Abstract
Cucumis melo L., better known by its popular cultivar cantaloupe, is an economically significant crop in the Cucurbitaceae family. Melon peel and seeds have shown medicinal potential due to their numerous biological qualities, including anti-inflammatory, anti-cancer, antibacterial, hepatoprotective and immunomodulatory effects to treat cardiovascular disease, diabetes and oedema. This scoping review aims to broaden the research scope on the cancer-fighting abilities of melon extract and its half maximal inhibitory concentration (IC50). Three databases which are Scopus, ScienceDirect and PubMed were used to locate relevant publications utilising the keywords ‘melon’, ‘Cucumis melo’, ‘inhibitory activity’, ‘cancer’ and ‘anti-cancer’. The Preferred Reporting Items for Systematic and Meta-analyses extension for Scoping Review (PRISMA-ScR) framework was used in conducting this study. Out of 904 articles, 14 articles met the inclusion criteria and were used in this analysis. These articles were published in English between 2000 and 2023 with full text accessibility, specifically addressed the fruit cantaloupe (Cucumis melo L.) or melon and reported on any type of cancer. Cucumis melo extract showed promising anti-cancer action in both in vitro and in vivo investigations on eight different cancer types: cervical, colon, prostate, leukaemia, multiple myeloma, breast, hepatoma and ovarian cancer. A thorough analysis shows that some of the IC50 values were significantly low, especially in cases of colon and prostate cancer, indicating a significant anti-cancer effect. The substantial anti-cancer benefits of Cucumis melo fruit extracts point to the necessity for additional investigation into their potential for cancer therapy on each form of cancer.
Keywords: anti-cancer, Cucumis melo, half maximal inhibitory concentration (IC50), in vitro, in vivo
Introduction
Cucumis melo L., commonly referred to as the well-known melon cultivar cantaloupe, is a commercially important crop belonging to the Cucurbitaceae family (1–3). The fruit parts are eaten, while the peels and seeds are discarded. According Rolim et al. (4), increasing fruit consumption increases the volume of waste generated, notably the peels and seeds. These melon peels and seeds have shown medicinal potential (4–6). For example, the oil extracted from seed contains a high concentration of vitamin E and polyunsaturated fatty acids, primarily linoleic acid (5). Melon seeds are also an excellent source of natural antioxidants and may serve as nutritional components or as a fortifying material to extend shelf life (6). For instance, melon seed flour (MSF) was shown to be a promising by-product with high nutritional value by Çağındı et al. (7). It can be used as a good source of protein, fat and fibre for the creation of fortified functional foods. These findings demonstrate that MSF has a low moisture content, which lowers spoiling and preserves microbiological quality while also aiding in the preservation of nutrients (8). It has been discovered that all components of the melon, even the seeds and peels, have their own health benefits.
Melon varieties have shown several biological properties towards human health. For many years, numerous traditional medicine systems have employed various melon species including Cucumis melo L. to cure a variety of illnesses, such as cardiovascular disease, diabetes and oedema. The melon also possesses anti-inflammation, anti-cancer, antibacterial, hepatoprotective and immunomodulatory properties (9, 10). Despite this, few studies have highlighted the anti-cancer properties of Cucumis melo for each form of cancer. The cytotoxicity of muskmelon fruit and seed extract has been studied (4, 11) though these studies were only conducted on one cancer cell type and extract from one plant part either seeds, peel or whole fruit (12). Therefore, a thorough scoping study of Cucumis melo and its anti-cancer properties was performed by reviewing and compiling the current literature on the anti-cancer effects of melon extracts and the half-maximal inhibitory concentration (IC50) values of these extracts.
Methods
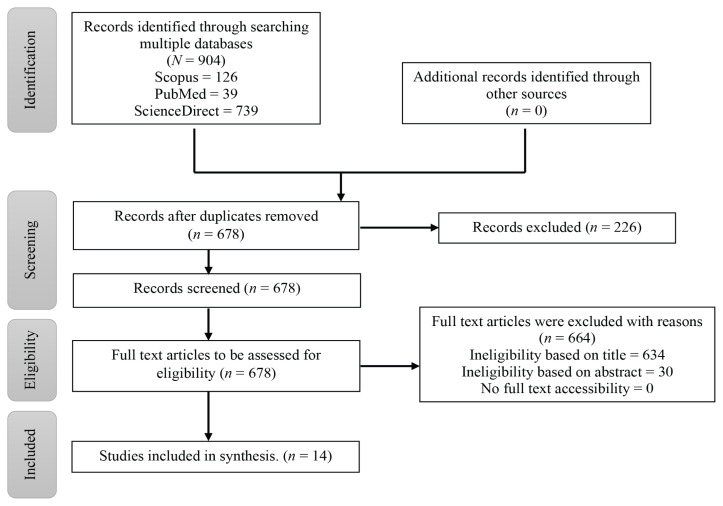
The purpose of this study was to provide a broad review of the most recent research on Cucumis melo extract and its anti-cancer properties. This scoping review was carried out in compliance with the Preferred Reporting Items for Systematic and Meta-analyses extension for Scoping Review (PRISMA-ScR) by Tricco et al. (13) which comprise identification, screening and eligibility, and included articles in the flow diagram (Figure 1).
