Summary
Background
Radiology-based prognostic biomarkers play a crucial role in patient counseling, enhancing surveillance, and designing clinical trials effectively. This study aims to assess the predictive significance of preoperative CT-based tumor contour irregularity in determining clinical outcomes among patients with renal cell carcinoma (RCC).
Methods
We conducted a retrospective multi-institutional review involving 2218 patients pathologically diagnosed with RCC. The training and internal validation sets included patients at Zhongshan Hospital between January 2009 and August 2019. The external test set comprised patients from the First Affiliated Hospital, Zhejiang University School of Medicine (January 2016 to January 2018), the Xiamen Branch of Zhongshan Hospital (November 2017 to June 2023), and the Cancer Imaging Archive. The contour irregularity degree (CID), quantified as the ratio of irregular cross-sections to the total tumor cross-sections, was analyzed for its prognostic relevance across different subgroups of RCC patients. A novel CID-based scoring system was developed, and its predictive efficacy was evaluated and compared with existing prognostic models.
Findings
The CID exhibited significant discriminatory power in predicting overall survival (OS), recurrence-free survival (RFS), and disease-specific survival (DSS) among patients with RCC tumors measuring 3 cm or larger (all p < 0.001). Multivariate analyses confirmed the CID as an independent prognostic indicator. Notably, the CID augmented prognostic stratification among RCC patients within distinct risk subgroups delineated by SSIGN models and ISUP grades. The CID-based nomogram (C-Model) demonstrated robust predictive performance, with C-index values of 0.88 (95%CI: 0.84–0.92) in the training set, 0.92 (95%CI: 0.88–0.98) in the internal validation set, and 0.86 (95%CI: 0.81–0.90) in the external test set, surpassing existing prognostic models.
Interpretation
Routine imaging-based assessment of the CID serves as an independent prognostic factor, offering incremental prognostic value to existing models in RCC patients with tumors measuring 3 cm or larger.
Funding
This study was funded by grants from National Natural Science Foundation of China; Shanghai Municipal Health Commission; China National Key R&D Program and Science and Technology Commission of Shanghai Municipality.
Keywords: Renal cell carcinoma, Prognosis, SSIGN
Research in context.
Evidence before this study
We conducted a PubMed search using the terms “(Renal cell carcinoma) AND (Surgery) AND (Computed Tomography) AND (Survival) AND (Predict)” to identify relevant articles published up to May 1, 2024. Our review revealed that most imaging-based models for predicting prognosis in renal cell carcinoma (RCC) patients are developed using radiomics or deep learning principles, but there is a lack of investigation into user-friendly imaging indicators. To address these gaps, our study aims to identify effective and readily accessible indicators based on routine CT imaging within a large, multicenter cohort encompassing various histologic types of RCC.
Added value of this study
In this multicenter study, we demonstrated that the CT image-based tumor contour irregularity degree (CID), quantified as the ratio of irregular cross-sections to total tumor cross-sections, serves as an independent predictor of survival outcomes for patients with RCC tumors measuring 3 cm or larger. Additionally, the CID enables further risk stratification within low-, intermediate-, and high-SSIGN subgroups. By combining the CID with pathological features, we developed a novel nomogram, achieving impressive C-index values of 0.88, 0.92, and 0.86 in the training, internal validation, and external test sets, respectively, representing significant improvements over the SSIGN model.
Implications of all the available evidence
Our findings underscore that the CT image-based CID offers substantial prognostic differentiation for RCC tumors larger than 3 cm and can mitigate the limitations of current pathology-based scoring systems. CID is a readily accessible indicator from routine CT scans, providing additional insights for preoperative counseling, postoperative monitoring, and guiding decisions on adjuvant therapies.
Introduction
Renal cell carcinoma (RCC) stands as a prevalent malignancy within urology, impacting over 400,000 individuals globally each year and accounting for 2%–3% of adult malignancies.1 Despite advances, the unpredictable nature of RCC complicates personalized follow-up treatment strategies for affected patients.2 Notably, even small tumors harbor metastatic potential, and the overall mortality rate associated with RCC remains a challenge to mitigate.3 Precise prediction of tumor recurrence and post-surgery mortality holds paramount importance for patient counseling, tailored surveillance, and the identification of suitable candidates for adjuvant therapies.
Traditionally, risk stratification in RCC patients has relied on features such as TNM classification4 and nuclear grade.5 However, these features alone do not yield perfect accuracy. Hence, an increasing number of prognostic models integrate various prognostic features to enhance predictive accuracy, among which the Stage, Size, Grade and Necrosis (SSIGN) model is the most widely used.6, 7, 8, 9 The SSIGN model incorporates additional pathologic features (tumor size, nuclear grade, and histological tumor necrosis) beyond TNM staging, thereby improving its predictive capacity for survival outcomes in RCC.6 However, some clinicians observed significant survival disparities persist among patients within the same SSIGN classification. One limitation of the SSIGN model is its development solely based on clear cell renal cell carcinoma (ccRCC), disregarding other histological variations.6 Furthermore, SSIGN primarily incorporates pathological features, and the assessment of pathological features often involves multiple-point evaluations at critical sites potentially leading to the loss of comprehensive tumor information. Therefore, there is an urgent need to develop superior indicators to further complement existing pathology-based staging systems in stratifying the prognosis of RCC patients.
Computed tomography (CT) serves as a dependable non-invasive diagnostic modality, extensively utilized in the detection of renal masses.10, 11, 12 Recent studies have hinted at the prognostic value inherent in the morphological properties of RCC tumors as observed through imaging.13, 14, 15 However, the lack of standardization in assessing shape irregularities impedes objective and quantitative analysis, thereby limiting its clinical utility. Previous research has established tumor contour irregularity degree (CID) as the ratio of irregular cross-sections to total tumor cross-sections, playing a crucial role as a radiological feature in assessing tumor heterogeneity.16, 17, 18 However, the prognostic relevance of CID across diverse histologic subtypes of RCC requires validation within a comprehensive, multi-institutional patient cohort.
This study aims to leverage a multicenter dataset to validate the prognostic relevance of the CID across diverse subgroups of RCC patients. Additionally, we seek to ascertain whether the CID can complement pathology-based characteristics to enhance individual risk stratification in RCC patients.
