Abstract
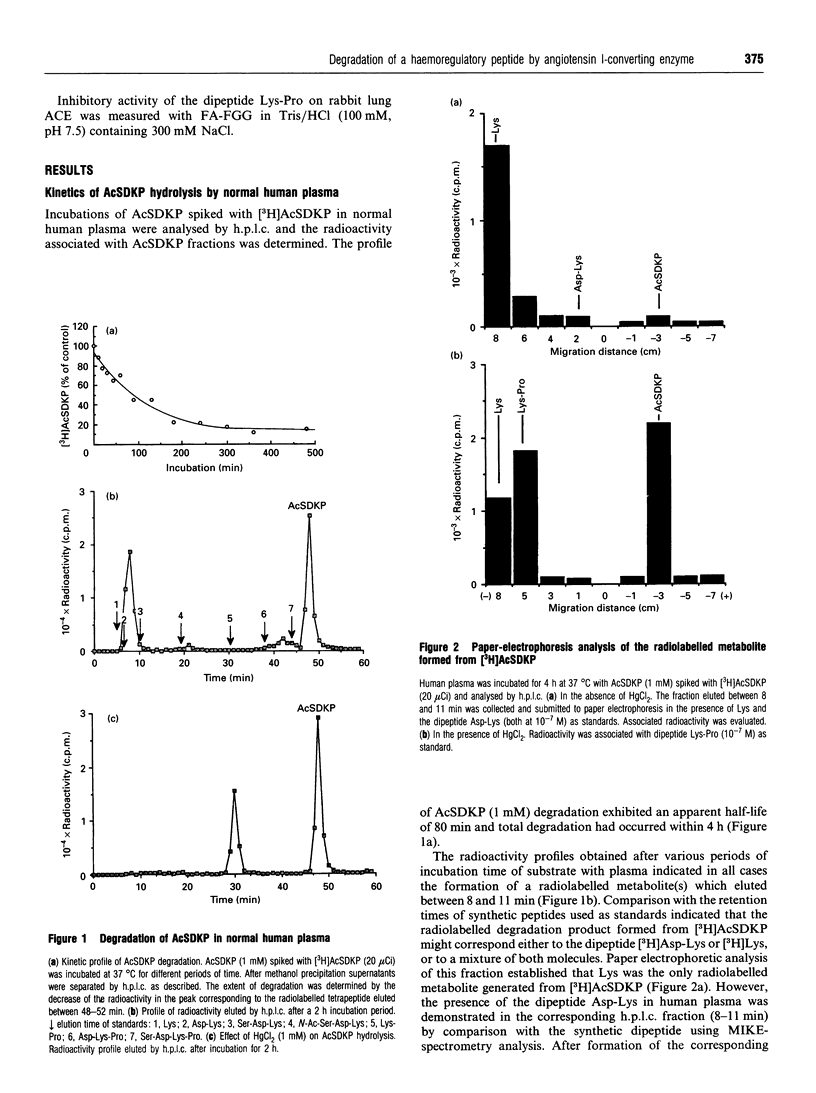
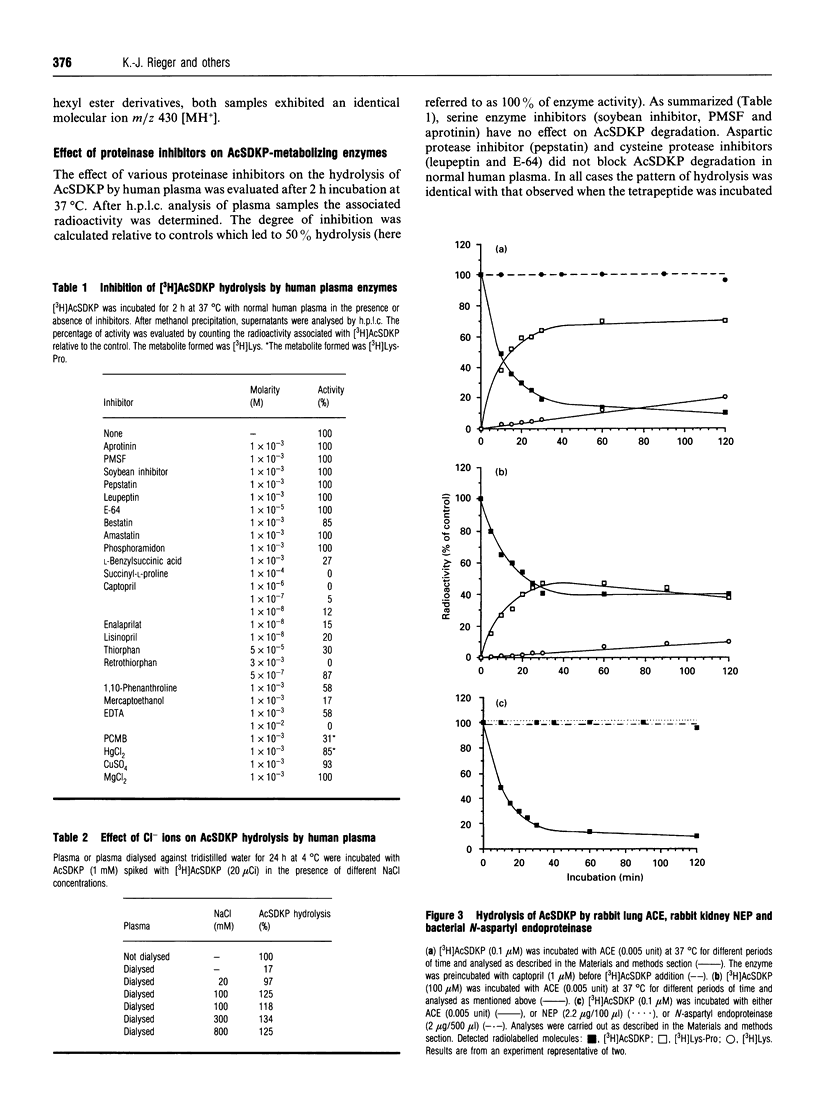
The degradation of N-Ac-Ser-Asp-Lys-Pro (AcSDKP), a negative regulator controlling the proliferation of the haematopoietic stem cell, by enzymes present in human plasma, has been investigated. Radiolabelled AcSD[4-3H]KP ([3H]AcSDKP, 1 mM) was completely metabolized in human plasma with a half-life of 80 min, leading exclusively to the formation of radiolabelled lysine. The cleavage of AcSDKP was insensitive to classical proteinase inhibitors including leupeptin, but sensitive to metalloprotease inhibitors. The degradation was completely blocked by specific inhibitors of angiotensin I-converting enzyme (ACE; kininase II; peptidyldipeptide hydrolase, EC 3.4.15.1), showing that the first step of the hydrolysis was indeed due to ACE. In dialysed plasma, the hydrolysis proceeded at only 17% of the maximal rate, whereas addition of 20 mM NaCl led to the recovery of the initial rate observed with normal plasma. Hydrolysis of AcSDKP by commercial rabbit lung ACE generated the C-terminal dipeptide Lys-Pro. Thus, ACE cleaves AcSDKP by a dipeptidyl carboxypeptidase activity. In fact the formation of Lys-Pro was observed when AcSDKP was incubated in human plasma in the presence of HgCl2. These results suggest that ACE is involved in the first limiting step of AcSDKP degradation in human plasma. The second step seems to be under the control of a leupeptin- and E-64-insensitive, HgCl2-sensitive plasmatic enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumont A., Brouet J. C., Roques B. P. Neutral endopeptidase 24.11 and angiotensin converting enzyme like activity in CALLA positive and CALLA negative lymphoid cells. Biochem Biophys Res Commun. 1989 May 15;160(3):1323–1329. doi: 10.1016/s0006-291x(89)80148-2. [DOI] [PubMed] [Google Scholar]