Figure 1.
PRISMA-ScR by Tricco et al. (13) flow diagram of study selection
Identifying the Research Question
The review questions are: i) What are the anti-cancer properties of the melon? ii) What are the half-maximal inhibitory concentrations (IC50) values of melon extracts in the treatment of cancer cells?
Distinguishing Relevant Studies
English academic journal articles published between 2000 and 2023 were searched using electronic database searches with PubMed, Scopus and ScienceDirect. The search included all study types but did not include systematic reviews or review articles. The researchers separately assessed the eligibility of titles, abstracts and keywords. Out of 904 articles found using electronic databases, 14 studies were included in this review. Figure 1 shows the flow diagram of data selection. The keywords used were ‘melon’ AND ‘inhibitory activity’, ‘melon’ AND ‘anti-cancer’, ‘melon’ AND ‘inhibitory activity’ AND ‘cancer’, and ‘Cucumis melo’ AND (‘anti-cancer’ OR ‘MTT assay’ OR ‘cell viability’) with Boolean terms AND/OR to combine or separate the keywords as shown in Table 1 and Table 2.
Table 1.
Keywords and search strings used in electronic database search
| Keywords | Search strings |
|---|---|
| #1 melo | (Cucumis melo) OR (melon) |
| #2 anti-cancer | (anti-cancer) OR (cancer) OR (inhibitory activity) OR (MTT assay) OR (cell viability) |
| #3 | #1 AND #2 |
Table 2.
The process of selecting keywords in electronic databases through the utilisation of Boolean search operators
| Keywords | Scopus | PubMed | ScienceDirect | Total |
|---|---|---|---|---|
| “melon” AND “inhibitory activity” | 42 | 7 | 285 | 334 |
| “melon” AND “anti-cancer” | 55 | 25 | 269 | 349 |
| “melon” AND “inhibitory activity” AND “cancer” | 2 | 0 | 100 | 102 |
| “Cucumis melo” AND (“anti-cancer” OR “MTT assay” OR “cell viability”) | 27 | 7 | 85 | 119 |
|
| ||||
| Total | 904 | |||
Selecting Studies
The identified studies must satisfy the following eligibility criteria to be included in this review: i) fruits involved were of cantaloupe, Cucumis melo L. or melon; ii) reported on any type of cancer and iii) has full text accessibility.
Charting the Data
The data were summarised in a table form which includes the author, year of publication, country, fruit parts, pure compound/crude extract, human cancer line/tumour xenograft, IC50, concentration used and main findings.
Collating, Summarising and Reporting the Results
The collected results were subsequently compiled and summarised. Based on the study’s limitations that were discovered in the chosen articles, the research gaps were emphasised.
Results
Three databases yielded a total of 904 publications: 739 publications in ScienceDirect, 126 publications in Scopus and 39 publications in PubMed (Figure 1). The articles were uploaded to Mendeley and checked for duplication. A total of 226 articles were removed and the remaining 678 articles were evaluated for eligibility based on the title and abstract. A total of 634 articles were excluded due to ineligibility determined by their titles and an additional 30 articles were excluded based on the ineligibility criteria outlined in their abstracts. Those articles were disregarded since they did not underline the anti-cancer properties or focus on Cucumis melo. Only 14 articles were included and further analysed because they matched the predetermined inclusion requirements.
Characteristics of Study
From the year 2000 to 2010, only two studies (n = 2, 14.2%) were conducted regarding Cucumis melo and its anti-cancer properties. Since then, from 2011 to 2023, there have been a considerable rise in research (n = 12, 85.7%) evaluating the effectiveness of Cucumis melo extract as an anti-cancer agent (Table 3). China has the most discoveries in this field of study (n = 7, 50.0%) followed by Egypt (n = 2, 14.2%), Brazil, Iran, India, Japan and Taiwan (n = 1, 7.1%). All studies conducted in vitro evaluation (n = 14, 100%) including both human and animal cell lines and four of these also incorporated in vivo studies (n = 4, 28.6%)
Table 3.
Summary of the included studies (n = 14)
| Characteristics | Number of studies |
|---|---|
| Year of publication | |
| January 2000 to December 2010 | 2 (14.1%) |
| January 2011 to September 2023 | 12 (85.7%) |
| Study location | |
| China | 7 (50.0%) |
| Egypt | 2 (14.1%) |
| Brazil, Iran, India, Japan, Taiwan | 1 (7.1%) |
| Type of experiments | |
| in vitro | 14 (100%) |
| in vivo | 4 (28.6%) |
A variety of human cancer cell lines were employed in an extensive number of in vitro research evaluations. The most used cancer cell lines were cervical cancer cells (HeLa), adenocarcinomic human alveolar basal epithelial cells (A549), (n = 3, 21.4%), followed by breast cancer cells (MCF-7), colorectal adenocarcinoma cells (HT-29), colon adenocarcinoma cells (HCT-116), prostate cancer cells (PC-3) (n = 2, 14.2%) and hepatoma cancer cells (BEL-7402), human non-small cell lung carcinoma (H1299), human lung fibroblast cells (HLF), human glioblastoma multiforme cells (GBM8401), colorectal carcinoma cells (RCM-1), lymphoblast cells (K562), cervical carcinoma cells (SiHa), kidney carcinoma cells (786-0), mouse lymphoma cells (L5178Y) and rat brain cells (PC12) (n = 1, 7.1%) (Table 4). Meanwhile, the in vivo assessments incorporated tumour xenograft in mice.
