Abstract
Background
Evidence suggests a connection between DNA methylation (DNAm) aging and reproductive aging. However, the causal relationship between DNAm and age at menopause remains uncertain.
Methods
Employing established DNAm epigenetic clocks, such as DNAm Hannum age acceleration (Hannum), Intrinsic epigenetic age acceleration (IEAA), DNAm-estimated granulocyte proportions (Gran), DNAm GrimAge acceleration (GrimAgeAccel), DNAm PhenoAge acceleration (PhenoAgeAccel), and DNAm-estimated plasminogen activator inhibitor-1 levels (DNAmPAIadjAge), a bidirectional Mendelian randomization (MR) study was carried out to explore the potential causality between DNAm and menopausal age. The primary analytical method used was the inverse variance weighted (IVW) estimation model, supplemented by various other estimation techniques.
Results
DNAm aging acceleration or deceleration, as indicated by Hannum, IEAA, Gran, GrimAgeAccel, PhenoAgeAccel, and DNAmPAIadjAge, did not exhibit a statistically significant causal effect on menopausal age according to forward MR analysis. However, there was a suggestive positive causal association between age at menopause and Gran (Beta = 0.0010; 95% confidence interval (CI): 0.0004, 0.0020) in reverse MR analysis.
Conclusion
The observed increase in granulocyte DNAm levels in relation to menopausal age could potentially serve as a valuable indicator for evaluating the physiological status at the onset of menopause.
Keywords: epigenetic clocks, DNA methylation, menopause, Mendelian randomization, causal association
Introduction
With the advancement of technology, biological age can be measured using various molecular or phenotypic biomarkers. For instance, research has found that the epigenetic clock, based on DNA methylation (DNAm) values and mathematical algorithms (1), can accurately quantify human aging (2), including reproductive senescence (3, 4) and immunosenescence (2). DNAm, as a significant epigenetic modification, primarily entails methylation at the fifth carbon position of cytosine, commonly observed in cytosine-guanine dinucleotides (CpG) in mammals (5, 6). Epigenetics typically includes modifications that influence the expression of genes (7, 8), and DNAm plays an important role in the development of various diseases (9–14). The commonly used epigenetic clocks include DNAm Hannum age acceleration (Hannum), Intrinsic epigenetic age acceleration (IEAA), DNAm-estimated granulocyte proportions (Gran), DNAm GrimAge acceleration (GrimAgeAccel), DNAm PhenoAge acceleration (PhenoAgeAccel), and DNAm-estimated plasminogen activator inhibitor-1 levels (DNAmPAIadjAge) measures (15, 16). Moreover, in recent years, there has been a heightened emphasis on understanding how DNAm impacts various physiological processes, with a particular focus on reproductive aging in women (17, 18). While previous studies have linked changes in DNAm patterns to conditions like cancer and neurological diseases, additional research is required to clarify the role of DNAm in the menopausal transition (19–22). We aim to investigate the causal connection between DNAm and menopausal age using established DNAm-based epigenetic clocks.
Menopause occurs due to the depletion of the limited supply of ovarian follicles, leading to a decrease in the production of the ovarian hormones progesterone and estrogen (23). Confirmation of the natural menopause typically occurs after 12 consecutive months of amenorrhea, in the absence of pathological causes (24). Nevertheless, research suggests that several factors, such as age at menarche, parity, body mass index, oral contraceptive use, alcohol consumption, smoking, and level of physical activity, often influence the onset age of menopause (25, 26). Genetic variables play a significant role in influencing menopause (27, 28), with genetic factors accounting for only 47% of the onset age, indicating that non-genetic factors also influence this hormonal aging process (29). For example, Lu et al. found that women undergoing early menopause exhibit higher levels of methylation in Alu and long interspersed nuclear element 1 (LINE-1) repetitive sequences, whereas those experiencing later menopause display lower levels of methylation (3). Conversely, data supports a nonlinear “U-shaped” relationship between the number of menstrual cycles in females and LINE-1 methylation (30). There is currently insufficient systematic study on the impact of DNAm on menopause age, and the available data does not prove a causal relationship between DNAm and menopause.
Studies employing Mendelian randomization (MR) aim to assess whether the observed association between an exposure and an outcome may suggest causation. This is achieved by investigating whether genetic variations associated with the exposure are also linked to the outcome (31–34). Our goal is to investigate the existence of a causal link between DNAm biomarkers and menopausal age, as well as to uncover potential underlying mechanisms. By using MR validation to evaluate the impact of DNAm on women’s menopausal transition, we may better comprehend the intricate relationship between reproductive aging and epigenetic regulation, providing a scientific basis for better understanding and treatment of women’s menopause.
Methods
Study design
In this study, bidirectional MR analyses were conducted. Initially, forward MR analysis utilized data from genome-wide association study (GWAS) datasets with six DNAm phenotypes as the exposure variables and menopause (age at onset) as the outcomes. Subsequently, reverse MR was performed to investigate the causal associations between the exposure variable (menopause (age at onset)) and the outcome variables (six DNAm phenotypes).
Data sources
In the bidirectional MR analysis, a range of summary datasets were employed (refer to Supplementary Table 1 ). The GWAS data for the six DNAm phenotypes were extracted from the study by McCartney et al. (15). Additionally, the GWAS data for menopause (age at onset) were obtained from the study by Loh et al. (35).
Statistical method
Instrumental variables (IVs) were chosen based on specific criteria, including: (1) IVs and exposure were significantly associated; (2) identification of independent IVs through clumping within a 10 Mb window and linkage disequilibrium (LD) of R2 < 0.001; and (3) the minor allele frequency (MAF) > 0.01, as previously reported (36–39). F-statistics were computed to assess the strength of IVs, with a value exceeding 10 indicating a reduced risk of weak IV bias (40).
