Abstract
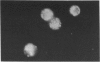
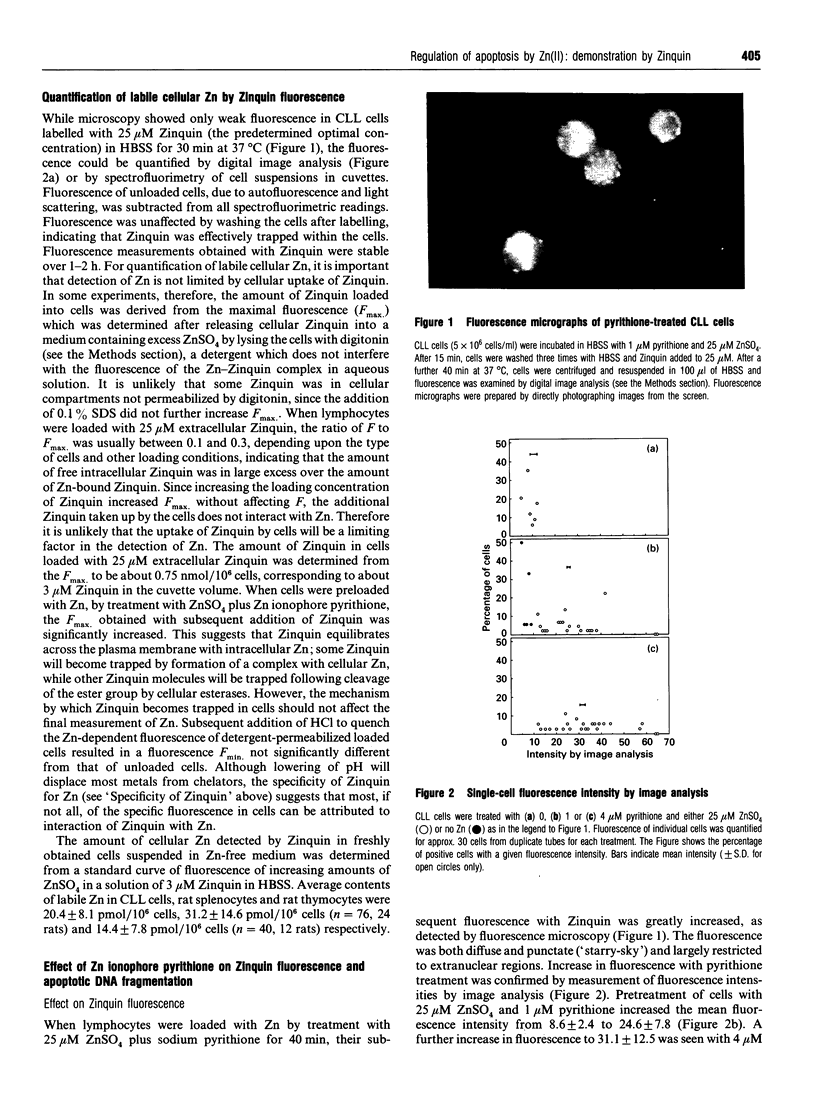
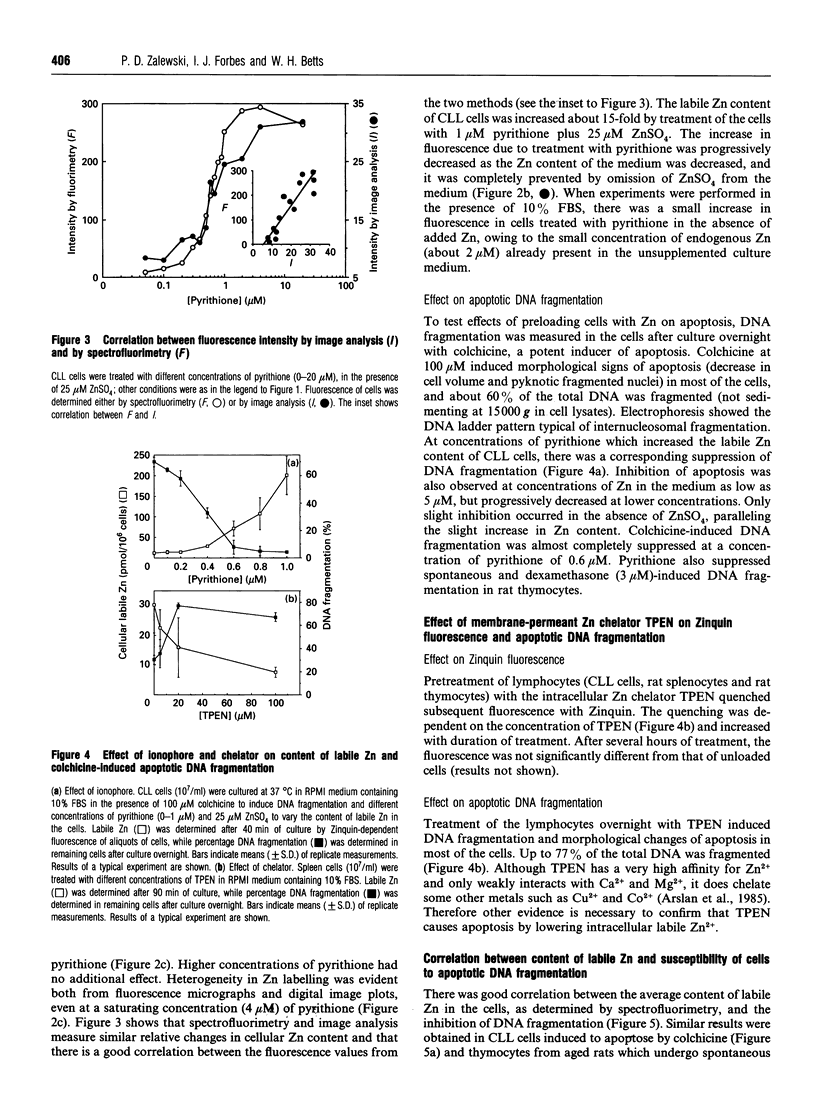
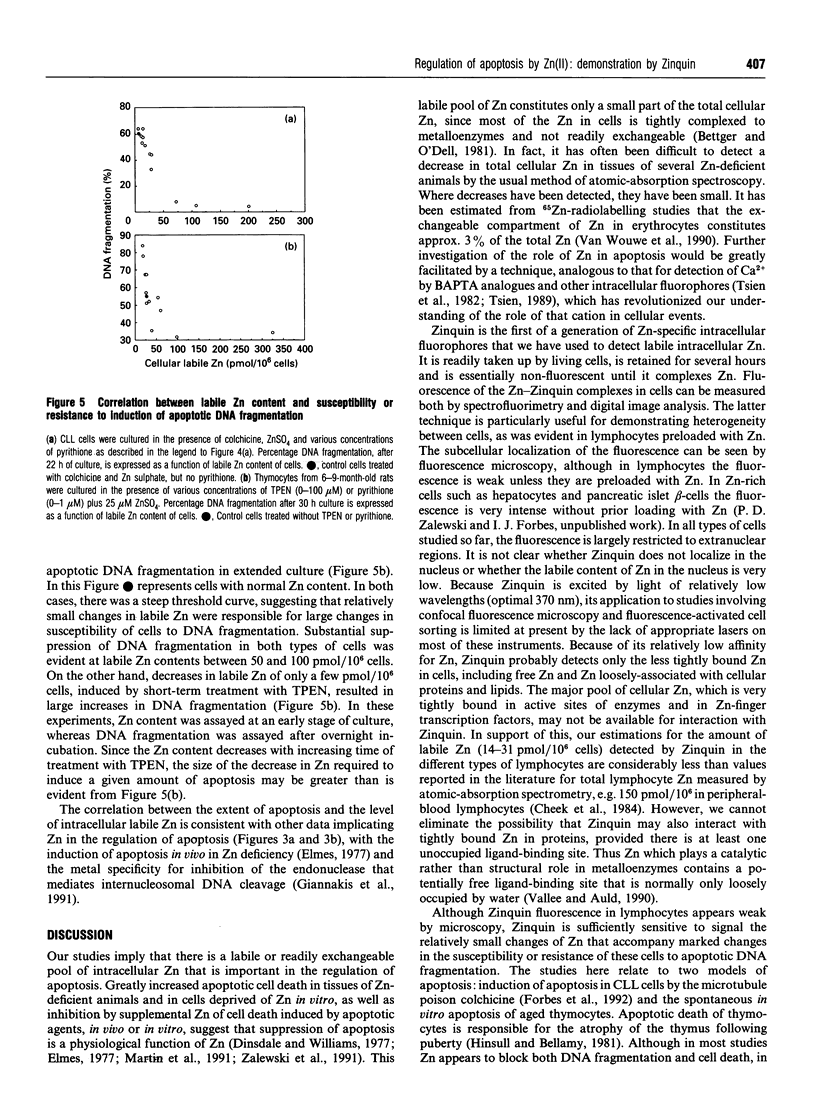
Zinquin [(2-methyl-8-p-toluenesulphonamido-6-quinolyloxy)-acetic acid], a membrane-permeant fluorophore specific for Zn(II), was used with spectrofluorimetry and video image analysis to reveal and quantify labile intracellular Zn. Zinquin labelled human chronic-lymphocytic-leukaemia lymphocytes, rat splenocytes and thymocytes with a weak diffuse fluorescence that was quenched when intracellular Zn was chelated with NNN'N'-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN) and was greatly intensified by pretreatment of cells with the Zn ionophore pyrithione and exogenous Zn. There was substantial heterogeneity of labile Zn among ionophore-treated cells, and fluorescence was largely extranuclear. The average contents of labile Zn in human leukaemic lymphocytes, rat splenocytes and rat thymocytes were approx. 20, 31 and 14 pmol/10(6) cells respectively. Morphological changes and internucleosomal DNA fragmentation indicated substantial apoptosis in these cells when the level of intracellular labile Zn was decreased by treatment with TPEN. Conversely, increasing labile Zn by pretreatment with Zn plus pyrithione suppressed both spontaneous DNA fragmentation and that induced by the potent apoptosis-induced agents colchicine and dexamethasone. These results suggest that prevention of apoptosis is a function of labile Zn, and that a reduction below a threshold concentration in this Zn pool induces apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arslan P., Di Virgilio F., Beltrame M., Tsien R. Y., Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem. 1985 Mar 10;260(5):2719–2727. [PubMed] [Google Scholar]
