tumor DNA analysis depicts subclonal architecture and genomic evolution of small cell lung cancer. Nat Commun 9:3114, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iams WT, Kopparapu PR, Yan Y, et al. : Blood-Based Surveillance Monitoring of Circulating Tumor DNA From Patients With SCLC Detects Disease Relapse and Predicts Death in Patients With Limited-Stage Disease. JTO Clinical and Research Reports [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devarakonda S, Sankararaman S, Herzog BH, et al. : Circulating Tumor DNA Profiling in Small-Cell Lung Cancer Identifies Potentially Targetable Alterations. Clin Cancer Res 25:6119–6126, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du M, Thompson J, Fisher H, et al. : Genomic alterations of plasma cell-free DNAs in small cell lung cancer and their clinical relevance. Lung Cancer 120:113–121, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Rago C, Huso DL, Diehl F, et al. : Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res 67:9364–70, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Newman AM, Bratman SV, To J, et al. : An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 20:548–54, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman AM, Lovejoy AF, Klass DM, et al. : Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 34:547–555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chabon JJ, Hamilton EG, Kurtz DM, et al. : Integrating genomic features for non-invasive early lung cancer detection. Nature 580:245–251, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azad TD, Chaudhuri AA, Fang P, et al. : Circulating Tumor DNA Analysis for Detection of Minimal Residual Disease After Chemoradiotherapy for Localized Esophageal Cancer. Gastroenterology 158:494–505.e6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD 17:251–264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature medicine 23:703–713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moding EJ, Liu Y, Nabet BY, et al. : Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nature Cancer 1:176–183, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellmann MD, Nabet BY, Rizvi H, et al. : Circulating Tumor DNA Analysis to Assess Risk of Progression after Long-term Response to PD-(L)1 Blockade in NSCLC. Clin Cancer Res 26:2849–2858, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Bai H, Hong C, et al. : Analyzing epidermal growth factor receptor mutation status changes in advanced non-small-cell lung cancer at different sampling time-points of blood within one day. Thorac Cancer 8:312–319, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma G, Wang J, Huang H, et al. : Identification of the plasma total cfDNA level before and after chemotherapy as an indicator of the neoadjuvant chemotherapy response in locally advanced breast cancer. Cancer Med 9:2271–2282, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi L, Pham TH, Payabyab EC, et al. : Circulating Tumor DNA as an Early Indicator of Response to T-cell Transfer Immunotherapy in Metastatic Melanoma. Clin Cancer Res 22:5480–5486, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riediger AL, Dietz S, Schirmer U, et al. : Mutation analysis of circulating plasma DNA to determine response to EGFR tyrosine kinase inhibitor therapy of lung adenocarcinoma patients. Sci Rep 6:33505, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phallen J, Leal A, Woodward BD, et al. : Early Noninvasive Detection of Response to Targeted Therapy in Non-Small Cell Lung Cancer. Cancer Res 79:1204–1213, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tie J, Kinde I, Wang Y, et al. : Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 26:1715–22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Comparison of CAPP-Seq and MSK-IMPACT. a. Number of patients for whom somatic mutations to track were identified in pre-treatment plasma (by CAPP-Seq), tumor tissue (by MSK-IMPACT), or both. b. Overlap of genomic regions, determined by comparison of contiguous spaces of chromosomal positions for genes covered by the CAPP-Seq and MSK-IMPACT panels.
Figure S2: VAFs of individual mutations for patients included in Figure 4.
Figure S3: Lack of association of overall survival or progression free survival with baseline ctDNA levels. Overall and progression free survival stratified by baseline plasma sample a. mean variant allele fraction (median VAF = 13.1%) or b. absolute concentration (median hGE/mL=1767.2).
Figure S4: Fold change in absolute ctDNA concentration after one cycle of chemotherapy is associated with patient outcomes. a. Progression-free survival of patients grouped according to whether the patient achieved a >5 fold-decrease in absolute ctDNA concentration comparing C2D1A to pretreatment plasma samples. b. Overall survival of patients grouped according to whether the patient achieved a >5 fold-decrease in absolute ctDNA concentration comparing C2D1A to pretreatment plasma samples.
Figure S5: Clinical and ctDNA factors associated with overall and progression free survival. Results from the univariable Cox proportional hazards models including C2/C1 VAF ratio, platinum-based treatment regimen received, age, sex, and pack-years of smoking history.
Figure S6: ctDNA concentration prior to cycle 2 of chemotherapy is associated with response. a. Mean VAF in C2D1A samples of responders and non-responders. b. Overall survival and c. progression free survival of patients grouped according to C2D1A mean VAF%. All panels include patients LUP880 and LUP884 for whom no baseline plasma was available but who had C2D1A samples.
Figure S7: Results of current study compared to prior studies examining ctDNA in SCLC. L = limited stage SCLC; E = extensive stage SCLC.
Data Availability Statement
Sequencing data will be deposited at the European Genome-Phenoma Archive (EGA), which is hosted by The European Bioinformatics Institute (EBI) and the Centre for Genomic Regulation (CRG), upon acceptance. Further information about EGA can be found on https://ega-archive.org.


