ABSTRACT
Pneumatosis cystoides intestinalis (PCI) is an uncommon condition characterized by the presence of a collection of individual gas cysts in the submucosa and subserosa of the intestine. The etiology of PCI is still unclear. We experienced 3 cases with PCI during treatment for pulmonary Mycobacterium avium complex (MAC) infection. Each case was treated conservatively. We believe our case series will highlight the importance of examining the gastrointestinal tract of patients with MAC infection and hopefully elucidate the clinical characteristics of PCI which developed during MAC treatment.
KEYWORDS: endoscopy, mycobacterium avium complex infection, pneumatosis cystoides intestinalis, colon, antibiotics
INTRODUCTION
Pneumatosis cystoides intestinalis (PCI) is an uncommon condition characterized by the presence of a collection of individual gas cysts in the submucosa and subserosa of the intestine. The overall incidence is 0.03%.1 Most patients are asymptomatic or develop nonspecific gastrointestinal symptoms.2,3 Although the clinical etiology remains unknown, a few theories were proposed, one of which suggests a link with pulmonary disease. We encountered 3 cases that developed PCI during treatment for Mycobacterium avium complex (MAC) pulmonary disease. By reporting them, we can hopefully provide clues as to the etiology of PCI.
CASE REPORT
Case 1
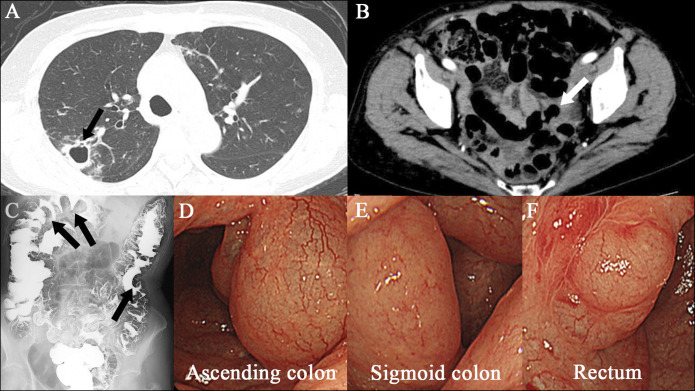
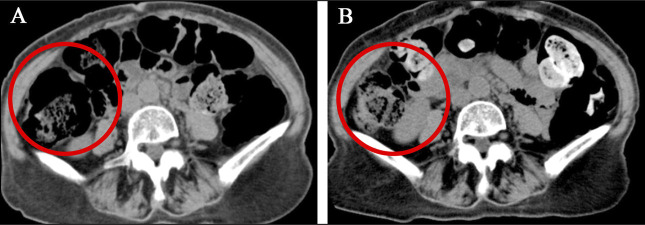
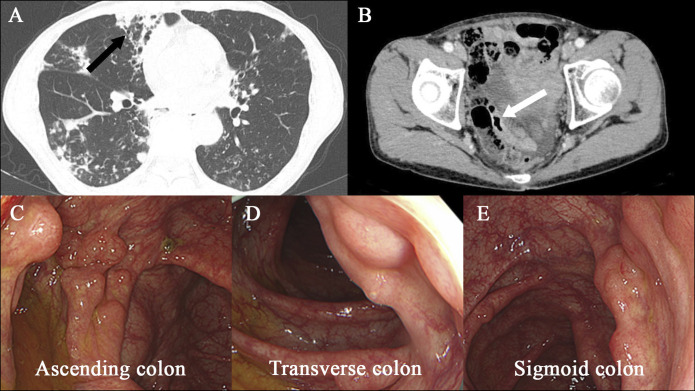
A 75-year-old woman with a history of MAC pulmonary disease presented to this hospital for newly developed bloody sputum. She had been on treatment with clarithromycin (CAM), rifampicin (RIF), and ethambutol (ETB) until cutaneous drug eruptions developed. She was noted to have a recurrence of MAC pulmonary disease with cavity lung lesions (Figure 1). Empiric treatment against MAC lung disease with azithromycin (AZM) (250 mg once daily), RIF (150 mg once daily), and ETB (500 mg twice daily) was started and maintained for 3 and a half years with a dose change of RIF (300 mg once daily) 1 year after. Then, she reported loose stools and was referred to the department of gastroenterology of this hospital. On physical examination, the abdomen was soft and nondistended with mild tender across the lower abdomen. An abdominal computed tomography (CT) scan showed multiple gas cysts in the intestinal wall (Figure 1). A colonoscopy revealed a collection of gas cysts between the ascending colon and rectum. Biopsies of the colon revealed a submucosal cyst with a multinucleated giant cell, consistent with PCI (Figure 2).4 Antibiotic treatment was stopped, and low-dose oxygen therapy was administered.5 Three weeks later, her loose stools stopped, and abdominal CT showed improvement of PCI (Figure 3).