In both forward and reverse MR analyses, we applied three statistical methods, namely the inverse variance weighted (IVW) (41), weighted median (WM) (42), and MR-Egger methods (43). The MR-Egger intercept test was employed to evaluate horizontal pleiotropy, while MR-PRESSO’s pleiotropy correction data were utilized to detect and address potential outliers. Heterogeneity was assessed through Cochrane Q statistics. Furthermore, the influence of each IV on causal relationships was examined through a ‘leave-one-out’ approach. Given that all outcomes were continuous variables, causal estimates were derived using beta coefficients and their corresponding 95% confidence intervals (CIs). These analyses were conducted using the TwoSampleMR package within the R software environment (44).
Results
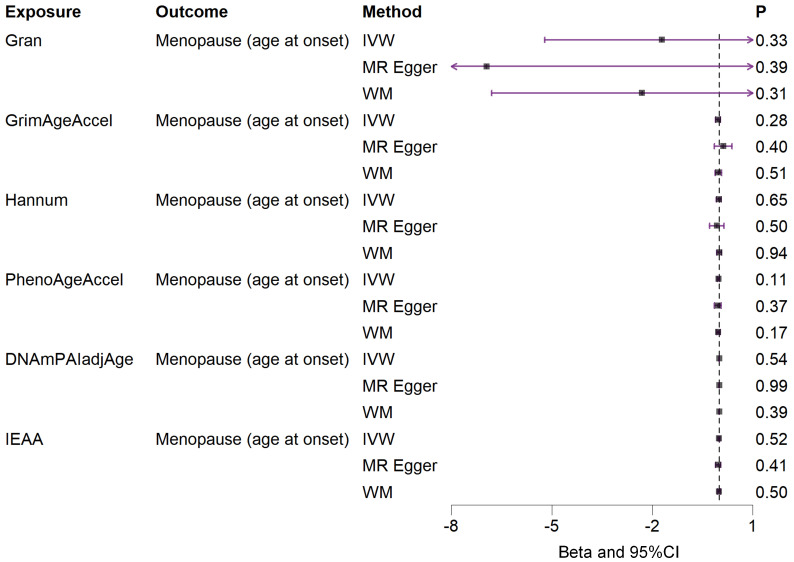
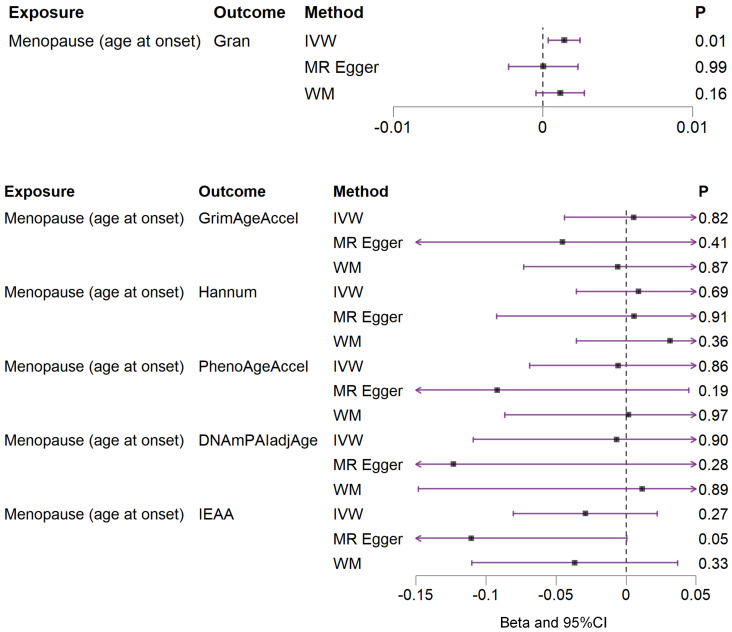
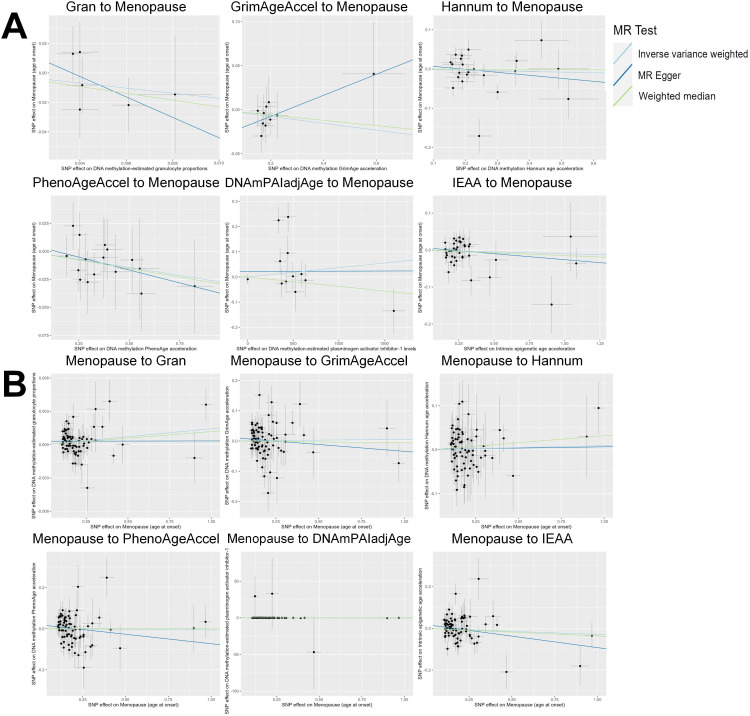
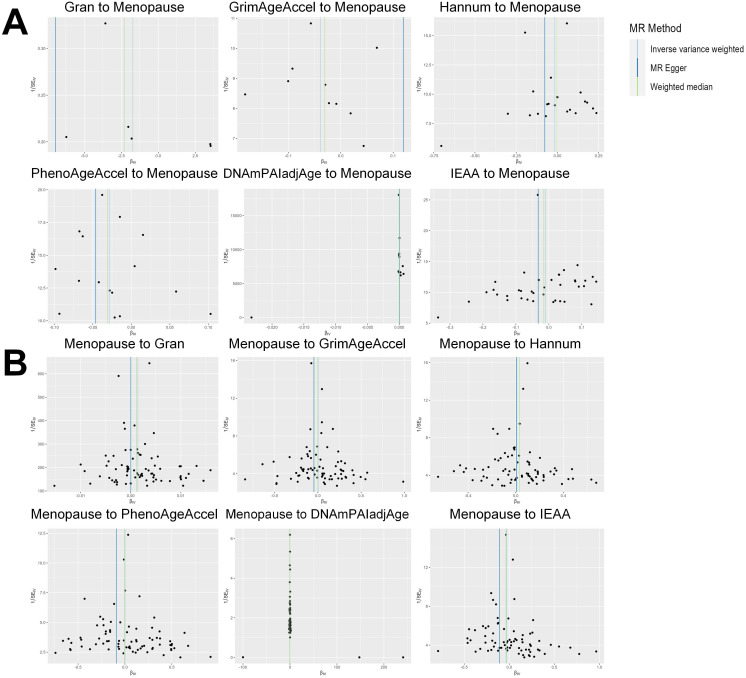
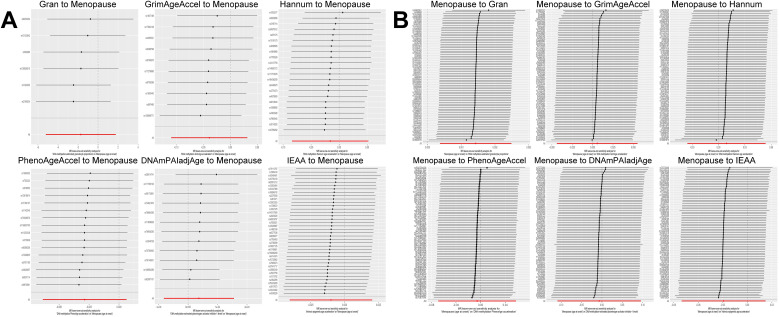
Based on the rigorous inclusion and exclusion criteria applied, all IVs included in our bidirectional MR analysis, both for the forward and reverse MR approaches, demonstrated robust instrument strength. This is evidenced by F-statistic values ranging from 23.99 to 651.62 (all > 10), as outlined in Supplementary Table 2 . The forward MR analysis showed no evidence of a causal relationship between any of the six DNAm phenotypes and menopause (age at onset) (all P > 0.05), as depicted in Figure 1 ; Supplementary Table 3 . Conversely, the IVW method in reverse MR indicated a significant association between genetically predicted menopause (age at onset) and one of the six DNAm phenotypes, specifically Gran (Beta = 0.0010; 95% CI: 0.0004, 0.0020) ( Figure 2 ; Supplementary Table 4 ). Consistency in the direction of the association