Methods
Ethics statement
The ethics committees of each participating institute approved the study (No. B2021-608R). Informed consents for patients from retrospective cohorts were waived.
Study design
This study was a multicenter retrospective analysis involving patients from three Chinese hospitals and The Cancer Imaging Archive (TCIA). Conducted in accordance with the Declaration of Helsinki, the study received approval from the ethics committees of each participating hospital, with informed consent waived. Firstly, the stability of the assessment method for the CID was tested through intra- and inter-observer analyses. Subsequently, the efficacy of the CID in predicting survival outcomes among patients with RCC of varying sizes and histopathological features was evaluated using a multicenter dataset. Further analyses involved stratification based on the SSIGN model to assess disease-specific mortality and recurrence risk among subgroups. Finally, a nomogram was developed and validated to predict clinical outcomes among patients with appropriately sized RCC.
Study participants
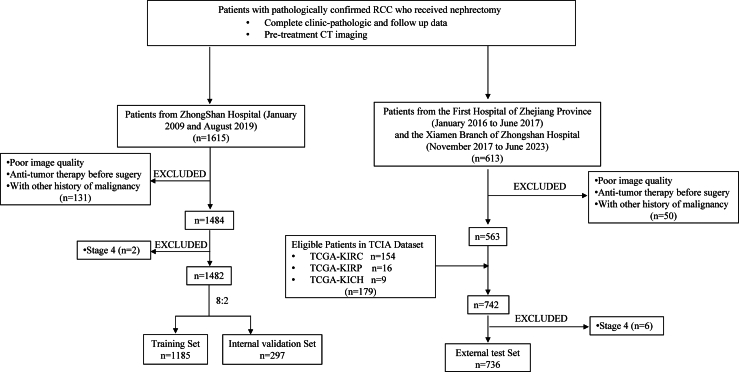
The study included patients diagnosed with malignant renal tumors who underwent nephrectomy at Zhongshan Hospital (Zhongshan Cohort) between January 2009 and August 2019, comprising the training and internal validation sets. The external test set comprised patients who underwent nephrectomy for malignant renal tumors at the First Affiliated Hospital, Zhejiang University School of Medicine (January 2016 to January 2018), the Xiamen Branch of Zhongshan Hospital (November 2017 to June 2023), and TCIA. Electronic medical records were thoroughly reviewed to extract personal characteristics. Patients with arterial or venous phase pre-operative computed tomography (CT) scans from TCIA were screened for inclusion. Clinical and radiological data from the TCIA dataset were obtained from the National Cancer Institute’s Cancer Imaging Program (https://www.cancerimagingarchive.net). Inclusion and exclusion criteria are outlined in Fig. 1. The study included a total of 2047 patients from the three medical centers mentioned above and 179 cases from the TCIA dataset.
Fig. 1.
Flowchart of the study population.
Exposure and outcomes
Definition of the CID
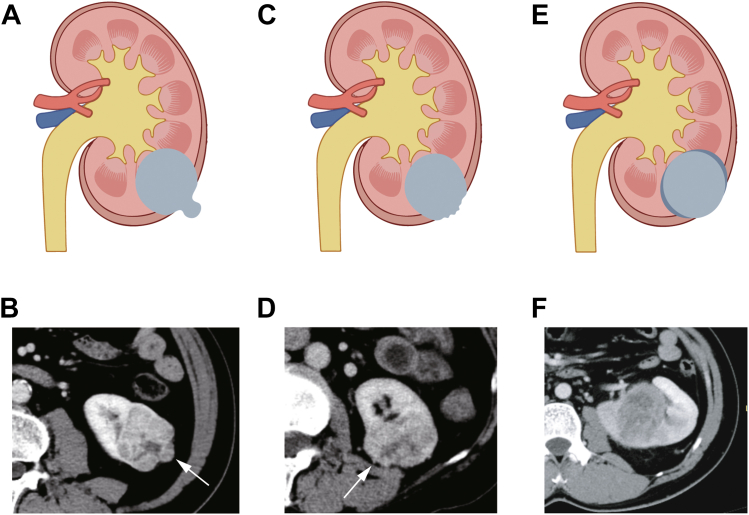
The CID was defined in our previous study.16 If a renal tumor met any of the following three criteria on cross-section CT, the contour of that layer was determined as irregular: 1) A mass with smooth but distorted margins, described as ‘lobular’ with an arc-shaped focal convex protrusion arising from part of the tumor (Fig. 2A–B); 2) A mass with unsmooth and sharp nodules, usually small with an acute margin (Fig. 2C–D); 3) A mass with blurred margins, where the margin between the tumor and renal parenchyma is unclear (Fig. 2E–F). The number of irregular cross-sections was denoted as X, and the total number of cross-sections occupied by the entire tumor from top to bottom was denoted as Y. The ratio between them (X/Y) was defined as the CID. Corticomedullary or nephrographic phase images with the clearest tumor delineation were selected, and only axial images were used. The CID assessment was performed by two radiologists blinded to clinical outcomes (Radiologist A with over 10 years of experience in genitourinary imaging, and Radiologist B with over 5 years of experience in genitourinary imaging). Radiologist B evaluated the images twice, with a three-month interval between evaluations, referred to as B1 and B2. Radiologists solely evaluated the anatomic morphology.
Fig. 2.
The contour irregularity with three criteria on cross-sectional imaging. (A and B) A mass with smooth but distorted margins, described as ‘lobular’ with an arc-shaped focal convex protrusion arising from a part of the tumor (arrow). (C and D) A mass with unsmooth and sharp nodules (arrow), usually small with an acute margin. (E and F) A mass with blurred margins, where the margin between the tumor and renal parenchyma is unclear.
Definition of clinical and pathological factors
Clinical data included age, sex, and surgical approach. Pathological features were evaluated by three experienced urologic pathologists blinded to patient outcomes, encompassing histologic subtype,19 the International Society of Urological Pathology (ISUP) grading system,5 2017 TNM stage,19 presence of perinephric or renal sinus fat invasion, tumor thrombus, lymph node involvement, and sarcomatoid differentiation. ISUP grade was evaluated in clear cell RCC and papillary RCC only.