- Barbieri D., Troiano L., Grassilli E., Agnesini C., Cristofalo E. A., Monti D., Capri M., Cossarizza A., Franceschi C. Inhibition of apoptosis by zinc: a reappraisal. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1256–1261. doi: 10.1016/0006-291x(92)90438-q. [DOI] [PubMed] [Google Scholar]
- Bettger W. J., O'Dell B. L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981 Mar 30;28(13):1425–1438. doi: 10.1016/0024-3205(81)90374-x. [DOI] [PubMed] [Google Scholar]
- Cheek D. B., Hay H. J., Lattanzio L., Ness D., Ludwigsen N., Spargo R. Zinc content of red and white blood cells in aboriginal children. Aust N Z J Med. 1984 Oct;14(5):638–642. doi: 10.1111/j.1445-5994.1984.tb05016.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Williams R. B. The enhancement by dietary zinc deficiency of the susceptibility of the rat duodenum to colchicine. Br J Nutr. 1977 Jan;37(1):135–142. doi: 10.1079/bjn19770013. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmes M. E. Apoptosis in the small intestine of zinc-deficient and fasted rats. J Pathol. 1977 Dec;123(4):219–223. doi: 10.1002/path.1711230404. [DOI] [PubMed] [Google Scholar]
- Fliss H., Ménard M., Desai M. Hypochlorous acid mobilizes cellular zinc. Can J Physiol Pharmacol. 