Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
NRG Oncology RTOG 0415 is a randomized phase III noninferiority (NI) clinical trial comparing conventional fractionation (73.8 Gy in 41 fractions) radiotherapy (C-RT) with hypofractionation (H-RT; 70 Gy in 28) in patients with low-risk prostate cancer. The study included 1,092 protocol-eligible patients initially reported in 2016 with a median follow-up of 5.8 years. Updated results with median follow-up of 12.8 years are now presented. The estimated 12-year disease-free survival (DFS) is 56.1% (95% CI, 51.5 to 60.5) for C-RT and 61.8% (95% CI, 57.2 to 66.0) for H-RT. The DFS hazard ratio (H-RT/C-RT) is 0.85 (95% CI, 0.71 to 1.03), confirming NI (P < .001). Twelve-year cumulative incidence of biochemical failure (BF) was 17.0% (95% CI, 13.8 to 20.5) for C-RT and 9.9% (95% CI, 7.5 to 12.6) for H-RT. The HR (H-RT/C-RT) comparing biochemical recurrence between the two arms was 0.55 (95% CI, 0.39 to 0.78). Late grade ≥3 GI adverse event (AE) incidence is 3.2% (C-RT) versus 4.4% (H-RT), with relative risk (RR) for H-RT versus C-RT 1.39 (95% CI, 0.75 to 2.55). Late grade ≥3 genitourinary (GU) AE incidence is 3.4% (C-RT) versus 4.2% (H-RT), RR 1.26 (95% CI, 0.69 to 2.30). Long-term DFS is noninferior with H-RT compared with C-RT. BF is less with H-RT. No significant differences in late grade ≥3 GI/GU AEs were observed between assignments (ClinicalTrials.gov identifier: NCT00331773).
INTRODUCTION
External beam radiotherapy (RT) is commonly used to treat localized prostate cancer. Preclinical and clinical research published over the past 20 years suggested that prostate cancer cell killing is sensitive to RT hypofractionation (H), fewer treatments of higher dose (>2.0 Gy) per treatment, which may increase the biologically effective dose and improve outcomes.1 Three randomized trials were designed to test whether H-RT was superior to conventional (C) RT fractionation (1.8-2.0 Gy per treatment). The results were mixed with one study demonstrating increased efficacy, but two larger studies did not have a similar conclusion.2-4 In the early 2000s, three large-scale phase III noninferiority (NI) trials were designed with more than 5,000 participants in total,5-7 and the initial results of these have been published previously. Each trial demonstrated NI of H-RT. This manuscript is an update of one of the NI trials, NRG Oncology/RTOG 0415.5 Updated results with a median follow-up of 12.76 years for surviving participants are now presented.
METHODS
Study Design
The details of the design of NRG Oncology RTOG 0415 have been published previously.5 Participants had low-risk prostate adenocarcinoma (T1-2a, Gleason score 6 or less and serum prostate-specific antigen [PSA] <10 ng/mL) and were randomly assigned 1:1 to C-RT (73.8 Gy in 41 treatments) or to H-RT (70 Gy in 28 treatments). Image guidance was required. Participants were stratified by PSA level, Gleason score, and RT modality (three-dimensional or intensity-modulated). Androgen deprivation therapy (ADT) was not allowed.
End Points
The primary study aim was to compare the disease-free survival (DFS) rate between the two assignments. DFS events included local progression, distant metastatic progression, biochemical failure (BF) defined by the Phoenix definition,8 or death from any cause. BF was declared on the date PSA rose to a level that was at least 2.0 ng/mL greater than the lowest post-RT PSA (termed the nadir PSA), and it was a key secondary end point that is the focus of this clinical trial update. Adverse events (AEs) were defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
The data for this report are through December 7, 2022. Statistical analysis methodology is reported in Appendix 1 (online only).