between menopause (age of onset) and Gran was observed when employing the MR-Egger and WM methods in reverse MR ( Figure 2 ; Supplementary Table 4 ). A scatter plot in Figure 3 illustrates a positive causal relationship between menopause (age of onset) and Gran. No evidence of heterogeneity or horizontal pleiotropy between menopause (age at onset) and Gran was detected in reverse MR ( Figure 4 ; Supplementary Table 4 ). Additionally, the results from MR-PRESSO indicated the absence of outliers. The leave-one-out method outcomes, as depicted in Figure 5 , demonstrate that individual SNPs do not unduly influence the results.
Figure 1.
Associations between genetically predicted six DNAm phenotypes and menopause (age at onset) examined by three MR methods. DNAm, DNA methylation; Hannum, DNA methylation Hannum age acceleration; IEAA, Intrinsic epigenetic age acceleration; Gran, DNA methylation-estimated granulocyte proportions; GrimAgeAccel, DNA methylation GrimAge acceleration; PhenoAgeAccel, DNA methylation PhenoAge acceleration; DNAmPAIadjAge, DNA methylation-estimated plasminogen activator inhibitor-1 levels; MR, Mendelian randomization; IVW, inverse-variance weighted; WM, weighted median; CI, confidence interval.
Figure 2.
Associations between genetically predicted menopause (age at onset) and six DNAm phenotypes examined by three MR methods. DNAm, DNA methylation; Hannum, DNA methylation Hannum age acceleration; IEAA, Intrinsic epigenetic age acceleration; Gran, DNA methylation-estimated granulocyte proportions; GrimAgeAccel, DNA methylation GrimAge acceleration; PhenoAgeAccel, DNA methylation PhenoAge acceleration; DNAmPAIadjAge, DNA methylation-estimated plasminogen activator inhibitor-1 levels; MR, Mendelian randomization; IVW, inverse-variance weighted; WM, weighted median; CI, confidence interval.
Figure 3.
