Abstract
Background
The anti-PD-L1 antibody durvalumab has been approved for use in first-line advanced biliary duct cancer (ABC). So far, predictive biomarkers of efficacy are lacking.
Methods
ABC patients who underwent gemcitabine-based chemotherapy with or without durvalumab were retrospectively enrolled, and their baseline clinical pathological indices were retrieved from medical records. Overall (OS) and progression free survival (PFS) were calculated and analyzed. The levels of peripheral biomarkers from 48 patients were detected with assay kits including enzyme-linked immunosorbent assay. Genomic alterations in 27 patients whose tumor tissues were available were depicted via targeted next-generation sequencing.
Results
A total of 186 ABC patients met the inclusion criteria between January 2020 and December 2022 were finally enrolled in this study. Of these, 93 patients received chemotherapy with durvalumab and the rest received chemotherapy alone. Durvalumab plus chemotherapy demonstrated significant improvements in PFS (6.77 vs. 4.99 months; hazard ratio 0.65 [95% CI 0.48–0.88]; P = 0.005), but not OS (14.29 vs. 13.24 months; hazard ratio 0.91 [95% CI 0.62–1.32]; P = 0.608) vs. chemotherapy alone in previously untreated ABC patients. The objective response rate (ORR) in patients receiving chemotherapy with and without durvalumab was 19.1% and 7.8%, respectively. Pretreatment sPD-L1, CSF1R and OPG were identified as significant prognosis predictors in patients receiving durvalumab. ADGRB3 and RNF43 mutations were enriched in patients who responded to chemotherapy plus durvalumab and correlated with superior survival.
Conclusion
This retrospective real-world study confirmed the clinical benefit of durvalumab plus chemotherapy in treatment-naïve ABC patients. Peripheral sPD-L1 and CSF1R are promising prognostic biomarkers for this therapeutic strategy. Presence of ADGRB3 or RNF43 mutations could improve the stratification of immunotherapy outcomes, but further studies are warranted to explore the underlying mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03796-1.
Keywords: ABC, Durvalumab, Soluble PD-L1, CSF1R, ADGRB3
Introduction
Biliary tract cancers (BTCs) are a heterogeneous group of malignancies arising from the epithelial cells of the biliary tree and the gallbladder that often present with locally advanced or distant metastatic diseases at diagnosis; therefore, the prognosis remains dismal [1]. The incidence of BTCs has increased annually in the past decade, and now accounts for approximately 3% of all gastrointestinal carcinomas [2]. Surgical resection holds the only curative chance; however, it is only suitable for less than 10% of BTCs [3]. Chemotherapy is the standard of care for patients with unresectable disease, and the five year survival rate is less than 5% [2, 3].
In 2010, the ABC-02 trial established gemcitabine plus cisplatin (GP) as the standard regimen for treatment-naïve advanced biliary duct cancer (ABC) given the strong evidence of a significant clinical benefit, including overall (median OS [mOS]: 11.7 vs. 8.1 months, HR = 0.64, P < 0.001), progression free survival (median PFS [mPFS]: 8.0 vs. 5.0 months, HR = 0.63, P < 0.001) and objective response rate (ORR: 26.1% vs. 15.5%), over gemcitabine alone [4]. Since then, other cytotoxic regimens, such as oxaliplatin combined with capecitabine (XELOX), gemcitabine (GEMOX) or S-1 plus gemcitabine (GS), were found to be noninferiority to GP [5–7]. However, the survival benefits and tumor response to chemotherapy are far from satisfactory, and novel therapeutic options are urgently warranted.
Immune checkpoint inhibitors (ICIs), including antibodies targeting programmed cell death 1/ligand 1 (PD-1/PD-L1), have revolutionized the therapeutic pattern of several malignancies [8]. The efficacy of pembrolizumab and nivolumab monotherapy has been investigated in patients refractory to chemotherapy [9, 10]; however, the ORRs are relatively low, ranging from 3–22% [9–11]. In the first-line setting, anti-PD-1 antibodies combined with the standard GP, GS or GEMOX regimen achieved an ORR between 30.6–54% with discrete results in phase II trials with small sample sizes [11–15].