Survival outcomes
Survival analysis comprised disease-specific survival (DSS), recurrence-free survival (RFS), and overall survival (OS). DSS was defined as the time from surgery to death specifically attributed to renal tumors. RFS was calculated from surgery to local recurrence, distant metastasis, or renal tumor-related death. OS represented the time from surgery to death from any cause. The last follow-up was in December 2023, with regular follow-ups scheduled every 6–12 months for the first 2 years post-surgery, followed by annual check-ups. Follow-up data were collected from medical records, including physical examinations and imaging, supplemented by telephone inquiries and medical insurance records.
Statistical analysis
Intra-observer (B1 and B2) and inter-observer (A and B1, A and B2) variability were assessed using the intraclass correlation coefficient, with a value >0.8 indicating exceptional reliability.20 Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR), while categorical variables were expressed as frequency and percentages. The optimal threshold value of the CID, based on DSS, was determined using X-tile statistical software (version 3.6.1, Yale University, New Haven, CT, USA),21 which systematically tests and compares all potential thresholds, selecting the one with the highest significance. Patients were then categorized into low-CID or high-CID groups according to the defined cut-off value. Group comparisons were evaluated using an unpaired Student’s t-test when normality and equal variance assumptions were met; otherwise, a non-parametric test (Mann–Whitney U test) was applied. Differences in constituent ratios between groups were assessed using the chi-square test. The impact of the CID on OS, RFS, and DSS was analyzed using the Kaplan–Meier method with log-rank testing. Univariate and multivariate Cox proportional hazards regression models were employed to identify the independent prognostic factors for DSS in RCC patients, with 95% confidence intervals (CIs) and hazard ratios (HRs) presented. Factors with a significance level of p < 0.05 in the univariate analysis were included in the multivariate analysis, with backward stepwise selection performed. A CID-based nomogram predicting 1-, 3-, and 5-year survival rates was constructed based on independent prognostic factors identified through multivariate analysis. To assess the incremental value of CID to SSIGN model for survival prediction, a combined nomogram was developed in the training set. The predictive value of the newly developed model was evaluated using calibration plots, the C-index, and the area under the receiver operating characteristic curve (AUC), compared to the SSIGN and Leibovich’s chromophobe renal cell carcinoma (chrRCC) risk stratification models.6,22 To assess the clinical usefulness of these models, a decision curve analysis (DCA) was applied.
All statistical analyses were performed using Stata software (version 15.1, StataCorp, College Station, TX) and R statistical software (Version 4.1.1, https://www.r-project.org). A two-sided p < 0.05 was considered statistically significant.
Role of the funding source
The funders played no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. All authors had full access to all data in the study and accept responsibility for the decision to submit for publication.
Results
Baseline clinical characteristics
The baseline characteristics of the patients were summarized in Table 1. Following the application of inclusion and exclusion criteria, a total of 2218 patients (mean age: 58 years ± 12 [SD]; 757 women) with pathologically confirmed renal cell carcinoma were retrospectively included in the study. The median follow-up duration was 62 months (Range: 6–154). Of all the patients enrolled, 248 (11.2%) patients had deceased, and 242 (11.0%) experienced tumor recurrence. The median overall survival (OS) was 62 months, with 1-, 3-, and 5-year OS rates of 99.3%, 95.0%, and 90.4%, respectively. The median recurrence-free survival (RFS) was 61 months, with corresponding 1-, 3-, and 5-year RFS rates of 97.0%, 92.6%, and 88.7%. The median disease-specific survival (DSS) was 62 months, with 1-, 3-, and 5-year DSS rates of 99.4%, 96.2%, and 92.4%, respectively.
Table 1.
Clinical characteristics of RCC patients from different cohorts.
| Variable | No. of patients (%) or Median (IQR) |
|||
|---|---|---|---|---|
| Zhongshan cohort (N = 1482) | Zhejiang cohort (N = 433) | Xiamen cohort (N = 126) | TCIA cohort (N = 177) | |
| Age | 58 (50–65) | 56 (48–64) | 56 (45–67) | 59 (50–70) |
| Sex | ||||
| Male | 970 (65.5) | 288 (66.5) | 85 (67.5) | 118 (66.7) |
| Female | 512 (34.5) | 145 (33.5) | 41 (32.5) | 59 (33.3) |
| Median size, cm | 3.5 (2.5–5) | 3.5 (2.5–5.5) | 3.0 (2.2–5.0) | 4.9 (3.4–6.9) |
| Surgical method | ||||
| Partial nephrectomy | 553 (37.3) | 289 (66.7) | 69 (54.8) | 65 (35.7) |
| Radical nephrectomy | 929 (62.7) | 144 (33.3) | 57 (45.2) | 112 (63.3) |
| Pathologic type | ||||
| Clear cell RCC | 1179 (79.6) | 344 (79.5) | 93 (73.8) | 152 (85.9) |