Table 4.
Type of cell lines and animals
| Type of cell lines/animals | Number of studies |
|---|---|
| in vitro | |
| HeLa, A549 | 3 (21.4%) |
| MCF-7, HT-29, HCT-116, PC-3 | 2 (14.2%) |
| Jurkat, HepG2, SKOV-3, MM1.S, MM1.R, U266, RPMI 8266, BEL-7402, H1299, HLF, GBM 8401, RCM-1, K562, SiHa, 786-0, mouse lymphoma (L5178Y), rat brain (PC12) | 1 (7.1%) |
| in vivo | |
| MC4-L2 cells in BALB/c inbred mice breast tissue, MM1.S cell in CB17-SCID mice, BEL-7402 tumour xenograft implant into nude mice, LLC cells into nude mice. | 4 (28.6%) |
Notes: HeLa = cervical cancer cells; A549 = adenocarcinomic human alveolar basal epithelial cells; MCF-7 = breast cancer cell lines; HT-29 = colorectal adenocarcinoma; HCT 116 = colon adenocarcinoma; PC-3 = prostate cancer; Jurkat = human T lymphocytes cell; HepG2 = hepatoblastoma cell line; SKOV-3 = human ovarian cancer cell line; MM1.S = proliferation of dexamethasone-sensitive; MM1.R = proliferation of dexamethasone-resistant; U266 = B lymphocyte isolated from the peripheral blood of a 53-year-old, male patient with myeloma; RPMI 8226 = B lymphocyte that was isolated from the peripheral blood of a 61-year-old, male with plasmacytoma; BEL-7402 = hepatoma cancer; H1299 = human non-small cell lung carcinoma cell line; HLF = human lung fibroblasts cell; GBM 8401 = human glioblastoma multiforme cells; RCM-1 = colorectal carcinoma; K562 = lymphoblast cells; SiHa = Cervical carcinoma cell line; 786-0 = kidney carcinoma; L5178Y = mouse lymphoma; PC12 = rat brain; MC4-L2 = mouse breast cancer cell line; SCID = severe combined immunodeficiency; LLC = Lewis lung carcinoma
A wide range of assays were employed to assess the effectiveness of Cucumis melo extract on various cancer cell lines. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT) (n = 8, 57.1%) was the most used assay in the in vitro research followed by the Cell Counting Kit-8 assay (CCK-8), neutral red dye (n = 2, 14.2%) and 3-(4,5-dimethylthiazol-2-yl) -5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, trypan blue exclusion assay, sulforhodamine B (SRB) assay (n = 1, 7.1%) (Table 5). Histological examination was done for in vivo studies. It comprised evaluation of tumour weight, assessment of angiogenesis of breast cancer in BALB/c mice using trypsin inhibitor from Cucumis melo seed extract and evaluation of morphology, which comprised evaluation of photomicrography (fluorescent staining method) and evaluation of histopathology (haematoxylin and eosin stain method).
Table 5.
Type of assays used to assess anti-cancer activity
| Type of assays | Number of studies |
|---|---|
| in vitro | |
| MTT assay | 8 (57.1%) |
| CCK-8 assay, neutral red dye | 2 (14.2 %) |
| MTS assay, trypan blue exclusion assay, SRB assay | 1 (7.1%) |
| in vivo | |
| Histopathological examination | 4 (28.6%) |
Notes: MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; CCK-8 = Cell Counting Kit-8 assay; MTS = [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]; SRB = sulforhodamine B
Assessment of Study Outcomes
All 14 articles reported positive findings on the anti-cancer properties of Cucumis melo extract on several cancer types (Table 6). Ten studies highlighted the anti-cancer properties of pure compounds such as cucumol A (14), cucumol B (15), cucurbitacin B (CuB) (16–19), protein trypsin inhibitor (20), cucurbitacin E (CuE) (21, 22) and (methylthio)acetic acid (MTA) (23) from the Cucumis melo. Seven studies highlighted the molecular pathways responsible for the cytotoxic and anti-proliferative effects of Cucumis melo extract on several cancer cell lines: triggering TLR4/NLRP3/GSDMD-dependent pyroptosis (inflammatory cell cytokines are released and cells inflate with bubbles as a form of programmed cell death) (19), arresting cell cycle at G2/M phase (18) via GADD45γ gene expression and blocking cyclin B1/CDC2 complex in GBM8401 (22), inhibiting expression of angiogenesis-related genes (20), reducing c-Raf (16), inducing STAT3-dependent apoptosis cell cycle (21) and triggering apoptosis (23).