Recently, survival benefit of combining chemotherapy and the PD-1 inhibitor pembrolizumab was demonstrated in the KEYNOTE-966 trial, adding further evidence to the first-line management of ABC [16]. Regarding antibodies targeting PD-L1, durvalumab plus GP was shown to result in significantly better mOS than GP alone (12.8 vs. 11.5 months; HR: 0.80, 95% CI 0.66–0.97, P = 0.021) in the TOPAZ-1 trial [17]; furthermore, the ORR increased from 18.7 to 26.7% after adding durvalumab to standard GP regimen [17]. However, the percentage of ABC patients from China enrolled into TOPAZ-1 was less than 20% and the efficacy of durvalumab in the real-world setting warrants further exploration. Moreover, identification of biomarkers predicting outcomes among patients receiving this combinational approach would further improve the therapeutic efficacy.
Currently, there is no definite evidence regarding the predicting values of PD-L1 expression and tumor mutation burden (TMB) in ABC, which have been well recognized as useful biomarkers in lung cancer and melanoma in the era of immunotherapy [12, 14, 15, and 18]. Several previous phase II trials testing PD-1 inhibitors in ABC have suggested the prognostic values of specific genetic alterations, such as KRAS [19] or SMARCA4 [14] mutations, loss-of-function mutations of chromatin remodeling genes [12, 13], alterations in the homologous recombination repair pathway [13] and immunologic characteristics, including the T cell signature labeled by genotyping [13] and blood TMB [15]. For example, Chen et al. found that PD-1 inhibitors (Camrelizumab) combined with gemcitabine-based regimen achieved an mPFS of 6.1 months and a mOS of 11.8 months in 37 ABC patients. The patients with ARID1A mutations showed prolonged mOS (13.2 vs. 7.2 months) and mPFS (6.3 vs. 4.3 months) than those without [15]. However, genetic as well as circulating biomarkers predicting the prognosis of durvalumab in ABC have not been reported.
In this study, we retrospectively enrolled 186 consecutive ABC patients in our center receiving gemcitabine-based chemotherapy with or without durvalumab and analyzed, for the first time, the real-world efficacy of this regimen in Chinese cohort. Potential circulating and genomic biomarkers were also explored to identify candidates most likely to benefit from durvalumab in the first-line setting.
Materials and methods
Study population and sample collection
Patients diagnosed with unresectable locally advanced or metastatic disease of the biliary tract, including that of gallbladder, bile duct or ampullary origin, who received antitumor regimens between January 2020 and December 2022 at Sun Yat-sen University Cancer Center were enrolled into our cohort. Comprehensive information, including patient demographics, primary and metastatic tumor characteristics, pretreatment blood test results, and follow-up data, was extracted from the electronic medical database.
The main inclusion criteria were as follows: (1) pathologically confirmed cholangiocarcinoma or gallbladder cancer or ampulla carcinoma (pancreatobiliary subtype); (2) treatment with gemcitabine-based mono, doublet (gemcitabine plus cisplatin) or triplet (gemcitabine, cisplatin and abraxane) palliative chemotherapy with or without durvalumab; (3) previously untreated patients with unresectable disease; and (4) complete medical record in our center. The main exclusion criteria were as follows: (1) neo-adjuvant or adjuvant settings; (2) a history of receiving target agents or PD-1 antibodies in the first-line setting and (3) a history of other tumors.
Tumor tissues fixed with formalin and embedded in paraffin were obtained from 17 patients and used for genomic profiling. A total of 66 peripheral plasma samples, including 48 at baseline and 18 at disease progression time point, were collected from patients receiving gemcitabine-based chemotherapy plus durvalumab. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethical Committee of Sun Yat-sen University Cancer Center. Written consent for the use of patient data was obtained from all participants during their hospitalization.
Cytokine arrays and determination of soluble PD-1 and PD‑L1 (sPD-1 and sPD-L1) concentrations in the plasma
A human cytokine kits (GSH-CAA-1000, Ray Biotech, Norcross, GA) was used to measure the relative expression levels of 80 circular cytokines according to the manufacturer’s instructions. Briefly, 100 μl samples (2 × dilution) were added into the microarray following overnight incubation at 4 °C, the supernatant was removed and the slides were then washed and incubated with biotinylated antibody cocktail and Cy3 Equivalent Dye-Streptavidin. Then the fluorescence was detected with the laser scanner (InnoScan 300 Microarray Scanner, Innopsys, France) and data were analyzed with the matched software. The concentrations of sPD-1 and sPD-L1 was detected with the Enzyme-linked immunosorbent assay (ELISA) kits (hPD-1 qKit and hB7-H1 qKit, R&D system, Minnesota, USA) per the manufacturer’s instructions. Standards (consisting of a seven-point serial dilution) and undiluted plasma samples were added to 96-well plates precoated with a monoclonal antibody specific for human PD-1 and human B7-H1, respectively. The specific binding protein was detected with an enzyme-linked monoclonal anti-PD1 antibody for sPD-1 and a polyclonal anti-PD-L1 antibody for sPD-L1. The plate was covered with a sealer, and incubated at room temperature for 2 h before adding of substrate solution. The reaction was stopped and measured at 450 nm by the microplate reader.