RESULTS
Baseline Characteristics
Between April 2006 and December 2009, 1,115 participants were enrolled, and 23 were excluded (16 assigned C-RT and seven assigned H-RT). Baseline characteristics of 1,092 analyzable participants were reported previously. Intensity modulation was used in 79.1%, 99.9% had Gleason score 5%-6%, and 80.4% had PSA 4 to <10 ng/mL (median 5.4); these stratification factors were well-balanced between groups, as were other characteristics. The median follow-up duration from random assignment is 11.9 years in all patients and 12.8 years in surviving patients.
DFS
At the time of analysis, 460 DFS events were reported: 240 in the C-RT arm and 220 in the H-RT arm. The estimated 12-year DFS is 56.1% (95% CI, 51.5 to 60.5) for C-RT and 61.8% (95% CI, 57.2 to 66.0) for H-RT. The DFS hazard ratio (HR; H-RT/C-RT) is 0.85 (95% CI, 0.71 to 1.03), confirming NI of H-RT (P < .001).
BF
At the time of analysis, BF was observed in 144 participants: 91 in the C-RT arm and 53 in the H-RT arm. The cumulative incidence of BF at 12 years was 17.0% (95% CI, 13.8 to 20.5) in the C-RT arm and 9.9% (95% CI, 7.5 to 12.6) in the H-RT arm as illustrated in Figure 1. The HR for H-RT/C-RT comparing BF between assignments was 0.55 (95% CI, 0.39 to 0.78), two-sided P < .001. The protocol-specified NI criterion was met (H-RT/C-RT HR ≥ 1.67 rejected; P < .001).
FIG 1.
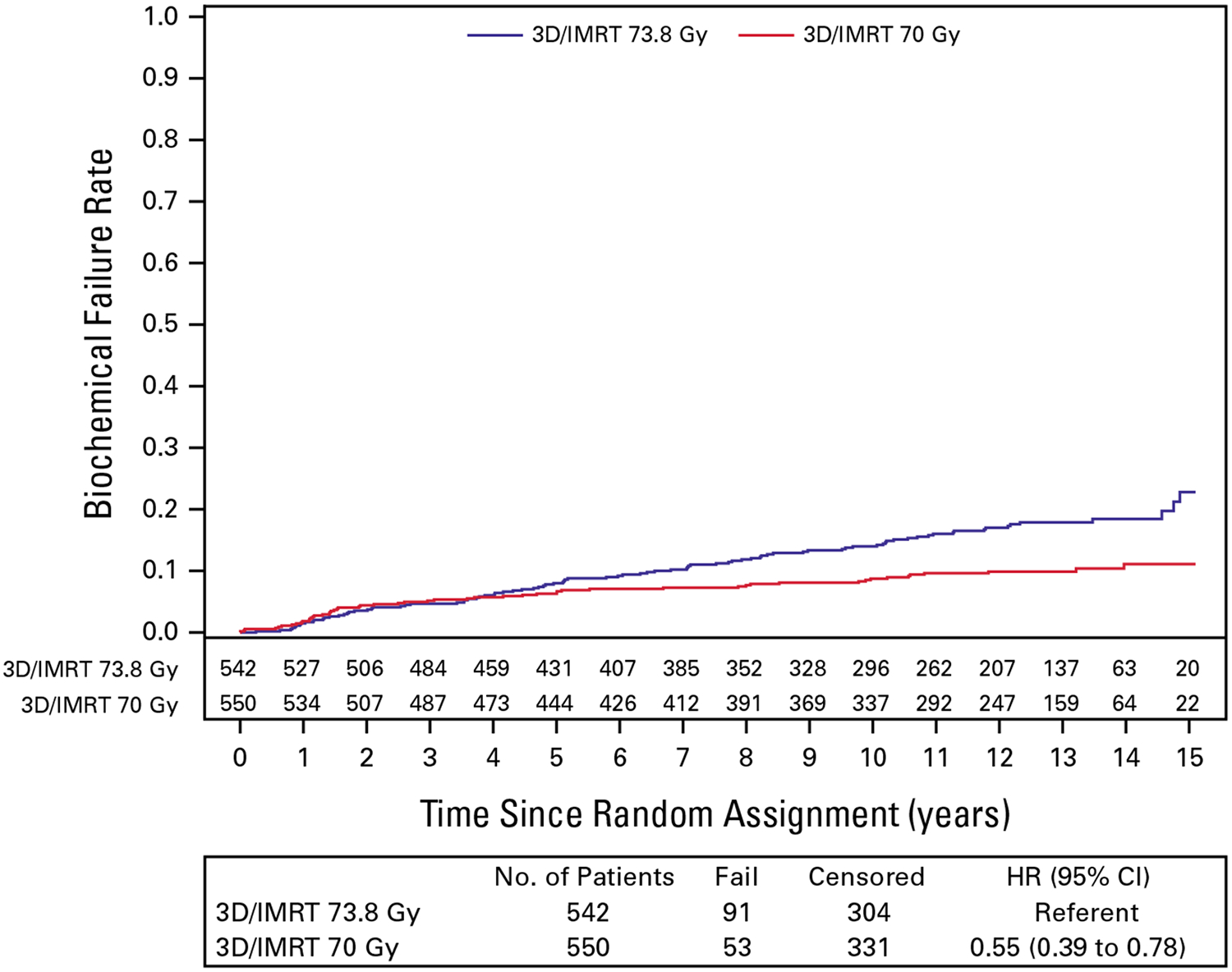