| Papillary RCC | 118 (8.0) | 19 (4.4) | 9 (7.1) | 16 (9.0) |
| Chromophobe RCC | 89 (6.0) | 30 (6.9) | 6 (4.8) | 9 (5.1) |
| Other Malignanta | 96 (6.4) | 40 (9.2) | 18 (14.3) | 0 (0.0) |
| T stage | ||||
| Ⅰ | 1243 (83.9) | 372 (85.9) | 107 (84.9) | 109 (61.6) |
| Ⅱ | 105 (7.1) | 35 (8.1) | 10 (8.0) | 20 (11.3) |
| Ⅲ | 134 (9.0) | 26 (6.0) | 9 (7.1) | 48 (27.1) |
| ISUP Gradeb | ||||
| Grade 1 | 88 (6.8) | 53 (14.5) | 7 (6.9) | 3 (1.8) |
| Grade 2 | 989 (76.2) | 245 (67.5) | 84 (82.3) | 80 (47.6) |
| Grade 3 | 203 (15.7) | 59 (16.3) | 6 (5.9) | 72 (42.9) |
| Grade 4 | 17 (1.3) | 6 (1.7) | 5 (4.9) | 13 (7.7) |
| Lymph node involvement | ||||
| Absent | 1463 (98.7) | 429 (99.1) | 125 (99.2) | 174 (98.3) |
| Present | 19 (1.3) | 4 (0.9) | 1 (0.8) | 3 (1.7) |
| Sarcomatoid differentiation | ||||
| Absent | 1463 (98.7) | 426 (98.4) | 124 (98.4) | 168 (94.9) |
| Present | 19 (1.3) | 7 (1.6) | 2 (1.6) | 9 (5.1) |
| Necrosis | ||||
| Absent | 1308 (88.3) | 393 (90.8) | 118 (93.7) | 156 (88.1) |
| Present | 174 (11.7) | 40 (9.2) | 8 (6.3) | 21 (11.9) |
| Perinephric or renal sinus fat invasion | ||||
| Absent | 1364 (92.0) | 409 (94.5) | 115 (91.3) | 129 (72.9) |
| Present | 118 (8.0) | 24 (5.5) | 11 (8.7) | 48 (27.1) |
| Tumor thrombus | ||||
| Absent | 1425 (96.2) | 420 (97.0) | 120 (95.2) | 158 (89.3) |
| Present | 57 (3.8) | 13 (3.0) | 6 (4.8) | 19 (10.7) |
| SSIGN | ||||
| Low risk | 1102 (79.1) | 334 (82.9) | 100 (83.3) | 84 (50.0) |
| Intermediate risk | 245 (17.6) | 56 (13.9) | 17 (14.2) | 70 (41.7) |
| High risk | 46 (3.3) | 13 (3.2) | 3 (2.5) | 14 (8.3) |
| Leibovich’Chromophobe RCC risk groups | ||||
| Group 1 | 81 (91.0) | 28 (93.3) | 5 (83.3) | 7 (77.8) |
| Group 2 | 7 (7.9) | 2 (6.7) | 1 (16.7) | 0 (0.0) |
| Group 3 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 2 (22.2) |
| Imaging appearance | ||||
| Cystic | 922 (62.2) | 209 (48.3) | 106 (84.1) | 80 (45.2) |
| Solid | 560 (37.8) | 224 (51.7) | 20 (15.9) | 97 (54.8) |
Other malignant tumors included mucinous tubular and spindle cell carcinoma, tubulocystic RCC, eosinophilic solid and cystic RCC, Xp11 translocation RCC, etc.
ISUP grade was evaluated in clear cell RCC and papillary RCC only. RCC, renal cell carcinoma; SSIGN, the Stage, Size, Grade and Necrosis.
Radiological consistency in the CID
The intra-observer agreement for radiologist B demonstrated excellence, with a correlation coefficient of 0.921 (95% CI = 0.915–0.955). The inter-observer correlation coefficients between radiologist A and B1, and between A and B2, were 0.866 (95% CI = 0.855–0.922) and 0.910 (95% CI = 0.903–0.917), respectively.
Correlation between the CID and survival outcomes in RCC patients
The CID showed significant value in predicting OS, RFS, and DSS in patients with RCC tumors measuring 3 cm or larger, a phenomenon not observed in tumors smaller than 3 cm in both the Zhongshan cohort and external test set (Table 2). Moreover, significant associations were observed between the CID and survival outcomes across different pathological types of RCC (Supplementary Table S1) as well as ISUP grades (Supplementary Table S2) in both the Zhongshan cohort and external test set.
Table 2.
The prognostic performance of CID in RCC across varying tumor sizes in Zhongshan cohort and external test set.
| Tumor Size (cm) | p-value |
|||||
|---|---|---|---|---|---|---|
| Zhongshan cohort |
External test set |
|||||
| DSS | RFS | OS | DSS | RFS | OS | |
| 0–2 | 1.000 | 1.000 | 0.874 | 0.460 | 1.000 | 0.888 |
| 2–3 | 0.953 | 0.314 | 0.990 | 0.868 | 0.979 | 0.956 |
| 3–4 | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
| 4–5 | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
| 5–6 | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
| 6–7 | <0.001a | <0.001a | <0.001a | 0.045a | 0.001a | 0.092 |
| ≥7 | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a | <0.001a |
CID, contour irregularity degree; RCC, renal cell carcinoma; DSS, disease-specific survival; RFS, recurrence-free survival; OS, overall survival.
Indicate statistical significance.
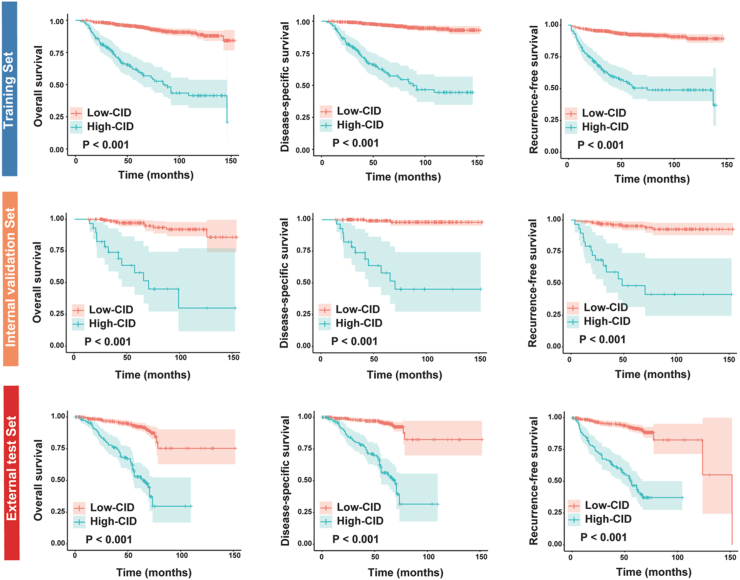
Considering these results, we focused our analysis on patients with RCC size ≥3 cm. The Zhongshan cohort was randomly divided into training and internal validation sets at an 8:2 ratio. We determined the optimal threshold value of the CID as 50% based on X-tile software regarding DSS in the training cohort. Patients were dichotomized into low-CID (<50%) and high-CID (≥50%) groups. Survival analyses revealed that high-CID patients exhibited significantly shorter DSS/OS/RFS compared to the low-CID group in RCC patients with tumor size ≥3 cm in both training, internal validation set, and external test set (Fig. 3, both p < 0.001), which was not observed in RCC patients with tumor size <3 cm (Supplementary Fig. S1). Furthermore, our analyses showed that patients with high-CID exhibited higher age (p < 0.001), more advanced T stage (p < 0.001), and a greater incidence of lymph node involvement (p < 0.001), sarcomatoid differentiation (p < 0.001), and necrosis (p < 0.001) compared to patients with low-CID.