Scatter plot of the MR analyses. (A): The influence of six DNAm phenotypes on the age of menopause; (B): The impact of age of menopause on six DNAm phenotypes. SNP, single nucleotide polymorphism; MR, Mendelian randomization; DNAm, DNA methylation; Hannum, DNA methylation Hannum age acceleration; IEAA, Intrinsic epigenetic age acceleration; Gran, DNA methylation-estimated granulocyte proportions; GrimAgeAccel, DNA methylation GrimAge acceleration; PhenoAgeAccel, DNA methylation PhenoAge acceleration; DNAmPAIadjAge, DNA methylation-estimated plasminogen activator inhibitor-1 levels.
Figure 4.
Funnel plot of the MR analyses, with each SNP acting as an IV. (A): The analyses of six DNAm phenotypes on the age of menopause; (B): The analyses of age of menopause on six DNAm phenotypes. SNP, single nucleotide polymorphism; IV, instrumental variable; MR, Mendelian randomization; DNAm, DNA methylation; Hannum, DNA methylation Hannum age acceleration; IEAA, Intrinsic epigenetic age acceleration; Gran, DNA methylation-estimated granulocyte proportions; GrimAgeAccel, DNA methylation GrimAge acceleration; PhenoAgeAccel, DNA methylation PhenoAge acceleration; DNAmPAIadjAge, DNA methylation-estimated plasminogen activator inhibitor-1 levels.
Figure 5.
Leave-one-out sensitivity analysis by the IVW method after exclude a specific SNP from the analysis. (A): The influence of six DNAm phenotypes on the age of menopause; (B): The impact of age of menopause on six DNAm phenotypes. MR, Mendelian randomization; SNP, single nucleotide polymorphism; IVW, inverse-variance weighted; DNAm, DNA methylation; Hannum, DNA methylation Hannum age acceleration; IEAA, Intrinsic epigenetic age acceleration; Gran, DNA methylation-estimated granulocyte proportions; GrimAgeAccel, DNA methylation GrimAge acceleration; PhenoAgeAccel, DNA methylation PhenoAge acceleration; DNAmPAIadjAge, DNA methylation-estimated plasminogen activator inhibitor-1 levels.
Discussion
Reproductive aging is a multifaceted process influenced by a combination of aging-related changes, environmental factors, and fluctuations in sex hormone levels (17). It is imperative to elucidate the precise causal link between menopausal age and epigenetic modifications. Conducting MR studies on DNAm and menopause provides a unique opportunity to clarify the intricate relationship between reproductive aging and epigenetic changes. The findings of this study indicated no statistically significant causal effects of epigenetic clocks on the timing of menopause. Intriguingly, our reverse causal MR analysis suggested a potential association where a later age at menopause could be linked to elevated Gran.
Women who undergo menopause at a later stage typically exhibit higher levels of sex hormones compared to those experiencing early menopause (45, 82). Fluctuations in sex hormone levels can impact DNAm. In women aged 20-30, the difference in DNAm levels between breast tissue and blood is most pronounced, and this disparity diminishes with age (46). In a comprehensive epigenome-wide study, sex hormone-binding globulin levels in teenage girls were found to be significantly associated with DNAm at three specific CpG sites and two differentially methylated regions, in contrast to men (47). Moreover, analysis of genome-wide DNAm reveals that females exhibit higher levels of DNAm than males at key loci in peripheral blood leukocytes (81.2%) (48). Therefore, we infer that sex hormone regulation could potentially account for the rise in Gran associated with delayed menopause.
We hypothesize that sex hormone levels linked to menopausal age play a role in the epigenetic alterations of immune-related granulocytes. Increased DNAm levels at CpG sites are considered a significant marker of aging (49). Studies have shown a substantial acceleration of the epigenetic age of the immune system in individuals with Parkinson’s disease, primarily associated with granulocytes (50). Therefore, we posit that the elevation in DNAm observed in granulocytes is intricately linked to the aging of the immune system. The impact of sex hormones on the immune system differs across genders, with women being more sensitive to specific infections and having a greater prevalence of autoimmune illnesses (51, 52). Therefore, the impact of female hormones on the immune system should not be overlooked. A systematic literature review indicates that progesterone levels in healthy non-pregnant women with regular menstrual cycles typically induce immunosuppression by stimulating T helper 2 cells (Th2) cytokines (53). Similarly, estrogen and its receptor signaling play a role in modulating inflammation and innate immune responses in neutrophils, with effects that vary based on gender and menopausal status (54, 55). Granulocytes, constituting approximately two-thirds of all white blood cells, represent an ideal group of immune cells for research purposes (56). Our current endeavor is to understand the mechanisms by which estrogen and progesterone impact epigenetic changes in circulating granulocytes.
DNA methyltransferases (DNMTs) may be involved in the effect of female hormones on neutrophil DNAm. DNMTs are crucial enzymes responsible for inducing DNAm, with key players being DNMT1 and DNMT3A (57). Some studies have demonstrated that the gonadal hormone 17β-estradiol (E2) can influence the methylation of CpG islands, by promoting the expression of DNMT mRNA and protein (58). For instance, microinjection of E2 into the dorsal hippocampus resulted in increased mRNA expression and protein levels of DNMT3A and DNMT3B in the hippocampus (59, 60). As individuals age, the gender disparity in DNMT expression diminishes, yet females consistently maintain methylation levels that are twice as high as males (61). Additional evidence indicates that on the initial day post-birth, females exhibit significantly higher DNMT3A mRNA expression in the amygdala, although this gender discrepancy diminishes over time (62). Nevertheless, the gender variations in DNMT activity are still a subject of debate (63, 64), and our hypothesis necessitates further validation through additional clinical investigations. Moreover, indicators of leukocyte DNAm include mitogen-activated protein kinase kinase kinase kinase 1 (MAP4K1) and homeobox A3 (HOXA3) (65). Studies have shown that progesterone promotes MAP4K1 expression in estrogen-driven breast cancer (66). The results indicate that the function of MAP4K1 is influenced by the levels of female sex hormones. Additionally, elevated levels of HOX mRNA are also typically linked to higher hormone (progesterone and estrogen) levels (67). Exploring the potential yet unexplored connection between sex hormones and HOXA3/MAP4K1 offers a novel avenue for studying the effects of menopausal age on granulocyte epigenetic changes, emphasizing the necessity for additional validation in future studies.