Biochemical failure. 3D/IMRT, three-dimensional radiation therapy and intensity modulated radiation therapy; HR, hazard ratio.
Other End Points
Cumulative incidence of local progression at 12 years was 4.7% (95% CI, 3.0 to 6.9) in the C-RT arm and 0.6% (95% CI, 0.2 to 1.7) in the H-RT arm. The observed HR (0.17 [95% CI, 0.06 to 0.48]) met the noninferiority criterion (HR > 1.28 rejected, P < .0001). Distant metastases were infrequent; the 12-year cumulative incidence was 1.7% (95% CI, 0.8 to 3.2) in the H-RT arm and 1.6% (95% CI, 0.8 to 3.0) in the C-RT arm. The HR contrasting H-RT to C-RT was 1.10 (95% CI, 0.42 to 2.85). No noninferiority criterion was specified for this end point.
Overall survival (OS) was similar in the two arms (HR 1.01 [95% CI, 0.82 to 1.24]), and the protocol-specified noninferiority criteria was met (HR > 1.54 rejected; P < .0001). The estimated 12-year OS was 68.7% (95% CI, 64.3 to 72.7) in the C-RT arm and 69.9% (95% CI, 65.5 to 73.9) in the H-RT arm. The most frequent causes of death were cardiovascular disease and other cancers.
AEs
Late GI and genitourinary (GU) AEs are provided in Table 1. The late grade ≥3 GI AE incidence is 3.2% (C-RT) versus 4.4% (H-RT), with relative risk (RR) for H-RT versus C-RT 1.39 (95% CI, 0.75 to 2.55). The late grade ≥3 GU AE incidence is 3.4% (C-RT) versus 4.2% (H-RT), RR 1.26 (95% CI, 0.69 to 2.30).
TABLE 1.