Fig. 3.
Kaplan–Meier estimate of overall survival (left panel), disease-specific survival (middle panel), and recurrence-free survival (right panel) stratified by contour irregularity degree in RCC patients with tumors measuring 3 cm or larger across the training set, internal validation set, and external test set. RCC, renal cell carcinoma.
Stratified analysis of the CID in subgroups defined by SSIGN models and ISUP grades
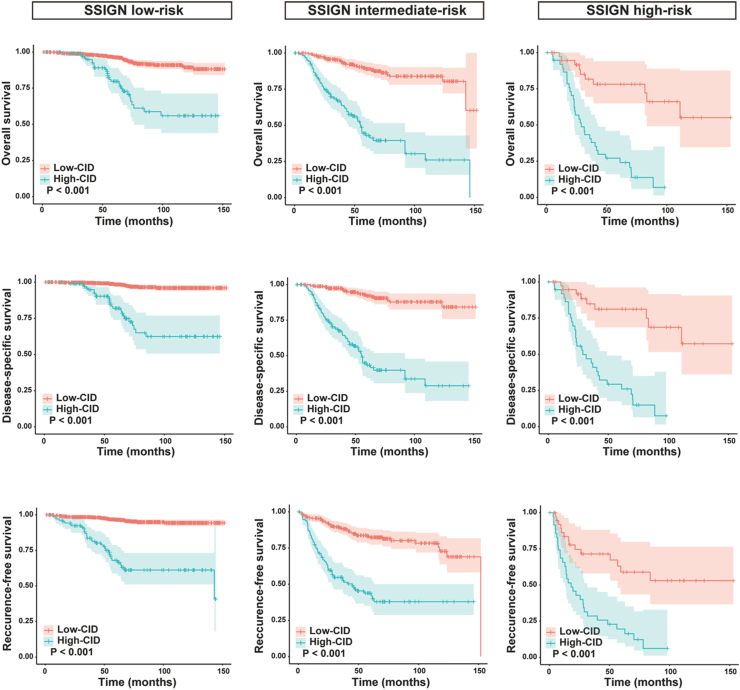
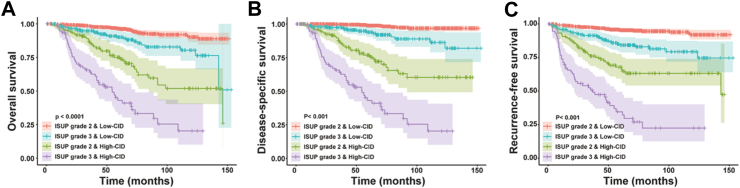
Kaplan–Meier survival analyses in all patients with tumor size ≥3 cm demonstrated that the CID could further stratify patients into prognostically distinct high-risk and low-risk groups within the low-, intermediate-, and high-risk categories identified by SSIGN (Fig. 4). Similarly, significant differences in survival outcomes stratified by the CID were observed between ISUP grades 2 and 3 (Fig. 5). Notably, the low-CID group among ISUP grade 3 patients demonstrated better survival outcomes compared to the high-CID group among ISUP grade 2 patients. Furthermore, multivariate analysis confirmed that CID serves as an independent prognostic stratification marker for patients with ISUP grade 2 and 3 tumors (Supplementary Table S3).
Fig. 4.
Kaplan–Meier estimates of overall survival (upper panel), disease-specific survival (middle panel), and recurrence-free survival (lower panel) are stratified by contour irregularity degree in RCC patients with tumors measuring 3 cm or larger, categorized by different SSIGN risk subgroups. RCC, renal cell carcinoma; SSIGN, the Stage, Size, Grade and Necrosis.
Fig. 5.
Kaplan–Meier estimates of disease-specific survival stratified by contour irregularity degree in RCC patients with tumors measuring 3 cm or larger, classified by ISUP grades 2 and 3. (A) Overall survival; (B) Disease-specific survival; (C) Recurrence-free survival. RCC, renal cell carcinoma; ISUP, the International Society of Urological Pathology.
Efficacy of CID-based nomograms in predicting prognosis
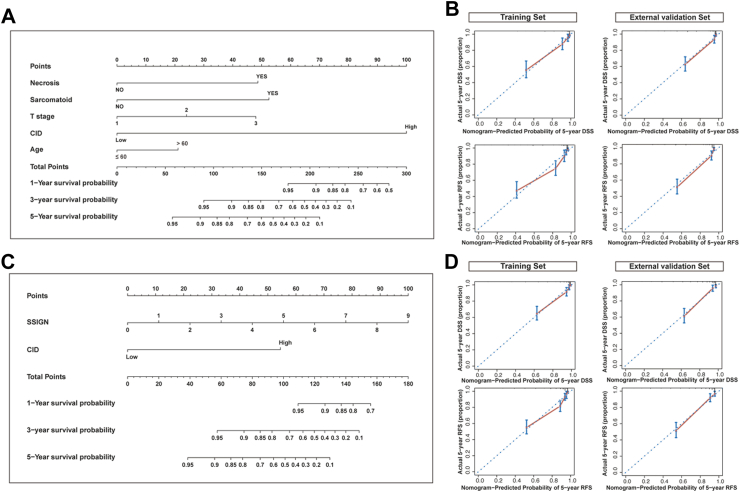
In the training set, Cox regression analysis of DSS is presented in Table 3. The CID (HR = 10.78, 95% CI = 6.63–17.53, p < 0.001), sarcomatoid differentiation (HR = 4.52, 95% CI = 2.23–9.16, p < 0.001), lymph node involvement (HR = 5.53, 95% CI = 2.47–12.38, p < 0.001), necrosis (HR = 2.69, 95% CI = 1.61–4.48, p < 0.001), T stage (HR = 1.80, 95% CI = 1.37–2.35, p < 0.001), and age (HR = 1.89, 95% CI = 1.17–3.05, p = 0.009) were identified as independent prognostic factors for DSS in multivariable analysis. Based on these findings, a nomogram (C-Model) predicting 1-, 3-, and 5-year DSS was constructed, with each variable assigned a score according to its β coefficients. The maximum variance inflation factor (VIF) was found to be 1.13, well below the threshold of 10, indicating no significant multicollinearity issues. To identify the incremental value of CID to SSIGN models for survival prediction, a combined nomogram (C-SSIGN) was also developed in the training set. Fig. 6 presents the two novel CID-based nomograms (C-Model and C-SSIGN) along with their calibration curves, demonstrating their excellent predictive performance.