Targeted NGS, bioinformatic analysis and online database
Genomic DNA was extracted from tumor samples, followed by purification and qualification prior to the construction of the DNA libraries. Unique indices were used to mark genomic DNA from each sample, which were then pooled together for probe incubation in preparation for targeted sequencing of 437 cancer related genes. The captured libraries were examined for quality and quantity using the KAPA library quantification kit (KAPA Biosystems) through qRT-PCR. Subsequently, the final libraries were sequenced on Hiseq 4000 platforms (Illumina) to achieve a mean coverage depth of 150 × for the tumor tissue samples after PCR duplicate removal (Geneseeq Technology Inc., Nanjing). Raw sequence data were obtained through base calling analysis of original image data. Single nucleotide variants (SNVs) and short insertions/deletions (indels) were identified using VarScan2 with a minimum variant allele frequency threshold set at 0.01, and a P value threshold of 0.05 was applied for variant calling to generate variant call format files. All SNVs/indels were annotated using Annotate Variation (ANNOVAR), with manual verification performed on each SNV/indel using the Integrative Genomics Viewer. Ten patients undergone targeted sequencing of 1027 cancer related genes in our center were also enrolled into our study. Specific gene lists of these two panels were shown in Table S3. Tumor mutation burden (TMB) in solid tumor and cholangiocarcinoma patients and associations with ADGRB3 mutations were retrieved from the cBioPortal database (http://www.cbioportal.org/).
Follow-up
Patients were followed up mainly through the outpatient clinic (or via telephone) every three months for the first two years after the first visit to our center. Progression-free survival (PFS) was defined as the time between diagnosis of unresectable or metastatic disease and recurrence or death from any cause or last follow-up. Overall survival (OS) was defined as the time from disease diagnosis (tumor recurrence, distant metastasis or unresectable locally advanced) to death from any cause or last follow-up.
Statistical analysis
The association between cytokines or sPD-1, sPD-L1 and other clinicopathological variables (i.e., demography and patient characteristics) was assessed by Student's T tests, Mann–Whitney U tests, Chi-square tests, or Fisher's exact tests based on the nature of the data. Statistical analyses were considered significant when the two-tailed P value was less than 0.05. OS and PFS were analyzed using the Kaplan–Meier method and logrank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) of time-to-event variables were estimated using univariate Cox proportional hazards analysis. The cutoff value of sPD-L1 and sPD-1 was generated via the X-tile software. The indices with a P value less than 0.05 in the univariate analysis or having clinical implications were included in the multivariate analysis. IBM SPSS version 20 (IBM, Armonk, NJ), PRISM v.8 (GraphPad Software Inc., USA) and R (4.2.1) were used for statistical analysis.