Table 6.
Anti-cancer properties of Cucumis melo and its half maximal inhibitory concentration (IC50) of reviewed studies (n = 14)
| No. | Author, year | Country | Fruit parts | Pure compound/crude extract | Human cancer cell line/tumour xenograft | IC50 | Concentration used | Main findings |
|---|---|---|---|---|---|---|---|---|
| 1. | Zhang et al., 2020 | Agricultural field in China |
Cucumis melo
|
Crude extract |
|
|
50, 150, 250, 350, 450 μg/mL |
|
|
|
**methanol extract |
||||||
| 2. | Li et al., 2013 | Markets in Guangzhou, China | Muskmelon (yellow pulp)
|
- |
|
*No record since the author only state the strongest anti-proliferative effects on 4 cancer line | 20, 40, 60, 80, 100 mg/mL from 200 mg/mL stock solution |
|
|
|
|
||||||
|
|
|
||||||
| 3. | Ibrahim et al., 2016 | El-Galaa village, Samalout, Minia, Egypt. |
Cucumis melo L. var reticulates
|
Pure compound- Cucumol A |
|
- | 50μL |
|
| 4. | Ibrahim et al., 2019 | Mankabad, Assiut, Egypt. |
Cucumis melo. L
|
Cucumol B |
|
|
- |
|
| 5. | Yang et al., 2017 | Sigma Aldrich (Cucurbitacins) |
Cucumis melo
|
Cucurbitacin B |
|
|
|
|
| 6. | Chan, Meng, et al., 2010 | Huisong Pharmaceutical |
Cucumis melo
Cucurbitacin B |
Cucurbitacin B |
|
|
0.01, 0.1, 1, 10, 100, 1000 μM |
|
| 7. | Yuan et al., 2021 | Chengdu, China |
Cucumis melo
|
Cucurbitacin B | A549 H1299 HLF LLC cells injected into mice |
0, 10, 100, 1000 nM |
|
|
| 8. | Chan et al., 2010 | Cucurbitacin B was obtained from ChromaDex, Inc |
|
1 μM to 50 μM |
|
|||
| 9. | Rezaei et al., 2022 |
Cucumis melo
|
Protein trypsin inhibitor (TI) Extract seed powder (EXT) |
|
TI - 0.3 mg/mL EXT-0.4 mg/mL |
5, 10, 25, 50, 100, 200, 300, 400, 800, 1200 μg/mL for both (TI) and (EXT) |
|
|
| 10. | Jing et al., 2020 | Shanghai, China | Cucumis melo. L | Cucurbitacin E |
|
*No cytotoxicity test done | 0, 0.25, 1.0, 2.5 μmol L−1 |
|
| 11. | Hsu et al., 2014 | Sigma, USA | Cucumis melo | CuE |
|
– | 0, 2.5, 5, 10 μM |
|
| 12. | Kamimura et al., 2023 | Kyoto, Japan | Cucumis melo var. conomon (Japanese pickling melon) | Methylthio acetic acid (MTA) |
|
– |
|
|
| 13. | Rolim et al., 2018 | - |
Cucumis melo L. reticulatus
|
Phenolic compounds |
|
|
0.1, 0.25, 0.5, 1.0 mg/mL |
|
|
|
|
||||||
| 14. | Ittiyavirah et al., 2014 | Cherthala, Alappuzha |
Cucumis melo. L.
|
Crude extract | PC-3 | 1.47 mg/mL | 100, 500, 1000 μg from 100 mg/mL |
|
Notes: Abbreviations used same as Table 4
In comparison to other cancer types, the anti-cancer activities of Cucumis melo extract against HCT-116 (12, 14) and HT-29 (4, 24) cell lines are the subject of the greatest research on melon anti-cancer properties (Table 7). In contrast to the study by Ibrahim et al. (14), which found an IC50 of 92.624 mg/mL for HCT-116, Zhang et al. (12) found the most potent IC50 at 0.25 mg/mL for seed chloroform extract and 0.26 mg/mL for whole fruit methanol extract (Table 8). Strong cytotoxic effects of Cucumis melo fruit extracts have also been reported by Ittiyavirah et al. (11) and Zhang et al. (12) against the PC-3 cell line. Ittiyavirah et al. (11) reported an IC50 of 1.47 mg/mL, whereas Zhang et al. (12) achieved an even more remarkable IC50 of about 0.34 mg/mL. These results demonstrate the noteworthy influence of Cucumis melo fruit extracts on prostate cancer and point to a promising direction for future studies in cancer therapy.
Table 7.