Table 3.
Univariate and multivariate analyses for DSS in the training set.
| Variable | Training set |
||
|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
||
| p-value | HR (95% CI) | p-value | |
| Age (≤60/>60) | <0.001a | 1.89 (1.17–3.05) | 0.009a |
| Sex (female/male) | 0.187 | ||
| T stage (Ⅰ/Ⅱ/Ⅲ/Ⅳ) | <0.001a | 1.80 (1.37–2.35) | <0.001a |
| Necrosis (no/yes) | <0.001a | 2.69 (1.61–4.48) | <0.001a |
| Lymph node involvement (no/yes) | <0.001a | 5.53 (2.47–12.38) | <0.001a |
| Sarcomatoid differentiation (no/yes) | <0.001a | 4.52 (2.23–9.16) | <0.001a |
| CID (<50%/≥50%) | <0.001a | 10.78 (6.63–17.53) | <0.001a |
| Tumor size, cm (4≤/4–7/>7) | <0.001a | ||
DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval; CID, contour irregularity degree.
Indicate statistical significance.
Fig. 6.
Development of the CID-based prognostic nomograms for RCC patients with tumors measuring 3 cm or larger. (A) The nomogram combining CID with pathological features (C-Model). (B) Calibration curves of the C-Model for predicting 5-year disease-specific survival (upper panel) and recurrence-free survival (lower panel) in the training and external validation sets. (C) The nomogram combining CID with SSIGN (C-SSIGN). (D) Calibration curves of the C-SSIGN for predicting 5-year disease-specific survival (upper panel) and recurrence-free survival (lower panel) in the training and external validation sets. CID, contour irregularity degree; RCC, renal cell carcinoma; SSIGN, the Stage, Size, Grade and Necrosis.
Our results suggested that the newly developed C-Model and C-SSIGN model showed superior performance to SSIGN, as evidenced by higher C-index and AUCs in the training, internal validation, and external test sets (Table 4). Moreover, DCA demonstrated that our newly developed models had superior clinical usefulness compared to SSIGN (Supplementary Fig. S2). Considering the inapplicability of SSIGN for chrRCC, the C-Model was compared with Leibovich’s chrRCC model in patients with chrRCC. Our nomogram exhibited a better AUC value of 0.75 (95% CI: 0.43–1.00) compared to Leibovich’s chrRCC model (AUC = 0.58, 95% CI: 0.31–0.85).
Table 4.
Performance of nomograms for DSS in patients with RCC in the training, internal validation, and external test sets compared with SSIGN.
| Performance parameter | Training set |
Internal validation set |
External test set |
||||||
|---|---|---|---|---|---|---|---|---|---|
| C-SSIGN | C-Model | SSIGN | C-SSIGN | C-Model | SSIGN | C-SSIGN | C-Model | SSIGN | |
| AUC | 0.89 | 0.88 | 0.82 | 0.95 | 0.93 | 0.84 | 0.85 | 0.85 | 0.75 |
| 95% CI low | 0.85 | 0.84 | 0.78 | 0.92 | 0.88 | 0.74 | 0.79 | 0.80 | 0.68 |
| 95% CI high | 0.93 | 0.93 | 0.87 | 0.99 | 0.98 | 0.93 | 0.90 | 0.90 | 0.81 |
| C-index | 0.89 | 0.88 | 0.83 | 0.95 | 0.92 | 0.83 | 0.86 | 0.86 | 0.79 |
| 95% CI low | 0.85 | 0.84 | 0.79 | 0.92 | 0.88 | 0.74 | 0.81 | 0.81 | 0.73 |
| 95% CI high | 0.92 | 0.92 | 0.86 | 0.98 | 0.96 | 0.91 | 0.90 | 0.90 | 0.84 |
| p-value | <0.001a | 0.016a | 0.013a | 0.070 | <0.001a | <0.001a | |||
p values correspond to comparisons of AUCs between the SSIGN score and each nomogram.
DSS, disease-specific survival; RCC, renal cell carcinoma; SSIGN, the Stage, Size, Grade and Necrosis; AUC, the area under the curve; CI, confidence interval.
Indicate statistical significance.
Discussion
In this study, we demonstrated that preoperative CT imaging-based CID could effectively predict both OS, RFS, and DSS in patients with RCC tumors measuring 3 cm or larger. Additionally, the CID can effectively discriminate survival outcomes among distinct subgroups of RCC patients following risk stratification according to the SSIGN model and ISUP grade. Our newly developed nomogram, integrating the CID and pathological features, shows significant superiority to existing staging systems.
Currently, prognostic differentiation for RCC patients widely relies on pathology-based prognostic models.4, 5, 6 The SSIGN model is the most widely used prognostic tool for localized RCC.6, 7, 8, 9 However, clinicians have observed significant survival disparities among patients within the same SSIGN classifications. Therefore, identifying user-friendly and effective prognostic indicators during routine examinations of RCC patients, to enhance the predictive performance of existing pathology-based prognostic models, is an urgent issue that needs to be addressed.