Results
Patient characteristics
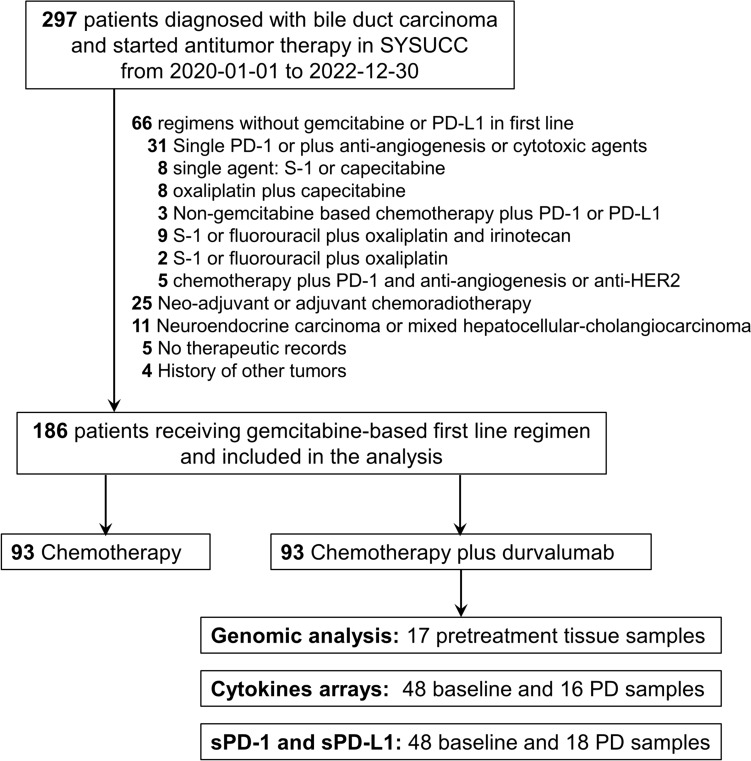
Between January 2020 and December 2022, a total of 297 consecutive patients diagnosed with biliary duct cancer in our center were retrospectively reviewed, of whom 186 met the inclusion and don’t meet the exclusion criteria were finally included in our study (Fig. 1). All patients in this cohort were of Chinese ethnicity. The demographic and clinicopathological characteristics of the patients are shown in Table 1. The cohort consisted of 96 males and 90 females, with a median age of 57 years. Among all patients, 58 (31.2%) had gallbladder cancer, while 80 (43.0%) and 35 (18.8%) had intrahepatic and extrahepatic cholangiocarcinoma, respectively. Thirteen (7.0%) patients diagnosed with ampulla carcinoma were also included. More than half of the patients (123/186, 66.1%) had liver metastasis, while only 20 (10.8%) patients were diagnosed with locally advanced disease, and 114 (61.3%) were pathologically confirmed as having poorly differentiated cancer. Resection of the primary tumor were performed in 99 (53.2%) patients. The number of patients receiving gemcitabine-based monotherapy, doublet and triplet regimen was 19 (10.2%), 161 (86.6%) and 6 (3.2%), respectively.
Fig. 1.
Flow diagram for included patients and the analysis process. PD-1, programmed death 1; PD-L1, programmed death ligand 1; HER2, human epidermal growth factor receptor; PD, progressive disease
Table 1.
The clinicopathological characteristics of patients receiving chemotherapy with or without durvalumab
| Characteristics | All patients | Chemo. + PD-L1 (n = 93) | Chemo. (n = 93) | P value |
|---|---|---|---|---|
| Age (Years) | ||||
| Mean (range) | 57 (28–82) | 57 (28–82) | 57 (32–82) | 0.836 |
| Gender, n (%) | ||||
| Female | 90 (48.4%) | 42 (45.2%) | 48 (51.6%) | 0.379 |
| Male | 96 (51.6%) | 51 (54.8%) | 45 (48.4%) | |
| Primary site, n (%) | ||||
| Gallbladder | 58 (31.2%) | 27 (29.0%) | 31 (33.3%) | 0.256 |
| Intrahepatic | 80 (43.0%) | 43 (46.2%) | 37 (39.8%) | |
| Extrahepatic | 35 (18.8%) | 14 (15.1%) | 21 (22.6%) | |
| Ampulla | 13 (7.0%) | 9 (9.7%) | 4 (4.3%) | |
| Liver metastasis, n (%) | ||||
| Absent | 63 (33.9%) | 34 (36.6%) | 29 (31.2%) | 0.536 |
| Present | 123 (66.1%) | 59 (63.4%) | 64 (68.8%) | |
| Disease stage, n (%) | ||||
| Locally advanced | 20 (10.8%) | 11 (11.8%) | 9 (9.7%) | 0.636 |