1991 Nov;69(11):1686–1691. doi: 10.1139/y91-250. [DOI] [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Giannakis C., Cowled P. A. Induction of apoptosis in chronic lymphocytic leukemia cells and its prevention by phorbol ester. Exp Cell Res. 1992 Feb;198(2):367–372. doi: 10.1016/0014-4827(92)90393-m. [DOI] [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D., Hurst N. P., Giannakis C., Whitehouse M. W. Zinc increases phorbol ester receptors in intact B-cells, neutrophil polymorphs and platelets. FEBS Lett. 1989 Apr 24;247(2):445–447. doi: 10.1016/0014-5793(89)81388-2. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Kasarskis E. J., Ringo D., Frederickson R. E. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987 Jun;20(2):91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Giannakis C., Forbes I. J., Zalewski P. D. Ca2+/Mg(2+)-dependent nuclease: tissue distribution, relationship to inter-nucleosomal DNA fragmentation and inhibition by Zn2+. Biochem Biophys Res Commun. 1991 Dec 16;181(2):915–920. doi: 10.1016/0006-291x(91)91278-k. [DOI] [PubMed] [Google Scholar]
- Lutwak-Mann C., McIntosh J. E. Zinc and carbonic anhydrase in the rabbit uterus. Nature. 1969 Mar 22;221(5186):1111–1114. doi: 10.1038/2211111a0. [DOI] [PubMed] [Google Scholar]
- MASKE H. Interaction between insulin and zinc in the islets of Langerhans. Diabetes. 1957 Jul-Aug;6(4):335–341. doi: 10.2337/diab.6.4.335. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Mazdai G., Strain J. J., Cotter T. G., Hannigan B. M. Programmed cell death (apoptosis) in lymphoid and myeloid cell lines during zinc deficiency. Clin Exp Immunol. 1991 Feb;83(2):338–343. doi: 10.1111/j.1365-2249.1991.tb05639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S., Jondal M. Cellular signalling in programmed cell death (apoptosis). Immunol Today. 