Radiological morphology offers a comprehensive depiction of the tumor, compensating for the inherent partial sampling in pathology. Despite previous studies proposing prognostic models based on radiomics or artificial intelligence, their clinical applications lack practicality due to small patient cohorts and limited generalizability.23, 24, 25, 26 It’s well established that contour analysis could aid in distinguishing between benign and malignant renal tumors.27 Our previous research successfully standardized renal tumor contour irregularity using the CID and demonstrated its prognostic value in papillary renal cell carcinoma (pRCC).16 In this study, by employing a large-scale and multicenter cohort encompassing 2218 patients pathologically diagnosed with ccRCC, pRCC, and chrRCC, we substantiate the potential of the CID in risk stratification for RCC patients across different histological subtypes. Notably, we found the CID to be an independent predictor of survival outcomes for patients with RCC tumors measuring 3 cm or larger, a phenomenon not observed in tumors smaller than 3 cm. This discrepancy may be attributed to the limited scan layers of tumors smaller than 3 cm, impacting the accuracy of the CID.
Furthermore, our results suggest that the application of the CID allows for further risk stratification within the low-, intermediate-, and high-SSIGN subgroups. Previous studies have highlighted significant differences in outcomes between ISUP grades 1–3 tumors and ISUP grade 4 tumors.5,28,29 Interestingly, ISUP grade 1 and grade 2 tumors were amalgamated for analytical purposes, as their outcomes were not significantly different from those of grade 3 tumors, indicating challenges in stratifying the prognosis between patients with grade 2 and grade 3 tumors.28 Our study reveals that RCC patients with high-CID within ISUP grade 2 exhibit poorer clinical outcomes compared to those with low-CID within ISUP grade 3. This underscores the potential of the CID in further differentiating the prognosis between RCC patients with ISUP grade 2 and grade 3. Collectively, our findings demonstrate that the CID serves as an effective preoperative imaging-based user-friendly biomarker for complementing the current pathology-based staging system.
Additionally, we developed a CID-based nomogram (C-Model) for predicting survival outcomes in the training set, incorporating several patient- and pathology-level features alongside the CID. The nomogram demonstrated strong predictive performance with C-index values of 0.88, 0.92, and 0.86 in the training, internal validation, and external test sets, respectively. Furthermore, we integrated CID into the SSIGN model to create a novel prognostic model (C-SSIGN) for survival prediction. Our results demonstrated that the C-SSIGN model exhibited significantly improved predictive efficacy compared to the SSIGN model in training (p < 0.001), internal validation (p = 0.013), and external test sets (p < 0.001).
Multiple clinical trials have demonstrated that adjuvant pembrolizumab significantly improves both disease-free survival and overall survival compared with placebo among high-risk RCC patients after nephrectomy.30, 31, 32 Therefore, our newly developed prognostic model could help identify high-risk patient populations who may benefit from more aggressive adjuvant therapy. By using effective prognostic models, we can identify high-risk RCC patients for closer follow-up, provide guidance for adjuvant therapy selection, and offer more objective information during doctor-patient communication. This aids in informed decision-making, improving treatment adherence. Additionally, the predictive model can assist doctors in communicating with patients and their families, providing objective information about disease progression and prognosis. Our findings could further optimize personalized treatment for RCC, improving patient outcomes and quality of life.
However, our study has certain limitations that warrant acknowledgment. Firstly, being retrospective, our investigation necessitates further prospective studies to validate our findings, ensuring reproducibility and generalizability. Secondly, for the sake of simplicity and practicality, we only included patients with preoperative CT imaging data to avoid potential biases associated with pooling CT and MRI scanners during the evaluation process. Future validation could be conducted in a large-scale cohort with preoperative MRI data. Thirdly, as our study is semi-quantitative, future integration with artificial intelligence could further advance it into a quantitative study, enhancing its predictive efficacy. Fourth, the majority of patients in this study are from China, highlighting the need for future validation in external cohorts from additional countries. Fifth, the positive surgical margin, recognized as a potential prognostic factor, was not included in this study, which might impact the predictive accuracy of newly developed model.
In conclusion, as a non-invasive and easily assessable biomarker, the CID emerges as a robust independent predictor of prognosis and complements existing pathology-based staging systems in forecasting survival outcomes among patients with RCC tumors measuring 3 cm or larger. Our newly devised nomogram serves as a practical and reliable tool, offering crucial prognostic insights for routine clinical application, thereby advancing precision medicine and personalized therapy in RCC management.
Contributors
P. Zhu, Y. Xiong, D. Chen, F. Chen, and J. Zhou was responsible for overall study design. P. Zhu, Y. Xiong, D. Chen, J. Qu, R. Wang, L. Yao, and J. Hou accessed and verified the underlying data. P. Zhu, F. Zhang, and S. Wang were responsible for analyses and interpretation of data. P. Zhu, Y. Xiong, D. Chen, F. Zhang, and J. Zhou were responsible for writing, reviewing, and/or revision of the manuscript. J. Zhou, J. Guo, F. Chen, C. Dai, and M. Zeng were study supervisions and guarantors. All the authors have read, discussed, and unanimously approved the final version of the manuscript. All authors had full access to the data and had the final responsibility for the decision to submit for publication.
Data sharing statement
All data of TCIA could be downloaded from the Cancer Imaging Archive. Other data are available upon reasonable request.
Declaration of interests
There are no conflicts of interest.
Acknowledgements
This study was funded by grants from National Natural Science Foundation of China (82171897, 82202106, 82371923); Shanghai Municipal Health Commission (202240152); China National Key R&D Program (2022YFC2401605); Science and Technology Commission of Shanghai Municipality (23Y11907400).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102775.
Contributor Information
Feng Chen, Email: chenfenghz@zju.edu.cn.
Jianjun Zhou, Email: zhoujianjunzs@126.com.