| Distant metastasis | 166 (89.2%) | 82 (88.2%) | 84 (90.3%) | |
| Prior surgery of primary tumor, n (%) | ||||
| No surgery | 87 (46.8%) | 41 (44.1%) | 46 (49.5%) | 0.464 |
| Radical surgery | 78 (41.9%) | 43 (46.2%) | 35 (37.6%) | |
| Palliative surgery | 21 (11.3%) | 9 (9.7%) | 12 (12.9%) | |
| Differentiation, n (%) | ||||
| High or intermediate | 72 (38.7%) | 41 (44.0%) | 31 (33.3%) | 0.132 |
| Poor | 114 (61.3%) | 52 (56.0%) | 62 (66.7%) | |
| ECOG, n (%) | ||||
| 1 | 164 (88.2%) | 81 (87.1%) | 83 (89.2%) | 0.650 |
| 0 | 22 (11.8%) | 12 (12.9%) | 10 (10.8%) | |
| Chemo. regimen, n (%) | ||||
| Doublet | 161 (86.6%) | 78 (83.9%) | 83 (89.2%) | 0.029 |
| Triplet | 6 (3.2%) | 1 (1.1%) | 5 (5.4%) | |
| Mono | 19 (10.2%) | 14 (15.0%) | 5 (5.4%) | |
The bold p value indicates statistically significant
Real-world efficacy of durvalumab plus gemcitabine-based chemotherapy in ABC
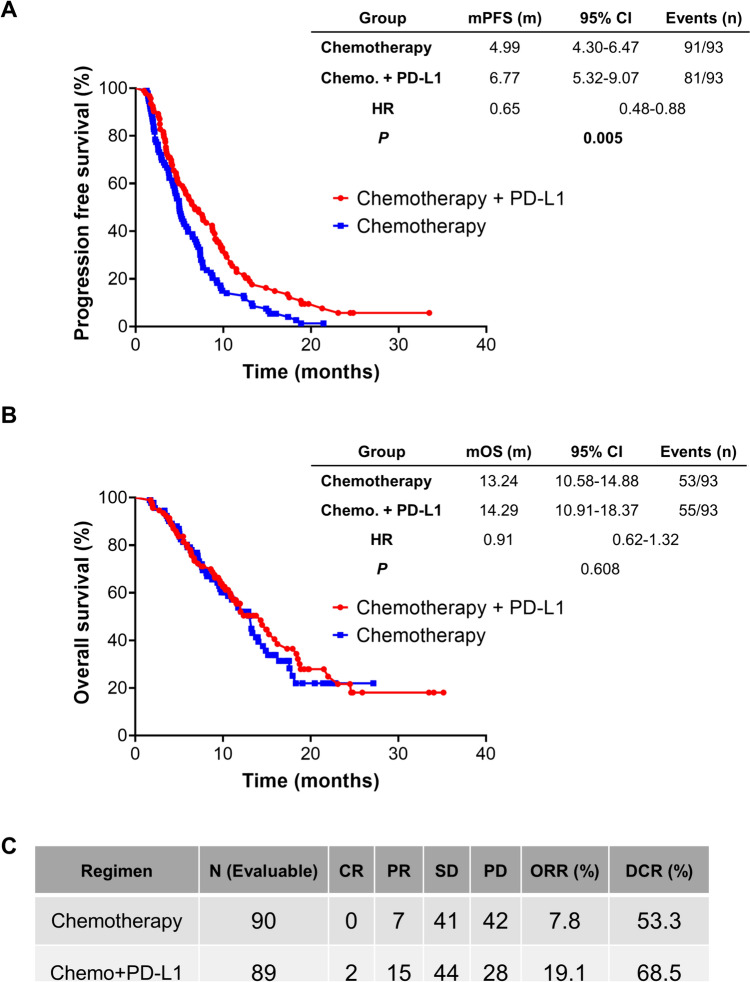
At the data cutoff date (31 July 2023), 172 patients experienced disease progression or death, while 108 died of this disease. Among these patients, PFS was significantly longer for patients in the chemoimmunotherapy group than for those in the chemotherapy alone group (mPFS: 6.77 vs. 4.99 months; HR, 0.65; 95%CI 0.48–0.88; P = 0.005, Fig. 2A), while the OS in the combinational arm was numerically but not significantly longer than that in the chemotherapy alone arm (mOS: 14.29 vs. 13.24 months; HR, 0.91; 95%CI 0.62–1.32; P = 0.608, Fig. 2B). Estimated 6-month PFS was 55.2% in the durvalumab group and 38.7% in the chemotherapy alone group; estimated 12 month PFS was 21.8% in the durvalumab group and 11.8% in the chemotherapy alone group (Fig. 2A). Estimated 12 month OS rates were 53.3% in the durvalumab group and 50.8% in the chemotherapy alone group; estimated 18 month overall survival rates were 35.8% in the durvalumab group and 23.2% in the chemotherapy alone group (Fig. 2B).
Fig. 2.
The efficacy of adding durvalumab to chemotherapy. PFS A, OS B and tumor response C in ABC patients receiving chemotherapy with or without durvalumab. PFS, progression free survival; OS, overall survival; ABC, advanced biliary tract cancer; PD-L1, programmed death ligand 1; Chemo., chemotherapy; HR, hazard ratio; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate
The investigator-assessed ORR per Response Evaluation Criteria in Solid Tumors v1.1 in patients receiving chemoimmunotherapy was 19.1%, with two patients achieving complete response (CR) and 15 partial response (PR). The percentage of patients achieving PR was 7.8% (7/90) in the chemotherapy group, and none achieved CR. The disease control rate (DCR) in patients receiving chemotherapy with or without durvalumab was 68.5% and 53.3%, respectively (Fig. 2C).