- Bogden A. E., Carde P., de Paillette E. D., Moreau J. P., Tubiana M., Frindel E. Amelioration of chemotherapy-induced toxicity by cotreatment with AcSDKP, a tetrapeptide inhibitor of hematopoietic stem cell proliferation. Ann N Y Acad Sci. 1991;628:126–139. doi: 10.1111/j.1749-6632.1991.tb17230.x. [DOI] [PubMed] [Google Scholar]
- Bünning P., Holmquist B., Riordan J. F. Substrate specificity and kinetic characteristics of angiotensin converting enzyme. Biochemistry. 1983 Jan 4;22(1):103–110. doi: 10.1021/bi00270a015. [DOI] [PubMed] [Google Scholar]
- Dubreuil P., Fulcrand P., Rodriguez M., Fulcrand H., Laur J., Martinez J. Novel activity of angiotensin-converting enzyme. Hydrolysis of cholecystokinin and gastrin analogues with release of the amidated C-terminal dipeptide. Biochem J. 1989 Aug 15;262(1):125–130. doi: 10.1042/bj2620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindel E., Guigon M. Inhibition of CFU entry into cycle by a bone marrow extract. Exp Hematol. 1977 Jan;5(1):74–76. [PubMed] [Google Scholar]
- Grillon C., Rieger K., Bakala J., Schott D., Morgat J. L., Hannappel E., Voelter W., Lenfant M. Involvement of thymosin beta 4 and endoproteinase Asp-N in the biosynthesis of the tetrapeptide AcSerAspLysPro a regulator of the hematopoietic system. FEBS Lett. 1990 Nov 12;274(1-2):30–34. doi: 10.1016/0014-5793(90)81322-f. [DOI] [PubMed] [Google Scholar]
- Hinman L. M., Stevens C., Matthay R. A., Bernard J., Gee L. Angiotensin convertase activities in human alveolar macrophages: effects of cigarette smoking and sarcoidosis. Science. 1979 Jul 13;205(4402):202–203. doi: 10.1126/science.221980. [DOI] [PubMed] [Google Scholar]