It is crucial to emphasize that our study did not report a significant causal link between six DNAm-based epigenetic aging markers and menopausal age. These findings might be influenced by potential confounding factors such as genetics (68), environment (69, 70), and hormones (71, 72), which also affect the age of menopause. The differentiation between menopausal age, menopause, and aging stems from the understanding that menopausal age predominantly reflects the physiological functions of the female reproductive system, whereas DNAm captures age-related alterations influenced by lifelong environmental factors and feedback mechanisms on methylation status (73, 74). While there could be a potential association between DNAm and menopausal age, assessing this influence at a population level may yield inconsistent results. Moreover, while DNAm is a crucial component of aging, it represents only a fraction of the overall process. Additional biological factors influencing menopausal age encompass gene expression, protein synthesis, and cellular signaling pathways (75–77), which may engage in intricate and not fully understood interactions with DNAm. Conversely, fluctuations in related hormones and changes in other endocrine substances due to prolonged menopause could affect the methylation of various body tissues. In future research, animal experiments could be conducted to validate whether elevated levels of hormones, DNMTs, and immune system changes are associated with increased DNAm-estimated granulocyte levels.
We are at the forefront of establishing the causal link between DNAm biomarkers and menopausal age, offering essential scientific insights to enhance our comprehension of menopause and its related conditions. Furthermore, by utilizing readily available extensive genomic and clinical epidemiological datasets, our study not only reduces experimental costs but also leverages genotype advantages to address common confounding and reverse causality issues in observational studies, thereby increasing the robustness of causal inference.
Our research design has several limitations. Firstly, our sample primarily consists of individuals of European descent, which limits the applicability of our observations in non-European women. Further validation in other ethnic groups is necessary. Secondly, the assessment of DNAm aging biomarkers may be influenced by technical issues and biological differences such as diet (78), education (79), environmental factors (80, 81), and genetic variations. These factors could introduce biases, potentially causing DNAm-based epigenetic clocks to inaccurately capture the physiological changes associated with age at menopause, despite being considered among the most reliable predictors of DNAm aging. Moreover, menopause-related diseases have not been considered as potential confounders, which may contribute to the altered metabolic and inflammatory levels in women. Lastly, our MR assessments can only determine linear causal effects, which may not fully reflect the complex relationship between DNAm and age at menopause.
Conclusion
Our bidirectional MR analysis indicates the lack of a statistically significant causal link between DNAm aging and menopause age. Conversely, delayed menopause may be correlated with higher estimated granulocyte levels in DNAm. However, the participants in this study were all of European descent. Future research should validate these findings in diverse populations and conduct experimental studies to elucidate the underlying mechanisms. Furthermore, additional exploration of additional biomarkers is needed to better assist in the early clinical detection and intervention of female aging processes.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grant 82100040, 82302277 from National Natural Science Foundation of China, grant 2022RC1232 from the Science and Technology Innovation Program of Hunan Province, grant 23A0662, 23B0872, 22A0658 from Scientific Research Foundation of Hunan Provincial Education Department, and grant 2022CYY012, 2022CYY029 from ESI Special Project of Changsha Medical University.
Abbreviations
DNAm, DNA methylation; Hannum, DNA methylation Hannum age acceleration; IEAA, Intrinsic epigenetic age acceleration; Gran, DNA methylation-estimated granulocyte proportions; GrimAgeAccel, DNA methylation GrimAge acceleration; PhenoAgeAccel, DNA methylation PhenoAge acceleration; DNAmPAIadjAge, DNA methylation-estimated plasminogen activator inhibitor-1 levels; MR, Mendelian randomization; IVW, inverse-variance weighted; CI, confidence interval; CpG, cytosine-guanine dinucleotides; LINE-1, long interspersed nuclear element 1; GWAS, genome-wide association study; IVs, instrumental variables; LD, linkage disequilibrium; MAF, minor allele frequency; SNP, single nucleotide polymorphism; WM, weighted median; DNMTs, DNA methyltransferases; E2, gonadal hormone 17β-estradiol; MAP4K1, mitogen-activated protein kinase kinase kinase kinase 1; HOXA3, homeobox A3.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author contributions
LW: Writing – original draft. SX: Writing – original draft. RC: Writing – original draft. YD: Writing – original draft. ML: Writing – original draft. CH: Writing – original draft. ZW: Writing – original draft. XM: Writing – original draft. MB: Writing – review & editing. BH: Writing – review & editing. SL: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1429514/full#supplementary-material