Studies of anti-cancer effects of melon on several types of cancer
| Parts of melon/phytochemical | Types of cancer | Number of studies |
|---|---|---|
| Seed | Colorectal cancer/colon cancer | 4 |
| Cervical cancer, breast cancer | 3 | |
| Prostate cancer, leukaemia, lung cancer, hepatoma, lymphoma, brain cancer, ovary cancer, kidney carcinoma | 1 | |
| Fruit/pulp | Prostate cancer, colon cancer | 2 |
| Leukaemia, cervical cancer, hepatoma, breast cancer | 1 | |
| Peel | Lung cancer, breast cancer, hepatoma, colon cancer, cervical cancer, colorectal cancer, kidney carcinoma | 1 |
| Stem - CuB | Multiple myeloma, hepatoma, non-small cancer lung cancer, leukaemia | 1 |
| Others: | ||
| •CuE | Lung cancer, brain malignant glioma | 1 |
| •MTA | Colorectal cancer | 1 |
Notes: CuB = Cucurbitacin B; CuE = Cucurbitacin E; MTA = methylthio acetic acid
Table 8.
Anti-cancer effects of melon on several type of cancer with its half maximal inhibitory concentration (IC50)
| Types of cancer | IC50 |
|---|---|
| Colon adenocarcinoma (HCT-116) | |
| Colorectal adenocarcinoma (HT-29) |
|
| Prostate cancer (PC-3) | |
| Cervical carcinoma (SiHa) |
|
| Cervical cancer (HeLa) | |
| Breast cancer (MCF-7) | |
| Multiple myeloma |
|
| Hepatoma cancer (BEL-7402) |
|
| Ovarian cancer (SKOV-3) |
|
| Leukaemia (jurkat) | |
| Kidney carcinoma (786-0) |
|
Notes:
chloroform extract,
methanol extract,
in vivo, MC4-L2 cells in BALB/c mice
Discussion
Research findings indicate that phytochemicals offer remarkable health advantages and are crucial in preventing human illnesses (12). The entire Cucurbitaceae family has several health benefits, with each fruit or vegetable having a distinct impact on human health (25). Cucumis melo is widely recognised for its advantageous pharmacological properties, which include anti-inflammatory, antioxidant, hepatoprotective, diuretic, anti-cancer, anti-ulcer and immunomodulatory effects (26). Notably, Cucumis melo contains bioactive substances that have shown anti-cancer effects, including cucumol A, cucumol B, phenolic compounds, protein trypsin inhibitor, MTA, CuB and CuE.
CuB is the most common and active compound among the cucurbitacin class and has attracted much interest from researchers worldwide (27). According to Zhang et al. (28) and Garg et al. (29), CuB activates the JAK/STAT, NF-κB, PI3K/AKT, Wnt/β-catenin and MAPK/ERK signalling pathways, causing apoptosis in a variety of cancer types. Particularly, the anti-cancer activity of CuB surpasses that of CuE, another well-studied component of cucurbitacin, as demonstrated by Jing et al. (21) and shows potential as a therapy agent for non-small cell lung cancer (30, 31). Further investigation by Yuan et al. (19) showed that CuB induced TLR4/NLRP3/GSDMD-dependent pyroptosis to suppress non-small cell lung cancer by elevating reactive oxygen species (ROS) and Ca2+, which may represent a viable target for treatment for this cancer type.
Additionally, Bajcsik et al. (32) identified CuE as a notable phytomolecule that is frequently present in therapeutic food plants from the Cucurbitaceae family. CuE is well known for its strong therapeutic potential. It has anti-inflammatory, immunomodulatory, hepatoprotective, and anti-cancer properties. For instance, an in vitro study by Hsu et al. (33) revealed that CuE therapy prevented brain metastasis of non-small cell lung cancer in mice model experiments, as well as the expression of yes-associated protein (YAP) and its downstream signalling genes in non-small cell lung cancer. CuE also had an anti-proliferative effect on A549 cells by acting as a tyrosine kinase inhibitor and interfering with the EGFR/MAPK signalling pathway (21).
This comprehensive scoping study evaluated the cytotoxic effects of Cucumis melo extract and its IC50 on several different cancer types. Cucumis melo might be capable of inhibiting nine different cancer types. The IC50 values of the extracts on the different cancer types ranged widely from 0.247 mg/mL to 178.384 mg/mL. Notably, the extract showed an outstanding IC50 of 0.24693 mg/mL on HCT 116 cell lines, indicating its potent inhibitory effect. The extract had the most inhibitory potency on HCT 116 when compared to the other cell lines (PC-3, Jurkat and HeLa) in the same investigation carried out by Zhang et al. (12). Furthermore, Li et al. (24) highlighted the correlation between stronger anti-proliferative efficiency and lower IC50 values.
Remarkably, the chloroform seed extract showed the greatest cytotoxicity, indicating an enhanced concentration of seed-derived bioactive substances. This association between higher levels of bioactive chemicals and enhanced cytotoxicity is consistent with previous research highlighting the direct connection between the quantity of active metabolites in plant extracts and their biological activities (34–36). Cucumol A, which is isolated from the seeds, showed significant cytotoxic action against HeLa cells and murine lymphoma cells (L5178Y) in a study conducted by Ibrahim et al. (14). Additionally, cucumol B, a new triterpene benzoate from Cucumis melo seeds, also acted as a cytotoxic agent on SKOV-3, MCF-7 and HCT 116 cells, as demonstrated by Ibrahim et al. (15). Terpenoids, especially those from cucurbitaceous plants, have been shown to exhibit chemopreventive and chemotherapeutic effects against various cancer types (15, 37).