Late Adverse Events
| AE Class | 73.8 Gy (n = 533) | 70 Gy (n = 542) |
|---|---|---|
| No. (%) | No. (%) | |
| Maximum grade of late GI AEs | ||
| None reported | 342 (64.2) | 285 (52.6) |
| Grade 1 | 109 (20.5) | 128 (23.6) |
| Grade 2 | 65 (12.2) | 105 (19.4) |
| Grade 3 | 16 (3.0) | 24 (4.4) |
| Grade 4 | 1 (0.2) | 0 (0.0) |
| <Grade 2 | 451 (84.6) | 413 (76.2) |
| ≥Grade 2 | 82 (15.4) | 129 (23.8) |
| <Grade 3 | 516 (96.8) | 518 (95.6) |
| ≥Grade 3 | 17 (3.2) | 24 (4.4) |
| Maximum grade of late GU AEs | ||
| None reported | 234 (43.9) | 214 (39.5) |
| Grade 1 | 156 (29.3) | 147 (27.1) |
| Grade 2 | 125 (23.5) | 158 (29.2) |
| Grade 3 | 16 (3.0) | 23 (4.2) |
| Grade 4 | 2 (0.4) | 0 (0.0) |
| <Grade 2 | 390 (73.2) | 361 (66.6) |
| ≥Grade 2 | 143 (26.8) | 181 (33.4) |
| <Grade 3 | 515 (96.6) | 519 (95.8) |
| ≥Grade 3 | 18 (3.4) | 23 (4.2) |
NOTE. Patients with no AEs reported are included in the <grade 2 and <grade 3 groups.
Abbreviations: AE, adverse event; GU, genitourinary.
DISCUSSION
The initial report of NRG Oncology RTOG 0415 concluded that the efficacy of moderate hypofractionation (70 Gy in 28 fractions over 5.6 weeks) was noninferior to conventional fractionation (73.8 Gy in 41 fractions over 8.2 weeks) in patients with low-risk prostate cancer with a modest increase in grade 2 AEs.5 The current update confirms NI of the primary DFS end point. To our knowledge, for the first time, however, there is strong evidence that moderate hypofractionation results in improved efficacy as measured by lesser long-term incidence of BF.
In the initial report, there was no difference in BF according to assigned arm, but the median follow-up was 5.8 years. The updated cumulative incidence estimate plots for BF show a separation starting 5 years after treatment with a 44% RR reduction of BF over more extended follow-up. This delay in the separation of the curves is expected given that participants had low-risk disease, and the most likely pattern of tumor recurrence is local.
Updated results of the CHHiP trial with 12.1 years of follow-up were recently reported.9 NI of 60 Gy in 20 fractions H-RT compared with 74 Gy in 37 fractions C-RT was confirmed, and between-group comparison of BF favored the H-RT arm (HR, 0.84 [95% CI, 0.70 to 1.02]; P = .089). Unlike our trial, most (>95%) CHHiP trial participants received short-term ADT with RT. When taken together, these two trials provide evidence that H-RT either without or without short-term ADT is a suitable, and perhaps preferred, alternative to C-RT.
Our study as designed has some potential limitations in the context of contemporary practice. Active surveillance (AS) is the preferred initial strategy for men with low-risk disease in 2023, but evidence for AS was relatively weak in 2004 when our study was designed, and utilization of AS was low then.10 Use of AS has increased in the past decade, but some patients with low-risk disease opt for definitive treatment at initial diagnosis or in a delayed fashion. Our trial results remain relevant for these patients and may be used to inform selection of an external beam RT approach. Grade 2 AEs were marginally higher with H-RT than C-RT, but patient-reported outcomes were not different.11 We used organ-at-risk planning parameters available two decades ago, but current guidance12 allows less radiation exposure of adjacent normal tissues as does a prostate-rectal spacer; it is plausible that these changes can reduce AEs in today’s practice environment. The standard C-RT dose schedule in 2004 was 70 Gy in 35 or 39 treatments, and this standard was used in other trials evaluating higher doses (78 Gy or 79.2 Gy); we selected 73.8 Gy in 41 fractions as a midpoint between the standard and the investigational RT doses, and it remains an acceptable standard of care today. Although we cannot know whether 70 Gy in 28 fractions is noninferior to 79.2 Gy for BF, there is no evidence that higher C-RT dose levels improve survival outcomes in low-risk prostate cancer.13-15
In conclusion, in low-risk prostate cancer, long-term DFS is noninferior with 70 Gy in 28 fractions compared with 73.8 Gy in 41 fractions. H-RT reduces the RR of BF by 44%. No differences in late grade ≥3 GI/GU AEs were observed between C-RT and H-RT.
Supplementary Material
SUPPORT
Supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA37422 (CCOP), CA81647 (ATC), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI).