References
- 1. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. (2018) 19:371–84. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 2. Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). (2018) 10:573–91. doi: 10.18632/aging.v10i4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu S, Niu Z, Chen Y, Tu Q, Zhang Y, Chen W, et al. Repetitive element DNA methylation is associated with menopausal age. Aging Dis. (2018) 9:435–43. doi: 10.14336/AD.2017.0810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yarde F, Broekmans FJ, van der Pal-de Bruin KM, Schönbeck Y, te Velde ER, Stein AD, et al. Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum Reprod. (2013) 28:3328–36. doi: 10.1093/humrep/det331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. (2013) 38:23–38. doi: 10.1038/npp.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He S, Feng X. DNA methylation dynamics during germline development. J Integr Plant Biol. (2022) 64:2240–51. doi: 10.1111/jipb.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. (2003) 33 Suppl:245–54. doi: 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- 8. Luo Y, Chen D, Xing XL. Comprehensive analyses revealed eight immune related signatures correlated with aberrant methylations as prognosis and diagnosis biomarkers for kidney renal papillary cell carcinoma. Clin Genitourin Cancer. (2023) 21:537–45. doi: 10.1016/j.clgc.2023.06.011 [DOI] [PubMed] [Google Scholar]
- 9. Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, de Magalhães JP. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. (2012) 15:483–94. doi: 10.1089/rej.2012.1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu W, Li H, Wang Z, Dai Z, Liang X, Luo P, et al. The tertiary lymphoid structure-related signature identified PTGDS in regulating PD-L1 and promoting the proliferation and migration of glioblastoma. Heliyon. (2024) 10:e23915. doi: 10.1016/j.heliyon.2023.e23915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lou Z, Gong YQ, Zhou X, Hu GH. Low expression of miR-199 in hepatocellular carcinoma contributes to tumor cell hyper-proliferation by negatively suppressing XBP1. Oncol Lett. (2018) 16:6531–9. doi: 10.3892/ol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Lin L, Wu Y, Bing P, Zhou J, Yu W. Upregulation of TIMM8A is correlated with prognosis and immune regulation in BC. Front Oncol. (2022) 12:922178. doi: 10.3389/fonc.2022.922178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang W, Liu H, Zeng Z, Liang Z, Xie H, Li W, et al. KRT17 promotes T-lymphocyte infiltration through the YTHDF2-CXCL10 axis in colorectal cancer. Cancer Immunol Res. (2023) 11:875–94. doi: 10.1158/2326-6066.CIR-22-0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pozojevic J, Sivaprasad R, Laß J, Haarich F, Trinh J, Kakar N, et al. LINE1-mediated epigenetic repression of androgen receptor transcription causes androgen insensitivity syndrome. Sci Rep. (2024) 14:16302. doi: 10.1038/s41598-024-65439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCartney DL, Min JL, Richmond RC, Lu AT, Sobczyk MK, Davies G, et al. Genome-wide association studies identify 137 genetic loci for DNA methylation biomarkers of aging. Genome Biol. (2021) 22:194. doi: 10.1186/s13059-021-02398-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu L, Pei H, Zhang Y, Zhang X, Feng M, Yuan L, et al. Association between dried fruit intake and DNA methylation: A multivariable mendelian randomization analysis. J Nutr Health Aging. (2023) 27:1132–9. doi: 10.1007/s12603-023-2030-x [DOI] [PubMed] [Google Scholar]
- 17. Wei S, Tang W, Chen D, Xiong J, Xue L, Dai Y, et al. Multiomics insights into the female reproductive aging. Ageing Res Rev. (2024) 95:102245. doi: 10.1016/j.arr.2024.102245 [DOI] [PubMed] [Google Scholar]
- 18. Levine ME, Lu AT, Chen BH, Hernandez DG, Singleton AB, Ferrucci L, et al. Menopause accelerates biological aging. Proc Natl Acad Sci USA. (2016) 113:9327–32. doi: 10.1073/pnas.1604558113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong T, Borgard H, Zhang Z, Chen S, Gao Z, Deng Y. Analysis and performance assessment of the whole genome bisulfite sequencing data workflow: currently available tools and a practical guide to advance DNA methylation studies. Small Methods. (2022) 6:e2101251. doi: 10.1002/smtd.202101251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. (2019) 20:590–607. doi: 10.1038/s41580-019-0159-6 [DOI] [PubMed] [Google Scholar]
- 21. Pratt D, Sahm F, Aldape K. DNA methylation profiling as a model for discovery and precision diagnostics in neuro-oncology. Neuro Oncol. (2021) 23:S16–s29. doi: 10.1093/neuonc/noab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang W, Li H, Yu Q, Xiao W, Wang DO. LncRNA-mediated DNA methylation: an emerging mechanism in cancer and beyond. J Exp Clin Cancer Res. (2022) 41:100. doi: 10.1186/s13046-022-02319-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson HD. Menopause. Lancet. (2008) 371:760–70. doi: 10.1016/S0140-6736(08)60346-3 [DOI] [PubMed] [Google Scholar]
- 24. Menopause (2022). Available online at: www.who.int/news-room/fact-sheets/detail/menopause (Accessed April 24, 2024).