Previously established research has demonstrated the efficacy of protease inhibitors in mitigating the risk of cancer development by inhibiting angiogenesis (38). A recent in vivo evaluation by Rezaei et al. (20) further substantiates this understanding, particularly focusing on a trypsin inhibitor extracted from Cucumis melo seeds. They showed that the trypsin inhibitor induced necrosis in tumour tissue, simultaneously suppressing the expression of pivotal genes associated with angiogenesis, such as matrix metalloproteinase genes (MMP-2, MMP-9) and vascular endothelial growth factor (VEGF). Moreover, the inhibitor manifested a dose-dependent positive influence on various tumour parameters, including height, width, depth and other crucial tissue characteristics.
This extensive body of research highlights the potential of Cucumis melo seed extracts as a source of various bioactive compounds with remarkable cytotoxic effects on a variety of cancer cell lines. These findings highlight the potential therapeutic use of trypsin inhibitors extracted from Cucumis melo seeds in treating cancer and provide insight into the complex ways in which these agents affect angiogenesis inhibition and other important aspects of tumour progression. The potentials of Cucumis melo extract as a powerful source of anti-cancer drugs are strengthened by this review. The data presented in this scoping review underscore the necessity of further investigation into the potential therapeutic uses of Cucumis melo in cancer treatment.
Even though most of the studies showed a positive effect of Cucumis melo on cancer treatment, this scoping review found a few research gaps. Firstly, the anti-cancer properties of melon extract have not been studied with respect to all types of cancer. This limitation in the scope of research raises the possibility that the observed treatment efficacy may be much more significant in malignancies that have not yet been investigated. Secondly, the publications did not emphasise the phytochemicals’ or the bioactive compound’s synergistic effects on prevention and treatment inside the crude extract. Thirdly, the evaluation of melon extract’s efficacy in treating cancer in vivo was limited to tumour xenografts. Prior to clinical trials, more information on in vivo study on dosages, administration modalities and immune system–cancer cell interactions is required. Finally, no clinical trial evaluations were provided despite the potent anti-cancer characteristics of melon extract in vitro and in vivo, highlighting the necessity for further investigation into the efficacy, efficiency, long-term impacts and safety prior to approving melon extract as a cancer treatment agent.
Conclusion
This scoping study highlights the remarkable anti-cancer activities of Cucumis melo extract against a range of malignancies, including leukaemia, multiple myeloma, breast, hepatoma, ovarian, colon, prostate and cervical cancer. The measured IC50 values, which ranged from 0.247 mg/mL to 178.384 mg/mL, show the difference in the potency of Cucumis melo extracts against different cancer cells. Additionally, some bioactive compounds found in Cucumis melo, including cucumol A, cucumol B, CuB, CuE, protein trypsin inhibitor and MTA, significantly suppress the growth of certain cancer kinds. Notably, the evaluation primarily employed human cancer cell lines for in vitro studies, while murine models were utilised for in vivo investigations.
The scoping review reveals several remarkable IC50 values of Cucumis melo extract, particularly against colon and prostate cancer cells, suggesting a prominent anti-cancer effect. A comparison of the IC50 values from two distinct investigations revealed a pattern, with the most recent studies providing a more potent IC50 inhibitor value in contrast to the earlier research. For a thorough understanding, further combination studies are necessary to determine whether the in vitro synergy is also present in animal models. It is crucial to investigate drug interactions, especially in cases of prostate and colon cancers as it shows potential for future cancer therapy. Further research aimed at bridging the gap between in vitro and in vivo settings and exploring pathways in various cancer types will help us better grasp the therapeutic potential of Cucumis melo and explore the safety issues.
Acknowledgements
The authors would like to thank the PhD students and lectures at the Biomedicine programme, School of Health Sciences, Universiti Sains Malaysia for the feedback on search strategy. The author’s cooperative efforts in reviewing and assisting with the final manuscript’s improvement are recognised for their significant contributions to the intellectual content.
Footnotes
Conflict of Interest: None.
Funds: None.