APPENDIX 1. STATISTICAL METHODS
Sample size was determined assuming 85% 5-year disease-free survival (DFS) with C-RT. The trial was designed to establish with 90% power and α = .05 that H-RT results in 5-year DFS that is not lower than C-RT by more than 7.65% (hazard ratio [HR] < 1.52). The noninferiority margin was chosen to be approximately one half of the absolute difference in 5-year DFS observed in contemporary superiority trials of dose-escalation (15%) in similar patients. Under-assumed failure rates and guarding against a 10% ineligible or lack of data rate, the final targeted accrual was 1,067 patients, with definitive analysis to occur after 238 DFS events. The trial was expected to accrue 20 patients per month and reach primary end point reporting at 11 years from start of accrual. Interim reports were provided to the external Data Monitoring Committee (DMC) every 6 months. Interim analyses were planned after 60, 120, and 179 DFS events for early rejection of both the null hypothesis and the alternative hypothesis. The boundaries for rejecting the null hypothesis were based on a Lan and DeMets alpha spending function approach with properties similar to the O’Brien-Fleming boundary.16 The futility testing followed the method of Harrington et al17 of testing to reject the alternative. At the third interim analysis, the DMC recommended that results of the trial be disclosed. Based on the event information at that time (185/238 or 78% of events required for definitive analysis), stopping to reject the null hypothesis of inferiority required a test statistic P value of .011 or smaller, and this condition was satisfied.
All eligible patients with follow-up were included and analyzed according to assignment (modified intention-to-treat analysis), with time-to-event duration originating at random assignment. Overall and DFS distributions were calculated using the Kaplan-Meier method.18 The cumulative incidence estimator was used for all other end points to account for competing risks.19 Treatment efficacy for DFS and other end points was tested by comparing cause-specific hazards with the log-rank statistic.20 Noninferiority hypotheses for each secondary end point were also defined in the study protocol. HRs with 95% CI were computed using the Cox proportional hazards model for the end point–specific hazard.21 Frequency distributions of grade category (none reported or grade 1 to 5) for selected adverse events were compared using chi-square tests. To evaluate differences in risk of grade 2 or greater or grade 3 or greater event frequency by treatment arm, 2 × 2 tables were formed, and relative risk estimates with 95% CIs were computed. Median follow-up time was computed using the Kaplan-Meier estimate of time to last follow-up date with death considered the censoring event (reverse Kaplan-Meier method).
Footnotes
PRIOR PRESENTATION
Presented at the American Society of Radiation Oncology Annual Scientific Meeting, San Diego, CA, October 1-4, 2023.
CLINICAL TRIAL INFORMATION
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/JCO.23.02445.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
W. Robert Lee
Patents, Royalties, Other Intellectual Property: UpToDate Editor
James J. Dignam
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Celgene, Bristol Myers Squibb/Sanofi, Duality Biologics
Mahul B. Amin
Employment: Labcorp of America
Leadership: Kidney Cancer Association
Stock and Other Ownership Interests: CORE Science Solutions, CellMax Life, Precipio, Karkinos Health, Morphle, Pathpresenter, IBEX Medical Analytics
Honoraria: Genomic Health
Consulting or Advisory Role: UroGen pharma, Advanced Clinical
Deborah W. Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society for Radiation Oncology, Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmot Cancer Center
Consulting or Advisory Role: University of Rochester
Daniel Low
Employment: University of California Los Angeles
Honoraria: Varian Medical Systems, ViewRay
Consulting or Advisory Role: Varian Medical Systems, ViewRay, TAE Life Sciences
Research Funding: Varian Medical Systems
Expert Testimony: ViewRay
Travel, Accommodations, Expenses: Varian Medical Systems
David D’Souza
Honoraria: Tersera
Consulting or Advisory Role: Knight Pharmaceuticals
Travel, Accommodations, Expenses: Tersera
Jeff M. Michalski
Leadership: American Society of Therapeutic Radiation Oncology
Travel, Accommodations, Expenses: American Society of Therapeutic Radiation Oncology
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Ian S. Dayes
Honoraria: Verity Pharmaceuticals
William A. Hall
Consulting or Advisory Role: Aktis Oncology
Research Funding: Elekta (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending for a wearable device for radiation treatment planning (Inst)
Travel, Accommodations, Expenses: Elekta (Inst)
Paul L. Nguyen
Stock and Other Ownership Interests: Volatilyx, Nanocan Therapeutics, Stratagen Bio, Reversal Therapeutics, Telerad Oncology
Consulting or Advisory Role: Bayer, Blue Earth Diagnostics, Boston Scientific, Janssen Oncology, Myovant Sciences, Nanocan Therapeutics, AIQ Solutions, Novartis, Theranano
Research Funding: Astellas Pharma, Janssen, Bayer
Patents, Royalties, Other Intellectual Property: Wife has a patent on volatile diagnostics of infections
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
No other potential conflicts of interest were reported.