Figure 1.
Images of case 1. An axial chest CT scan (panel A, arrow) shows right upper lobe cavitation. An axial abdominal CT scan (panel B, arrow) during antibiotic treatment for Mycobacterium avium complex pulmonary infection reveals air-filled cysts in the wall of the colon. Images from air-contrast barium enema show polypoid filling defects (panel C, arrows) consistent with pneumatosis cystoides intestinalis. Images obtained by colonoscopy (panels D, E, and F) during antibiotic treatment show multiple cystic lesions. CT, computed tomography.
Figure 2.
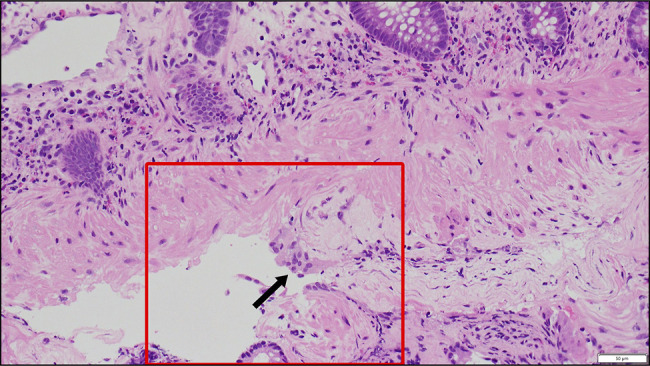
Biopsy specimen obtained from the transverse colon of case 1. Biopsy specimen from the transverse colon reveals a submucosal cyst with a multinucleated giant cell (arrow) on 40× hematoxylin and eosin staining, consistent with pneumatosis cystoides intestinalis.
Figure 3.
Abdominal CT of case 1 conducted before and after discontinuation of antibiotic treatment. An axial section of abdominal CT (panel A, circle) of case 1 conducted during antibiotic treatment for Mycobacterium avium complex pulmonary infection. It shows air-filled gas cysts in the wall of the ascending colon. After antibiotic treatment was stopped, abdominal CT (panel B, circle) shows improvement of pneumatosis cystoides intestinalis. CT, computed tomography.
Case 2
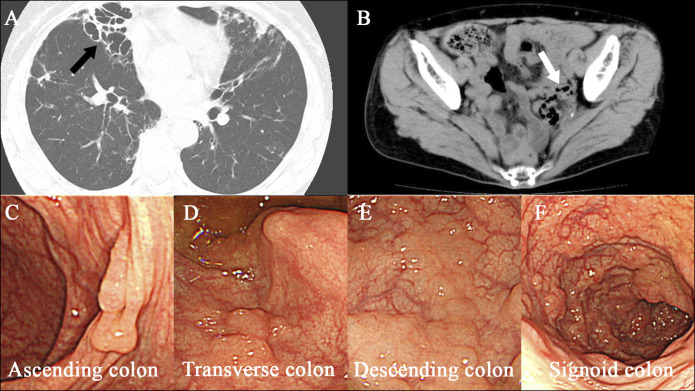
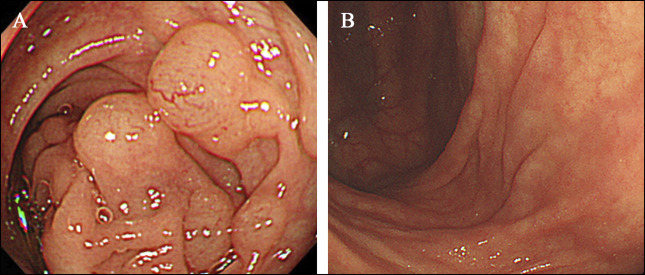
A 74-year-old woman was diagnosed with MAC pulmonary disease 2 years earlier and was referred to this hospital. She had been incidentally diagnosed with asymptomatic PCI on colonoscopy. She had been on treatment with CAM (800 mg twice daily), RIF (300 mg once daily), and ETB (500 mg twice daily) for 1 year until impaired vision was reported. Then, ETB was discontinued. Treatment with CAM, RIF, and sitafloxacin (250 mg once daily) was started and maintained for 4 years. One year later, hemoptysis developed, and she underwent bronchial arterial embolization. Mild hemoptysis persisted, and the routine follow-up chest x-ray showed a collapsed lung on the right side and treated conservatively. She was diagnosed with refractory MAC lung disease and treated with AZM (250 mg once daily), RIF (300 mg once daily), ETB (500 mg twice daily), and sitafloxacin (250 mg once daily). Then, she complained of persistent excessive gas and abdominal pain. Colonoscopy and abdominal CT revealed PCI in the sigmoid colon (Figure 4). Antibiotic treatment was stopped, and colonoscopy conducted 8 months later revealed normal-appearance mucosa and showed no sign of cystic lesions (Figure 5).