1990 Apr;11(4):120–121. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Owens G. P., Cohen J. J. Identification of genes involved in programmed cell death. Cancer Metastasis Rev. 1992 Sep;11(2):149–156. doi: 10.1007/BF00048061. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Hillyer C. D., Harten B., Schaeffer C. A., Dorminy M., Lackey D. A., 3rd, Kirsten E., Mendeleyev J., Buki K. G., Hakam A. Induction of endonuclease-mediated apoptosis in tumor cells by C-nitroso-substituted ligands of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7703–7707. doi: 10.1073/pnas.89.16.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. D., Montano C. Y., Kasarskis E. J. Quantitative histofluorescence of hippocampal mossy fiber zinc. Brain Res. 1989 Sep 4;496(1-2):257–267. doi: 10.1016/0006-8993(89)91073-1. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol Relat Areas Mol Biol. 1984;56:283–430. doi: 10.1002/9780470123027.ch5. [DOI] [PubMed] [Google Scholar]
- Van Wouwe J. P., Veldhuizen M., De Goeij J. J., Van den Hamer C. J. In vitro exchangeable erythrocytic zinc. Biol Trace Elem Res. 1990 Apr;25(1):57–69. doi: 10.1007/BF02990265. [DOI] [PubMed] [Google Scholar]
- Williams R. J. Zinc: what is its role in biology? Endeavour. 1984;8(2):65–70. doi: 10.1016/0160-9327(84)90040-1. [DOI] [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Giannakis C. Physiological role for zinc in prevention of apoptosis (gene-directed death). Biochem Int. 1991 Aug;24(6):1093–1101. [PubMed] [Google Scholar]