DATA SHARING STATEMENT
All data will be made available per the NCTN Data Archive rules. The link for the archive is https://nctn-data-archive.nci.nih.gov/
REFERENCES
- 1.Brenner DJ, Hall EJ: Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 43:1095–1101, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Hoffman KE, Voong KR, Levy LB, et al. : Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol 36:2943–2949, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Incrocci L, Wortel RC, Alemayehu WG, et al. : Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 17:1061–1069, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Pollack A, Walker G, Horwitz EM, et al. : Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 31:3860–3868, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WR, Dignam JJ, Amin MB, et al. : Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 34:2325–2332, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dearnaley D, Syndikus I, Mossop H, et al. : Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, noninferiority, phase 3 CHHiP trial. Lancet Oncol 17:1047–1060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catton CN, Lukka H, Gu CS, et al. : Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 35:1884–1890, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Roach M III, Hanks G, Thames H Jr, et al. : Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965–974, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Syndikus I, Griffin C, Philipps L, et al. : 10-Year efficacy and co-morbidity outcomes of a phase III randomised trial of conventional vs. hypofractionated high dose intensity modulated radiotherapy for prostate cancer (CHHiP; CRUK/06/016). J Clin Oncol 41, 2023. (6_suppl; abstr 304) [Google Scholar]
- 10.Cooperberg MR, Carroll PR: Trends in management for patients with localized prostate cancer, 1990-2013. JAMA 314:80–82, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Bruner DW, Pugh SL, Lee WR, et al. : Quality of Life in patients with low-risk prostate cancer treated with hypofractionated vs conventional radiotherapy: A phase 3 randomized clinical trial. JAMA Oncol 5:664–670, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekelman JE, Lee WR: Six questions to ask before we shorten radiation treatments for intact prostate cancer. Int J Radiat Oncol Biol Phys 97:718–721, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Kalbasi A, Li J, Berman A, et al. : Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol 1:897–906, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Kuban DA, Levy LB, Cheung MR, et al. : Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys 79:1310–1317, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Zietman AL, Bae K, Slater JD, et al. : Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 28:1106–1111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan KK, DeMets DL: Changing frequency of interim analysis in sequential monitoring. Biometrics 45:1017–1020, 1989 [PubMed] [Google Scholar]
- 17.Fleming TR, Harrington DP, O’Brien PC: Designs for group sequential tests. Control Clin Trials 5:348–361, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P: Nonparametric-estimation from incomplete observations. J Am Stat Assoc 53:457–481, 1958 [Google Scholar]
- 19.Korn EL, Dorey FJ: Applications of crude incidence curves. Stat Med 11:813–829, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170, 1966 [PubMed] [Google Scholar]
- 21.Prentice RL, Kalbfleisch JD, Peterson AV Jr, et al. : The analysis of failure times in the presence of competing risks. Biometrics 34:541–554, 1978 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be made available per the NCTN Data Archive rules. The link for the archive is https://nctn-data-archive.nci.nih.gov/