In the uni- and multivariate COX analysis, Eastern cooperative oncology group (ECOG) status and use of durvalumab were identified as independent predictors for PFS in ABC patients, while other indices including age, differentiation, and primary tumor location were not significantly associated with PFS (Table 2). The ECOG status was identified as independent predictors for OS (Table 3).
Table 2.
Uni and multivariate cox analysis of different clinical pathological indices with PFS
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 186 | 1.00 (0.98–1.01) | 0.754 | ||
| Sex | 186 | ||||
| Male | 96 | Reference | |||
| Female | 90 | 1.04 (0.77–1.41) | 0.790 | ||
| Differentiation | 186 | ||||
| Poor | 114 | Reference | Reference | ||
| High or intermediate | 72 | 0.66 (0.49–0.91) | 0.011 | 0.86 (0.62–1.21) | 0.387 |
| Disease stage | 186 | ||||
| Locally advanced | 20 | Reference | |||
| Distant metastasis | 166 | 1.55 (0.93–2.59) | 0.096 | ||
| Primary site | 186 | ||||
| Intrahepatic | 80 | Reference | |||
| Gallbladder | 58 | 0.95 (0.67–1.36) | 0.787 | ||
| Extrahepatic | 35 | 1.14 (0.76–1.72) | 0.529 | ||
| Ampulla | 13 | 0.82 (0.43–1.57) | 0.546 | ||
| Liver metastasis | 186 | ||||
| Present | 123 | Reference | Reference | ||
| Absent | 63 | 0.61 (0.44–0.85) | 0.003 | 0.66 (0.47–0.93) | 0.017 |
| Prior surgery of primary tumor | 186 | ||||
| No surgery | 87 | Reference | |||
| Radical surgery | 78 | 0.84 (0.61–1.16) | 0.282 | ||
| Palliative surgery | 21 | 0.79 (0.48–1.31) | 0.360 | ||
| ECOG | 186 | ||||
| 1 | 164 | Reference | Reference | ||
| 0 | 22 | 0.49 (0.30–0.80) | 0.004 | 0.49 (0.29–0.80) | 0.012 |
| Therapy | 186 | ||||
| Chemotherapy | 93 | Reference | Reference | ||
| Chemotherapy + PD-L1 | 93 | 0.65 (0.48–0.88) | 0.005 | 0.64 (0.47–0.87) | 0.005 |
| Chemotherapy regimen | 186 | ||||
| Mono | 19 | Reference | |||
| Doublet | 161 | 0.97 (0.59–1.59) | 0.900 | ||
| Triplet | 6 | 0.96 (0.38–2.43) | 0.930 | ||
The bold p values indicate statistically significant
Table 3.
Uni and multivariate cox analysis of different clinical pathological indices with OS
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 186 | 1.02 (1.00–1.04) | 0.138 | ||
| Sex | 186 | ||||
| Male | 96 | Reference | |||
| Female | 90 | 0.90 (0.62–1.32) | 0.593 | ||
| Differentiation | 186 | ||||
| Poor | 114 | Reference | Reference | ||
| High or intermediate | 72 | 0.65 (0.43–0.97) | 0.034 | 0.88 (0.57–1.35) | 0.551 |
| Disease stage | 186 | ||||
| Locally advanced | 20 | Reference | |||
| Distant metastasis | 166 | 0.97 (0.53–1.78) | 0.932 | ||
| Primary site | 186 | ||||
| Intrahepatic | 80 | Reference | |||
| Gallbladder | 58 | 0.70 (0.44–1.11) | 0.131 | ||
| Extrahepatic | 35 | 1.21 (0.74–1.98) | 0.447 | ||
| Ampulla | 13 | 0.59 (0.25–1.37) | 0.217 | ||
| Liver metastasis | 186 | ||||
| Present | 123 | Reference | Reference | ||
| Absent | 63 | 0.63 (0.42–0.96) | 0.031 | 0.79 (0.51–1.24) | 0.302 |
| Prior surgery of primary tumor | 186 | ||||
| No surgery | 87 | Reference | Reference | ||
| Radical surgery | 78 | 0.57 (0.38–0.86) | 0.007 | 0.70 (0.45–1.09) | 0.118 |
| Palliative surgery | 21 | 0.43 (0.20–0.89) | 0.024 | 0.45 (0.21–0.97) | 0.041 |
| ECOG | 186 | ||||
| 1 | 164 | Reference | Reference | ||
| 0 | 22 | 0.40 (0.21–0.76) | 0.006 | 0.42 (0.22–0.83) | 0.012 |
| Therapy | 186 | ||||
| Chemotherapy | 93 | Reference | |||
| Chemotherapy + PD-L1 | 93 | 0.91 (0.62–1.32) | 0.608 | ||
| Chemotherapy regimen | 186 | ||||
| Mono | 19 | Reference | |||
| Doublet | 161 | 0.70 (0.39–1.25) | 0.228 | ||
| Triplet | 6 | 1.20 (0.43–3.39) | 0.727 | ||
The bold p values indicate statistically significant
Soluble PD-1 (sPD-1) and soluble PD-L1 (sPD-L1) in ABC patients receiving chemotherapy plus durvalumab
The clinicopathological indices of patients receiving durvalumab were analyzed via COX methods to identify factors related to prognosis. As shown in Table S1 and Table S2, pretreatment basophil, Fbg (fibrinogen) and CA19-9 were identified as independent prognosis predictors for PFS, while only neutrophil to lymphocyte ratio (NLR) was an independent predictor for OS. We next sought to explore potential circulating biomarkers associated with the clinical response to durvalumab plus gemcitabine-based chemotherapy in 48 patients.