- 25. Peycheva D, Sullivan A, Hardy R, Bryson A, Conti G, Ploubidis G. Risk factors for natural menopause before the age of 45: evidence from two British population-based birth cohort studies. BMC Womens Health. (2022) 22:438. doi: 10.1186/s12905-022-02021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ceylan B, Özerdoğan N. Factors affecting age of onset of menopause and determination of quality of life in menopause. Turk J Obstet Gynecol. (2015) 12:43–9. doi: 10.4274/tjod [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Bruin JP, Bovenhuis H, van Noord PA, Pearson PL, van Arendonk JA, te Velde ER, et al. The role of genetic factors in age at natural menopause. Hum Reprod. (2001) 16:2014–8. doi: 10.1093/humrep/16.9.2014 [DOI] [PubMed] [Google Scholar]
- 28. Treloar SA, Do KA, Martin NG. Genetic influences on the age at menopause. Lancet. (1998) 352:1084–5. doi: 10.1016/S0140-6736(05)79753-1 [DOI] [PubMed] [Google Scholar]
- 29. Bacon ER, Mishra A, Wang Y, Desai MK, Yin F, Brinton RD. Neuroendocrine aging precedes perimenopause and is regulated by DNA methylation. Neurobiol Aging. (2019) 74:213–24. doi: 10.1016/j.neurobiolaging.2018.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyne DJ, Friedenreich CM, McIntyre JB, Stanczyk FZ, Courneya KS, King WD. Endogenous sex hormone exposure and repetitive element DNA methylation in healthy postmenopausal women. Cancer Causes Control. (2017) 28:1369–79. doi: 10.1007/s10552-017-0958-z [DOI] [PubMed] [Google Scholar]
- 31. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu Q, Chen R, Xu S, Ding Y, Huang C, He B, et al. Assessment of potential risk factors associated with gestational diabetes mellitus: evidence from a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1276836. doi: 10.3389/fendo.2023.1276836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han L, Xu S, Zhou D, Chen R, Ding Y, Zhang M, et al. Unveiling the causal link between metabolic factors and ovarian cancer risk using Mendelian randomization analysis. Front Endocrinol (Lausanne). (2024) 15:1401648. doi: 10.3389/fendo.2024.1401648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen R, Xu S, Ding Y, Li L, Huang C, Bao M, et al. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1307468. doi: 10.3389/fendo.2023.1307468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loh PR, Kichaev G, Gazal S, Schoech AP, Price AL. Mixed-model association for biobank-scale datasets. Nat Genet. (2018) 50:906–8. doi: 10.1038/s41588-018-0144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M, et al. Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1321576. doi: 10.3389/fendo.2023.1321576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang C, Xu S, Chen R, Ding Y, Fu Q, He B, et al. Assessing causal associations of bile acids with obesity indicators: A Mendelian randomization study. Med (Baltimore). (2024) 103:e38610. doi: 10.1097/MD.0000000000038610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang Y, Chen R, Xu S, Ding Y, Zhang M, Bao M, et al. Assessing causal associations of hyperparathyroidism with blood counts and biochemical indicators: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1295040. doi: 10.3389/fendo.2023.1295040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fu Q, Chen R, Ding Y, Xu S, Huang C, He B, et al. Sodium intake and the risk of various types of cardiovascular diseases: a Mendelian randomization study. Front Nutr. (2023) 10:1250509. doi: 10.3389/fnut.2023.1250509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 41. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. (2018) 27:R195–r208. doi: 10.1093/hmg/ddy163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–9. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santoro N, Roeca C, Peters BA, Neal-Perry G. The menopause transition: signs, symptoms, and management options. J Clin Endocrinol Metab. (2021) 106:1–15. doi: 10.1210/clinem/dgaa764 [DOI] [PubMed] [Google Scholar]
- 46. Sehl ME, Henry JE, Storniolo AM, Ganz PA, Horvath S. DNA methylation age is elevated in breast tissue of healthy women. Breast Cancer Res Treat. (2017) 164:209–19. doi: 10.1007/s10549-017-4218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harbs J, Rinaldi S, Keski-Rahkonen P, Liu X, Palmqvist R, Van Guelpen B, et al. An epigenome-wide analysis of sex hormone levels and DNA methylation in male blood samples. Epigenetics. (2023) 18:2196759. doi: 10.1080/15592294.2023.2196759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inoshita M, Numata S, Tajima A, Kinoshita M, Umehara H, Yamamori H, et al. Sex differences of leukocytes DNA methylation adjusted for estimated cellular proportions. Biol Sex Differ. (2015) 6:11. doi: 10.1186/s13293-015-0029-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Unnikrishnan A, Freeman WM, Jackson J, Wren JD, Porter H, Richardson A. The role of DNA methylation in epigenetics of aging. Pharmacol Ther. (2019) 195:172–85. doi: 10.1016/j.pharmthera.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horvath S, Ritz BR. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging (Albany NY). (2015) 7:1130–42. doi: 10.18632/aging.v7i12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sciarra F, Campolo F, Franceschini E, Carlomagno F, Venneri MA. Gender-specific impact of sex hormones on the immune system. Int J Mol Sci. (2023) 24(7):6302. doi: 10.3390/ijms24076302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. (2005) 11:411–23. doi: 10.1093/humupd/dmi008 [DOI] [PubMed] [Google Scholar]
- 53. Zwahlen M, Stute P. Impact of progesterone on the immune system in women: a systematic literature review. Arch Gynecol Obstet. (2024) 309:37–46. doi: 10.1007/s00404-023-06996-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoffmann JP, Liu JA, Seddu K, Klein SL. Sex hormone signaling and regulation of immune function. Immunity. (2023) 56:2472–91. doi: 10.1016/j.immuni.2023.10.008 [DOI] [PubMed] [Google Scholar]
- 55. Chakraborty B, Byemerwa J, Krebs T, Lim F, Chang CY, McDonnell DP. Estrogen receptor signaling in the immune system. Endocr Rev. (2023) 44:117–41. doi: 10.1210/endrev/bnac017 [DOI] [PubMed] [Google Scholar]
- 56. Granulocytes. Available online at: https://my.clevelandclinic.org/health/body/22016-granulocytes (Accessed April 24, 2024).