Figure 4.
Images of case 2. An axial chest CT scan (panel A, arrow) shows right lower lobe cavitation. An axial abdominal CT scan (panel B, arrow) during antibiotic treatment for Mycobacterium avium complex pulmonary infection reveals air-filled cysts in the wall of the colon. Images obtained by colonoscopy (panels C, D, E, and F) during antibiotic treatment show multiple cystic lesions. CT, computed tomography.
Figure 5.
Colonoscopy of case 2 conducted before and after discontinuation of antibiotic treatment. A colonoscopy (panel A) reveals cystic lesions through the ascending colon to the sigmoid colon of case 2 during antibiotic treatment. After antibiotic treatment was discontinued, no cystic lesions were seen on colonoscopy (panel B).
Case 3
A 68-year-old man was diagnosed with MAC pulmonary disease 9 years earlier and was referred to this hospital. He had been on treatment with CAM (800 mg twice daily), RIF (600 mg once daily), and ETB (750 mg once daily). He was incidentally diagnosed with asymptomatic PCI on colonoscopy 1 year after the initiation of the treatment (Figure 6). Treatment with AZM (250 mg once daily), RIF (450 mg once daily), and ETB (500 mg once daily) with the adjunctive use of inhaled amikacin was started. Ten months later, he reported persistent excessive gas and abdominal pain. Colonoscopy revealed worsening of PCI between the ascending and sigmoid colon. Antibiotic treatment was stopped, and 1 month later, colonoscopy showed improvement in the affected intestinal mucosa, with fewer and smaller cystic lesions.
Figure 6.
Images of case 3. An axial chest CT scan (panel A, arrow) shows right lower lobe cavitation. An axial abdominal CT scan (panel B, arrow) during antibiotic treatment for Mycobacterium avium complex pulmonary infection reveals air-filled cysts in the wall of the colon. Images obtained by colonoscopy (panels C, D, and E) during antibiotic treatment show multiple cystic lesions. CT, computed tomography.