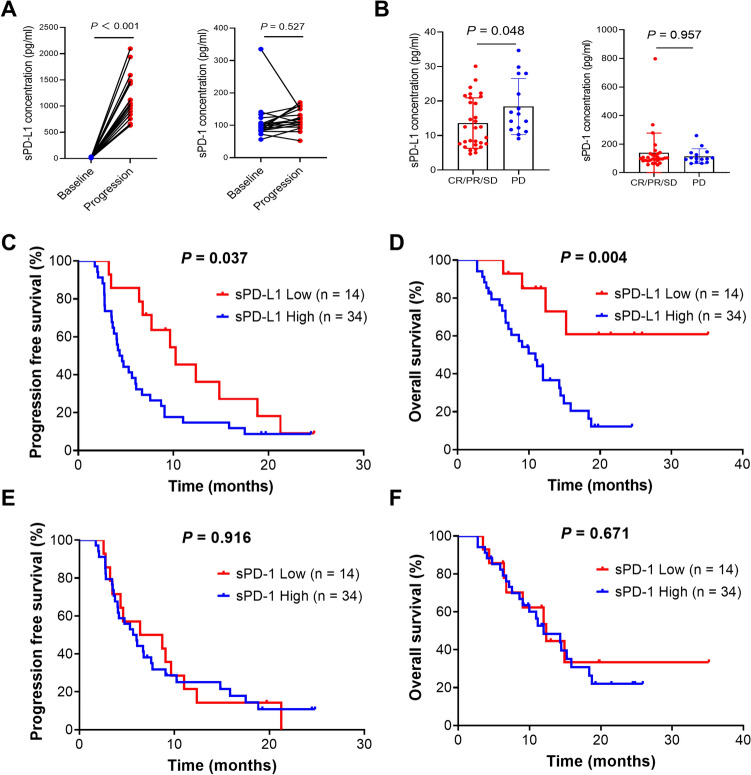
The median concentrations of sPD-L1 at baseline and disease progression was 14.643 pg/ml and 1032.8 pg/ml, respectively, while those of sPD-1 was 96.222 pg/ml and 118.35 pg/ml, respectively (Fig. 3A). The sPD-L1 at disease progression was approximately one hundred times that at baseline (P < 0.001) (Fig. 3A). The patients were divided into two groups according to the best tumor response. The level of sPD-L1 was significantly lower in patients with CR, PR or stable disease (SD) than in those with PD (Fig. 3B). However, there was no difference of sPD-1 level between these two groups (Fig. 3B). Nonsignificant associations of sPD-L1 and sPD-1 with disease stage or tumor location were observed (Supplementary Fig. S1A and S1B). Moreover, the patients with low baseline sPD-L1 had significantly better PFS (mPFS: 10.25 vs. 4.47 months, P = 0.037, Fig. 3C) and OS (mOS: NA vs. 10.91 months, P = 0.004, Fig. 3D) than those with high sPD-L1. However, sPD-1 did not demonstrate predictive value for either PFS (Fig. 3E) or OS (Fig. 3F).
Fig. 3.