- Lenfant M., Wdzieczak-Bakala J., Guittet E., Prome J. C., Sotty D., Frindel E. Inhibitor of hematopoietic pluripotent stem cell proliferation: purification and determination of its structure. Proc Natl Acad Sci U S A. 1989 Feb;86(3):779–782. doi: 10.1073/pnas.86.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord B. I., Mori K. J., Wright E. G., Lajtha L. G. Inhibitor of stem cell proliferation in normal bone marrow. Br J Haematol. 1976 Nov;34(3):441–445. doi: 10.1111/j.1365-2141.1976.tb03590.x. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Erdös E. G. Novel activity of human angiotensin I converting enzyme: release of the NH2- and COOH-terminal tripeptides from the luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1025–1029. doi: 10.1073/pnas.82.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry J., Papet M. P., Saez-Servent N., Plissonneau-Haumont J., Potier P., Lenfant M. Synthesis and activity of NAcSerAspLysPro analogues on cellular interactions between T-cell and erythrocytes in rosette formation. J Med Chem. 1990 Aug;33(8):2122–2127. doi: 10.1021/jm00170a012. [DOI] [PubMed] [Google Scholar]
- Wdzieczak-Bakala J., Fache M. P., Lenfant M., Frindel E., Sainteny F. AcSDKP, an inhibitor of CFU-S proliferation, is synthesized in mice under steady-state conditions and secreted by bone marrow in long-term culture. Leukemia. 1990 Mar;4(3):235–237. [PubMed] [Google Scholar]
- Wei L., Alhenc-Gelas F., Soubrier F., Michaud A., Corvol P., Clauser E. Expression and characterization of recombinant human angiotensin I-converting enzyme. Evidence for a C-terminal transmembrane anchor and for a proteolytic processing of the secreted recombinant and plasma enzymes. J Biol Chem. 1991 Mar 25;266(9):5540–5546. [PubMed] [Google Scholar]
- Wu Q., Li L., Cooper M. D., Pierres M., Gorvel J. P. Aminopeptidase A activity of the murine B-lymphocyte differentiation antigen BP-1/6C3. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):676–680. doi: 10.1073/pnas.88.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