Authors’ Contributions: Conception and design: HH
Analysis and interpretation of the data: RSSRS
Drafting of the article: RSSRS
Critical revision of the article for important intellectual content: HH, MZK
Final approval of the article: HH
Administrative, technical or logistic support: MZK
Collection and assembly of data: RSSRS
References
- 1.Chen S, Zhong K, Li Y, Bai C, Xue Z, Wu Y. Evolutionary analysis of the Melon (Cucumis melo L.) GH3 gene family and identification of GH3 genes related to fruit growth and development. Plants. 2023;12(6):1382. doi: 10.3390/plants12061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napolitano M, Terzaroli N, Kashyap S, Russi L, Jones-Evans E, Albertini E. Exploring heterosis in melon (Cucumis melo L.) Plants. 2020;9(2):282. doi: 10.3390/plants9020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores-León A, García-Martínez S, González V, Garcés-Claver A, Martí R, Julián C. Grafting snake Melon [Cucumis melo L. subsp. melo Var. flexuosus (L.) Naudin] in organic farming: effects on agronomic performance; resistance to pathogens; sugar, acid, and VOC profiles; and consumer acceptance. Front Plant Sci. 2021;12:613845. doi: 10.3389/fpls.2021.613845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolim PM, Fidelis GP, Padilha CE, Santos ES, Rocha HA, Macedo GR. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Braz J Med Biol Res. 2018;51(4):e6069. doi: 10.1590/1414-431X20176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabadán A, Nunes MA, Bessada SM, Pardo JE, Oliveira MB, Álvarez-Ortí M. From by-product to the food chain: melon (Cucumis melo L.) seeds as potential source for oils. Foods. 2020;9(10):1341. doi: 10.3390/foods9101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeb A. Phenolic profile and antioxidant activity of melon (Cucumis Melo L.) seeds from Pakistan. Foods. 2016;5(4):67. doi: 10.3390/foods5040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Çağındı Ö, Akca EE, Köse E. Melon seed: a nutritionally valuable by-product and its effects on cake quality. Food Chem. 2013;427:136679. doi: 10.1016/j.foodchem.2023.136679. [DOI] [PubMed] [Google Scholar]
- 8.Ntuli V, Mekbib SB, Asita AO, Molebatsi N, Makotoko M, Chatanga P. Microbial and physicochemical characterization of maize and wheat flour from a milling company, lesotho. Internet J Food Safety. 2013;15(2013):11–19. [Google Scholar]
- 9.Debnath P, Das B, Singha S, Kar A, Haldar PK, Sharma N, et al. Quantification of cucurbitacin E in different varieties of melon (Cucumis melo L.) fruit through validated RP-HPLC method. Nat Prod Res. 2022;38(7):1273–1279. doi: 10.1080/14786419.2022.2136656. [DOI] [PubMed] [Google Scholar]
- 10.Varela C, Melim C, Neves BG, Sharifi-Rad J, Calina D, Mamurova A, et al. Cucurbitacins as potential anticancer agents: new insights on molecular mechanisms. J Transl Med. 2022;20(1):630. doi: 10.1186/s12967-022-03828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ittiyavirah SP, George A, Santhosh AM, Kurian ST, Pappachan P, Jacob G. Studies of cytotoxic potential of aqueous fruit extract of Cucumis melo Linn in prostate cancer cell lines PC-3 using MTT and neutral red assay. Iranian J Pharmacol Therapeutics. 2014;13(1):19–25. [Google Scholar]
- 12.Zhang X, Bai Y, Wang Y, Wang C, Fu J, Gao L, et al. Anticancer properties of different solvent extracts of Cucumis melo L. Seeds and whole fruit and their metabolite profiling using HPLC and GC-MS. BioMed Res Int. 2020;2020:1–9. doi: 10.1155/2020/5282949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. https://www.acpjournals.org/doi/10.7326/M18-0850 . [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim S, Al-Haidari R, Mohamed G, Elkhayat E, Moustafa M. Cucumol A: a cytotoxic triterpenoid from Cucumis melo seeds. Rev Bras Farmacogn. 2016;26(6):701–704. doi: 10.1016/j.bjp.2016.03.012. [DOI] [Google Scholar]
- 15.Ibrahim SRM, Khedr AIM, Mohamed GA, Zayed MF, El-Kholy AAES, Al-Haidari RA. Cucumol B, a new triterpene benzoate from Cucumis melo seeds with cytotoxic effect toward ovarian and human breast adenocarcinoma. J Asian Nat Prod Res. 2019;21(11):1112–1118. doi: 10.1080/10286020.2018.1488832. [DOI] [PubMed] [Google Scholar]
- 16.Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, et al. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Letters. 2010;294(1):118–124. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Chan KT, Li K, Liu SL, Chu KH, Toh M, Xie WD. Cucurbitacin B inhibits STAT3 and the Raf/MEK/ERK pathway in leukemia cell line K562. Cancer Letters. 2010;289(1):46–52. doi: 10.1016/j.canlet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Liu J, Yang M, Huang N, Zhong Y, Zeng T, et al. Cucurbitacin B exerts anti-cancer activities in human multiple myeloma cells in vitro and in vivo by modulating multiple cellular pathways. Oncotarget. 2017;8(4):5800–5813. doi: 10.18632/oncotarget.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan R, Zhao W, Wang QQ, He J, Han S, Gao H, et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res. 2021;170:105748. doi: 10.1016/j.phrs.2021.105748. [DOI] [PubMed] [Google Scholar]