Appendix ASupplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Eichelberg C., Junker K., Ljungberg B., Moch H. Diagnostic and prognostic molecular markers for renal cell carcinoma: a critical appraisal of the current state of research and clinical applicability. Eur Urol. 2009;55(4):851–863. doi: 10.1016/j.eururo.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Hollingsworth J.M., Miller D.C., Daignault S., Hollenbeck B.K. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 4.Kim S.P., Alt A.L., Weight C.J., et al. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: results from a large, single institution cohort. J Urol. 2011;185(6):2035–2039. doi: 10.1016/j.juro.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 5.Delahunt B., Cheville J.C., Martignoni G., et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37(10):1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 6.Frank I., Blute M.L., Cheville J.C., Lohse C.M., Weaver A.L., Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 7.Zigeuner R., Hutterer G., Chromecki T., et al. External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. Eur Urol. 2010;57(1):102–109. doi: 10.1016/j.eururo.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Correa A.F., Jegede O., Haas N.B., et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation. J Clin Oncol. 2019;37(23):2062–2071. doi: 10.1200/JCO.19.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficarra V., Novara G., Galfano A., et al. The ‘Stage, Size, Grade and Necrosis’ score is more accurate than the University of California Los Angeles Integrated Staging System for predicting cancer-specific survival in patients with clear cell renal cell carcinoma. BJU Int. 2009;103(2):165–170. doi: 10.1111/j.1464-410X.2008.07901.x. [DOI] [PubMed] [Google Scholar]
- 10.Bektas C.T., Kocak B., Yardimci A.H., et al. Clear cell renal cell carcinoma: machine learning-based quantitative computed tomography texture analysis for prediction of fuhrman nuclear grade. Eur Radiol. 2019;29(3):1153–1163. doi: 10.1007/s00330-018-5698-2. [DOI] [PubMed] [Google Scholar]
- 11.Yamada T., Endo M., Tsuboi M., et al. Differentiation of pathologic subtypes of papillary renal cell carcinoma on CT. AJR Am J Roentgenol. 2008;191(5):1559–1563. doi: 10.2214/AJR.07.3181. [DOI] [PubMed] [Google Scholar]
- 12.Marcon J., Graser A., Horst D., et al. Papillary vs clear cell renal cell carcinoma. Differentiation and grading by iodine concentration using DECT-correlation with microvascular density. Eur Radiol. 2020;30(1):1–10. doi: 10.1007/s00330-019-06298-2. [DOI] [PubMed] [Google Scholar]
- 13.Hötker A.M., Karlo C.A., Zheng J., et al. Clear cell renal cell carcinoma: associations between CT features and patient survival. AJR Am J Roentgenol. 2016;206(5):1023–1030. doi: 10.2214/AJR.15.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlo C.A., Di Paolo P.L., Chaim J., et al. Radiogenomics of clear cell renal cell carcinoma: associations between CT imaging features and mutations. Radiology. 2014;270(2):464–471. doi: 10.1148/radiol.13130663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamshidi N., Jonasch E., Zapala M., et al. The radiogenomic risk score: construction of a prognostic quantitative, noninvasive image-based molecular assay for renal cell carcinoma. Radiology. 2015;277(1):114–123. doi: 10.1148/radiol.2015150800. [DOI] [PubMed] [Google Scholar]
- 16.Dai C., Huang J., Li Y., et al. Tumor contour irregularity on preoperative imaging: a practical and useful prognostic parameter for papillary renal cell carcinoma. Eur Radiol. 2021;31(6):3745–3753. doi: 10.1007/s00330-020-07456-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang J., Dai C., Zhang S., et al. Prognostic evaluation based on radiological morphological characteristic for tumors larger than 7 cm in renal cell carcinoma. J Cancer Res Clin Oncol. 2023;149(1):263–270. doi: 10.1007/s00432-022-04523-y. [DOI] [PubMed] [Google Scholar]
- 18.Xu P., Zhang S., Cheng J., et al. Prognostic value of tumour contour irregularity on surgical strategies for T1bN0M0 renal cell carcinoma: a multi-institutional study. Eur J Radiol. 2023;159 doi: 10.1016/j.ejrad.2022.110665. [DOI] [PubMed] [Google Scholar]
- 19.Ljungberg B., Albiges L., Abu-Ghanem Y., et al. European association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410. doi: 10.1016/j.eururo.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 21.Camp R.L., Dolled-Filhart M., Rimm D.L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 22.Leibovich B.C., Lohse C.M., Cheville J.C., et al. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur Urol. 2018;73(5):772–780. doi: 10.1016/j.eururo.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Mahootiha M., Qadir H.A., Bergsland J., Balasingham I. Multimodal deep learning for personalized renal cell carcinoma prognosis: integrating CT imaging and clinical data. Comput Methods Programs Biomed. 2024;244 doi: 10.1016/j.cmpb.2023.107978. [DOI] [PubMed] [Google Scholar]
- 24.He H., Jin Z., Dai J., Wang H., Sun J., Xu D. Computed tomography-based radiomics prediction of CTLA4 expression and prognosis in clear cell renal cell carcinoma. Cancer Med. 2023;12(6):7627–7638. doi: 10.1002/cam4.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G., Nie P., Yan L., et al. The radiomics-based tumor heterogeneity adds incremental value to the existing prognostic models for predicting outcome in localized clear cell renal cell carcinoma: a multicenter study. Eur J Nucl Med Mol Imaging. 2022;49(8):2949–2959. doi: 10.1007/s00259-022-05773-1. [DOI] [PubMed] [Google Scholar]
- 26.Nie P., Yang G., Wang Y., et al. A CT-based deep learning radiomics nomogram outperforms the existing prognostic models for outcome prediction in clear cell renal cell carcinoma: a multicenter study. Eur Radiol. 2023;33(12):8858–8868. doi: 10.1007/s00330-023-09869-6. [DOI] [PubMed] [Google Scholar]
- 27.Yap F.Y., Hwang D.H., Cen S.Y., et al. Quantitative contour analysis as an image-based discriminator between benign and malignant renal tumors. Urology. 2018;114:121–127. doi: 10.1016/j.urology.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Delahunt B., Eble J.N., Egevad L., Samaratunga H. Grading of renal cell carcinoma. Histopathology. 2019;74(1):4–17. doi: 10.1111/his.13735. [DOI] [PubMed] [Google Scholar]
- 29.Warren A.Y., Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World J Urol. 2018;36(12):1913–1926. doi: 10.1007/s00345-018-2447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choueiri T.K., Tomczak P., Park S.H., et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 31.Choueiri T.K., Tomczak P., Park S.H., et al. Overall survival with adjuvant pembrolizumab in renal-cell carcinoma. N Engl J Med. 2024;390(15):1359–1371. doi: 10.1056/NEJMoa2312695. [DOI] [PubMed] [Google Scholar]
- 32.Powles T., Tomczak P., Park S.H., et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133–1144. doi: 10.1016/S1470-2045(22)00487-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.