- 57. Hervouet E, Peixoto P, Delage-Mourroux R, Boyer-Guittaut M, Cartron PF. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin Epigenet. (2018) 10:17. doi: 10.1186/s13148-018-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kovács T, Szabó-Meleg E, Ábrahám IM. Estradiol-induced epigenetically mediated mechanisms and regulation of gene expression. Int J Mol Sci. (2020) 21(9):3177. doi: 10.3390/ijms21093177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. (2012) 32:2344–51. doi: 10.1523/JNEUROSCI.5819-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. (2010) 107:5605–10. doi: 10.1073/pnas.0910578107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cisternas CD, Cortes LR, Bruggeman EC, Yao B, Forger NG. Developmental changes and sex differences in DNA methylation and demethylation in hypothalamic regions of the mouse brain. Epigenetics. (2020) 15:72–84. doi: 10.1080/15592294.2019.1649528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kolodkin MH, Auger AP. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol. (2011) 23:577–83. doi: 10.1111/jne.2011.23.issue-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takasugi M, Hayakawa K, Arai D, Shiota K. Age- and sex-dependent DNA hypomethylation controlled by growth hormone in mouse liver. Mech Ageing Dev. (2013) 134:331–7. doi: 10.1016/j.mad.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 64. Raiche J, Rodriguez-Juarez R, Pogribny I, Kovalchuk O. Sex- and tissue-specific expression of maintenance and de novo DNA methyltransferases upon low dose X-irradiation in mice. Biochem Biophys Res Commun. (2004) 325:39–47. doi: 10.1016/j.bbrc.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 65. Dunnet MJ, Ortega-Recalde OJ, Waters SA, Weeks RJ, Morison IM, Hore TA. Leukocyte-specific DNA methylation biomarkers and their implication for pathological epigenetic analysis. Epigenet Commun. (2022) 2:5. doi: 10.1186/s43682-022-00011-z [DOI] [Google Scholar]
- 66. Boopalan T, Arumugam A, Delgado A, Parada J, Agullo P, Lakshmanaswamy R. Abstract P5-03-06: progesterone activates MAPK pathway in estrogen induced mammary carcinogenesis. Cancer Res. (2010) 70:P5–03-06. doi: 10.1158/0008-5472.SABCS10-P5-03-06 [DOI] [Google Scholar]
- 67. Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. (2006) 27:331–55. doi: 10.1210/er.2005-0018 [DOI] [PubMed] [Google Scholar]
- 68. Mishra G, Hardy R, Kuh D. Are the effects of risk factors for timing of menopause modified by age? Results from a British birth cohort study. Menopause. (2007) 14:717–24. doi: 10.1097/gme.0b013e31802f3156 [DOI] [PubMed] [Google Scholar]
- 69. Hardy R, Kuh D. Social and environmental conditions across the life course and age at menopause in a British birth cohort study. Bjog. (2005) 112:346–54. doi: 10.1111/j.1471-0528.2004.00348.x [DOI] [PubMed] [Google Scholar]
- 70. Lawlor DA, Ebrahim S, Smith GD. The association of socio-economic position across the life course and age at menopause: the British Women’s Heart and Health Study. Bjog. (2003) 110:1078–87. doi: 10.1111/j.1471-0528.2003.02519.x [DOI] [PubMed] [Google Scholar]
- 71. Hatch EE, Troisi R, Wise LA, Hyer M, Palmer JR, Titus-Ernstoff L, et al. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am J Epidemiol. (2006) 164:682–8. doi: 10.1093/aje/kwj257 [DOI] [PubMed] [Google Scholar]
- 72. Steiner AZ, D’Aloisio AA, DeRoo LA, Sandler DP, Baird DD. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am J Epidemiol. (2010) 172:140–8. doi: 10.1093/aje/kwq092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Matías-García PR, Ward-Caviness CK, Raffield LM, Gao X, Zhang Y, Wilson R, et al. DNAm-based signatures of accelerated aging and mortality in blood are associated with low renal function. Clin Epigenet. (2021) 13:121. doi: 10.1186/s13148-021-01082-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McCarthy M, Raval AP. The peri-menopause in a woman’s life: a systemic inflammatory phase that enables later neurodegenerative disease. J Neuroinflamm. (2020) 17:317. doi: 10.1186/s12974-020-01998-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thasneem K, Kalarani IB, Jayaprasad P, Mohammed V, Veerabathiran R. Genes linked with early menopause and the pathogenesis of its associated diseases: a systematic review. Middle East Fertility Soc J. (2022) 27:2. doi: 10.1186/s43043-021-00093-0 [DOI] [Google Scholar]
- 76. Liu Q, Wei F, Wang J, Liu H, Zhang H, Liu M, et al. Molecular mechanisms regulating natural menopause in the female ovary: a study based on transcriptomic data. Front Endocrinol (Lausanne). (2023) 14:1004245. doi: 10.3389/fendo.2023.1004245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, et al. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. (2009) 23:631–41. doi: 10.1096/fj.08-117200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Biemans Y, Bach D, Behrouzi P, Horvath S, Kramer CS, Liu S, et al. Identifying the relation between food groups and biological ageing: a data-driven approach. Age Ageing. (2024) 53:ii20–9. doi: 10.1093/ageing/afae038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sugden K, Moffitt TE, Arpawong TE, Arseneault L, Belsky DW, Corcoran DL, et al. Cross-national and cross-generational evidence that educational attainment may slow the pace of aging in european-descent individuals. J Gerontol B Psychol Sci Soc Sci. (2023) 78:1375–85. doi: 10.1093/geronb/gbad056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chang CJ, O’Brien KM, Kresovich JK, Nwanaji-Enwerem JC, Xu Z, Gaston SA, et al. Associations between use of chemical hair products and epigenetic age: Findings from the Sister Study. Environ Epidemiol. (2024) 8:e311. doi: 10.1097/EE9.0000000000000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lucia RM, Huang WL, Pathak KV, McGilvrey M, David-Dirgo V, Alvarez A, et al. Association of glyphosate exposure with blood DNA methylation in a cross-sectional study of postmenopausal women. Environ Health Perspect. (2022) 130:47001. doi: 10.1289/EHP10174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gracia CR, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. (2005) 12(2):128–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.