- 20.Rezaei S, Azarpira N, Koohpeyma F, Yousefi R, Doaei S, Heidari M, et al. Evaluation of morphology and angiogenesis of breast cancer in BALB/c mice using trypsin inhibitor from Cucumis melo seeds. In vitro and in vivo study. Contemp Oncol (Pozn) 2022;26(3):204–219. doi: 10.5114/wo.2022.120700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing S, Wu Z, Zhang T, Zhang J, Wei Z. In vitro antitumor effect of cucurbitacin E on human lung cancer cell line and its molecular mechanism. Chinese J Nat Med. 2020;18(7):483–490. doi: 10.1016/s1875-5364(20)30058-3. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YC, Chen MJ, Huang TYC. Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45γ in human brain malignant glioma (GBM) 8401 cells. Cell Death Dis. 2014;5(2):e1087. doi: 10.1038/cddis.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamimura M, Sasaki A, Otani Y, Nakamura Y, Nakamura T, Kuramochi K, et al. Methylthi oacetic acid, a derivative of aroma compounds from Cucumis melo var. conomon dose-dependently triggers differentiation and apoptosis of RCM-1 human colorectal cancer cells. J Toxicol Sci. 2023;48(1):25–35. doi: 10.2131/jts.48.25. [DOI] [PubMed] [Google Scholar]
- 24.Li F, Li S, Li HB, Deng GF, Ling WH, Wu S, et al. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J Funct Foods. 2013;5(3):1298–1309. doi: 10.1016/j.jff.2013.04.016. [DOI] [Google Scholar]
- 25.Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen, et al. Cucurbita plants: from farm to industry. Applied Sci. 2019;9(16):3387. doi: 10.3390/app9163387. [DOI] [Google Scholar]
- 26.Manchali S, Chidambara MKN, Vishnuvardana, Patil BS. Nutritional composition and health benefits of various botanical types of Melon (Cucumis melo L.) Plants. 2021;10(9):1755. doi: 10.3390/plants10091755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai S, Wang C, Zhao X, Ma C, Fu K, Li Y, et al. Cucurbitacin B: a review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol Res. 2022;187:106587. doi: 10.1016/j.phrs.2022.106587. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Bian Z, Zhang Y, Wang J, Kan L, Wang X, et al. Cucurbitacin B inhibits proliferation and induces apoptosis via STAT3 pathway inhibition in A549 lung cancer cells. Mol Med Rep. 2014;10(6):2905–2911. doi: 10.3892/mmr.2014.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg S, Kaul SC, Wadhwa R. Cucurbitacin B and cancer intervention: chemistry, biology and mechanisms (Review) Int J Oncol. 2018;52:19–37. doi: 10.3892/ijo.2017.4203. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Xiang Y, Liu X, Zhang T, Yang R, Chen S, et al. Cucurbitacin B induces the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/Akt signaling axis in gefitinib-resistant non-small cell lung cancer. Molecules. 2019;24(3):647. doi: 10.3390/molecules24030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg S, He H, Kumari A, Sundar D, Kaul SC, Wadhwa R. Induction of senescence in cancer cells by a novel combination of Cucurbitacin B and Withanone: molecular mechanism and therapeutic potential. J Gerontol. 2020;75(6):1031–1041. doi: 10.1093/gerona/glz077. [DOI] [PubMed] [Google Scholar]
- 32.Bajcsik N, Pfab R, Pietsch J. Simultaneous determination of cucurbitacin B, E, I and E-glucoside in plant material and body fluids by HPLC-MS. J Chromatogr B. 2017;1052:128–134. doi: 10.1016/j.jchromb.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Hsu P, Tian B, Yang Y, Wang Y, Liu S, Anatoly U, et al. Cucurbitacin E inhibits the Yes-associated protein signaling pathway and suppresses brain metastasis of human non-small cell lung cancer in a murine model. Oncol Rep. 2019;42(2):697–707. doi: 10.3892/or.2019.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohanty SK, Mallappa KS, Godavarthi A, Subbanarasiman B, Maniyam A. Evaluation of antioxidant, in vitro cytotoxicity of micropropagated and naturally grown plants of Leptadenia reticulata (Retz.) Wight & Arn.-an endangered medicinal plant. Asian Pac J Trop Med. 2014;7:S267–S271. doi: 10.1016/s1995-7645(14)60244-3. [DOI] [PubMed] [Google Scholar]
- 35.Perry PL, Wang Y, Lin J. Analysis of honeydew melon (Cucumis melovar inodorus) flavour and GC-MS/MS identification of (E,Z)-2,6-nonadienyl acetate. Flavour Fragr J. 2009;24(6):341–347. doi: 10.1002/ffj.1947. [DOI] [Google Scholar]
- 36.Swamy MK, Arumugam G, Kaur R, Ghasemzadeh A, Yusoff M, Mohd, et al. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid Based Complement Alternat Med. 2017;2017:1–10. doi: 10.1155/2017/1517683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs. 2009;20(10):880–892. doi: 10.1097/cad.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 38.Mills PK, Beeson WL, Abbey DE, Fraser GE, Phillips RL. Dietary habits and past medical history as related to fatal pancreas cancer risk among adventists. Cancer. 1988;61(12):2578–2585. doi: 10.1002/1097-0142(19880615)61:12<2578:aid-cncr2820611232>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]