DISCUSSION
The incidence of PCI has increased because of advancements in radiology and endoscopy.6,7 Three hypotheses of pathogenesis have been proposed; mechanical, pulmonary, and bacterial theories.1 Recently, there have been multiple reports of the incidence of PCI in patients receiving medication. One of them is glucosidase inhibitors, such as α-glucosidase inhibitor.8,9 Another medication is corticosteroids.10,11 In our case series, PCI can be attributable to MAC infection per se through pulmonary theory. Alveolar air leakage secondary to high airway pressure can cause an alveolar rupture, through which air may have traveled to the retroperitoneum and entered the mesentery of the bowel. Besides, all our patients develop lung cavities. Those cavities breach the alveolar barrier and may cause alveolar air leakage, too. Antibiotic treatment for MAC infection was also suspected as culprit of PCI. Refractory MAC pulmonary disease complicates its antibiotic treatment, and the period of treatment is likely to be longer. Two possible scenarios are as follows; one possible mechanism may be the interaction between antibiotics, which disrupts the mucosal integrity of the intestinal wall. The other possible mechanism can be alteration of gut microbiota by the combination of multiple antibiotics. Abnormal bacteria that would not be able to thrive under normal circumstances may grow and ferment, leading to high intraluminal pressure. To the best of our knowledge, 1 case has been reported by Yamasaki et al.12 The case was a 63-year-old woman who had been on antibiotic treatment with CAM, RIF, and ETB for MAC pulmonary infection for 2 years. During treatment, a colonoscopy revealed PCI in the colon. Hyperbaric oxygen therapy did not work, and only when antibiotic therapy was stopped did they confirm improvement of PCI. Their case was in line with our case series in a couple of ways; pulmonary MAC infection and its long-term antibiotic treatment predate development of PCI. Medications included the combination of macrolides and RIF. Discontinuation of antibiotics brought about improvement of PCI. In conclusion, we encountered 3 cases which developed PCI during long-term treatment for MAC pulmonary infection. Although the mechanism is still unknown, clinicians should raise awareness of a link between PCI and MAC treatment. When a patient on MAC treatment reports gastrointestinal symptoms, abdominal CT should be conducted to check whether or not PCI develops.
DISCLOSURES
Author contributions: H. Uehara treated the patients and drafted the manuscript; Y. Yamakazi treated the patients on MAC and provided the gastroenterology referral; T. Akamatsu has the expertise of PCI and made suggestions for drafting the manuscript. K. Shimodaira checked the manuscript, provided critical feedback, and helped shape the final version of the manuscript. M. Miyajima checked the manuscript, provided critical feedback, and helped shape the final version of the manuscript. T. Akamatsu is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Hiroyuki Uehara, Email: n_c_par7@yahoo.co.jp.
Yoshitaka Yamazaki, Email: yoshitakayamazaki@hotmail.com.
Taiji Akamatsu, Email: akamatus-taiji@pref-nagano-hosp.jp.
REFERENCES
- 1.Im J, Anjum F. Pneumatosis intestinalis 2023 Apr 27. In: StatPearls [Internet]. StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- 2.Wang YJ, Wang YM, Zheng YM, Jiang HQ, Zhang J. Pneumatosis cystoides intestinalis: Six case reports and a review of the literature. BMC Gastroenterol. 2018;18(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzaroli F, Turco L, Ceroni L, et al. Pneumatosis cystoides intestinalis. World J Gastroenterol. 2011;17(44):4932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsueh KC, Tsou SS, Tan KT. Pneumatosis intestinalis and pneumoperitoneum on computed tomography: Beware of non-therapeutic laparotomy. World J Gastrointest Surg. 2011;3(6):86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679–83. [DOI] [PubMed] [Google Scholar]
- 6.Ohmiya N, Hirata I, Sakamoto H, et al. ; Intractable Diseases, the Health and Labour Sciences Research Group. Multicenter epidemiological survey of pneumatosis intestinalis in Japan. BMC Gastroenterol. 2022;22(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzaniga G, Villa F, Tosi F, et al. Pneumatosis intestinalis induced by anticancer treatment: A systematic review. Cancers (Basel). 2022;14(7):1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKinley BJ, Santiago M, Pak C, Nguyen N, Zhong Q. Pneumatosis intestinalis induced by alpha-glucosidase inhibitors in patients with diabetes mellitus. J Clin Med. 2022;11(19):5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujimoto T, Shioyama E, Moriya K, et al. Pneumatosis cystoides intestinalis following alpha-glucosidase inhibitor treatment: A case report and review of the literature. World J Gastroenterol. 2008;14(39):6087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh SSN, Shelat V. Prednisolone induced pneumatosis coli and pneumoperitoneum. World J Gastroenterol. 2022;28:3739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammons MA, Bauling PC, Weil R, 3rd. Pneumatosis cystoides intestinalis with pneumoperitoneum in renal transplant patients on cyclosporine and prednisone. Transpl Proc. 1986;18(6):1868–70. [PubMed] [Google Scholar]
- 12.Yamasaki M, Teshima H, Okanobu H, Hattori N. Pneumatosis cystoides intestinalis in pulmonary mycobacterial disease. Br J Hosp Med (Lond). 2019;80(6):iii. [DOI] [PubMed] [Google Scholar]