Soluble PD-1 (sPD-1) and PD-L1 (sPD-L1) in patients receiving durvalumab. A Concentrations of sPD-1 and sPD-L1 at baseline and during disease progression. B Concentrations of sPD-1and sPD-L1 in patients achieving different responses to durvalumab. PFS C and OS D in ABC patients receiving chemotherapy with durvalumab according to sPD-L1 concentrations. PFS E and OS F in ABC patients receiving chemotherapy with durvalumab according to sPD-1 levels. PFS, progression free survival; OS, overall survival; sPD-1, soluble programmed death 1; sPD-L1, soluble programmed death ligand 1; ABC, advanced biliary tract cancer; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease
Circular cytokines in ABC patients receiving chemoimmunotherapy
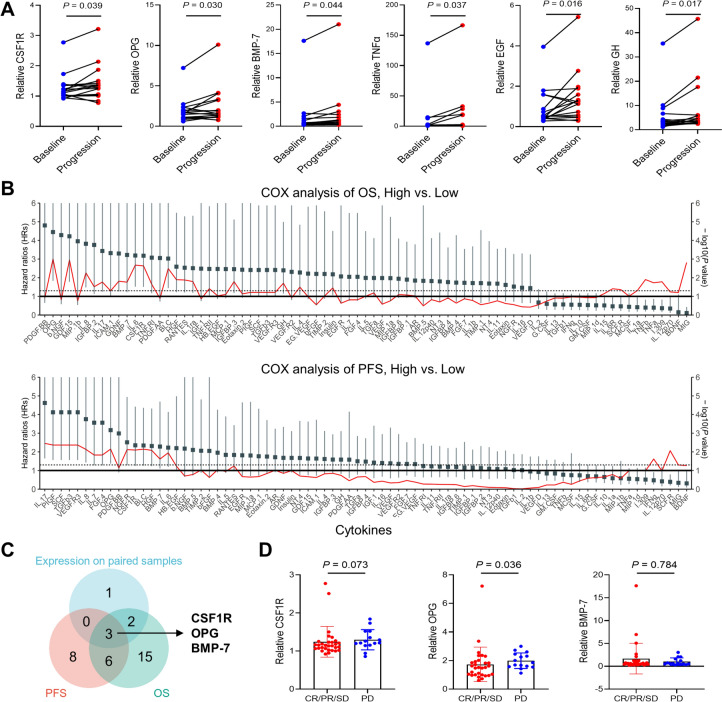
As peripheral blood is an easily accessible and noninvasive sample for predicting therapy outcomes in several cancers, we profiled a panel of 80 cytokines in 48 patients with 64 samples. Six cytokines, including CSF1R, OPG, BMP-7, TNFα, EGF and GH, were found to be significantly elevated over the course of the disease (Fig. 4A and Supplementary Fig. S1C). When correlating the concentrations of these cytokines with the survival of ABC patients receiving durvalumab, a panel of candidates, including BLC and IL-8, were significantly associated with OS and PFS (Fig. 4B). Of the six cytokines elevated at the disease progression time point, CSF1R, OPG and BMP-7 were found to be significantly associated with PFS and OS of ABC patients receiving durvalumab (Fig. 4C). Even after adjusted for multiple comparisons, CSF1R and OPG was significantly associated with OS in this cohort (Table S4). Baseline OPG level was significantly higher in patients evaluated as PD compared with those evaluated as CR, PR or SD, while there were no differences of CSF1R and BMP-7 between these groups (Fig. 4D). Specifically, the mPFS (7.69 vs. 4.26 months, P = 0.004) and mOS (15.87 vs. 8.30 months, P = 0.004) were significantly longer in patients with low CSF1R levels than in those with high CSF1R levels (Supplementary Fig. S2A). Patients with low OPG and BMP-7 levels also demonstrated survival advantages (Supplementary Fig. S2B and S2C).
Fig. 4.
Peripheral cytokines in patients receiving durvalumab. A Relative levels of CSF1R, OPG, BMP-7, TNFα, EGF and GH in paired blood samples collected at baseline and the disease progression time point. B OS and PFS in ABC patients receiving chemotherapy with durvalumab according to cytokines level. The red line indicates -log10(P value) of each cytokine. C Venn diagram of selected cytokines. D Associations of CSF1R, OPG and BMP-7 with clinical response to durvalumab. CSF1R, colony-stimulating factor 1 receptor; OPG, osteoprotegerin; BMP-7; bone morphogenetic protein 7; TNFα, tumor necrosis factor α; EGF, epidermal growth factor; GH, growth factor
ADGRB3 mutations were enriched in patients responding to durvalumab
To explore genetic mutations associating with efficacy of durvalumab, 17 patients with available tumor samples subjected to a target DNA sequencing containing a panel of 437 cancer related genes at Geneseeq Technology (Table S3) and ten patients undergone target sequencing with 1021 cancer related genes in our center (Table S3) were analyzed. Due to the limited sample size, the patients were grouped as those without or with clinical benefit according to whether the tumor progressed during first evaluation or not.
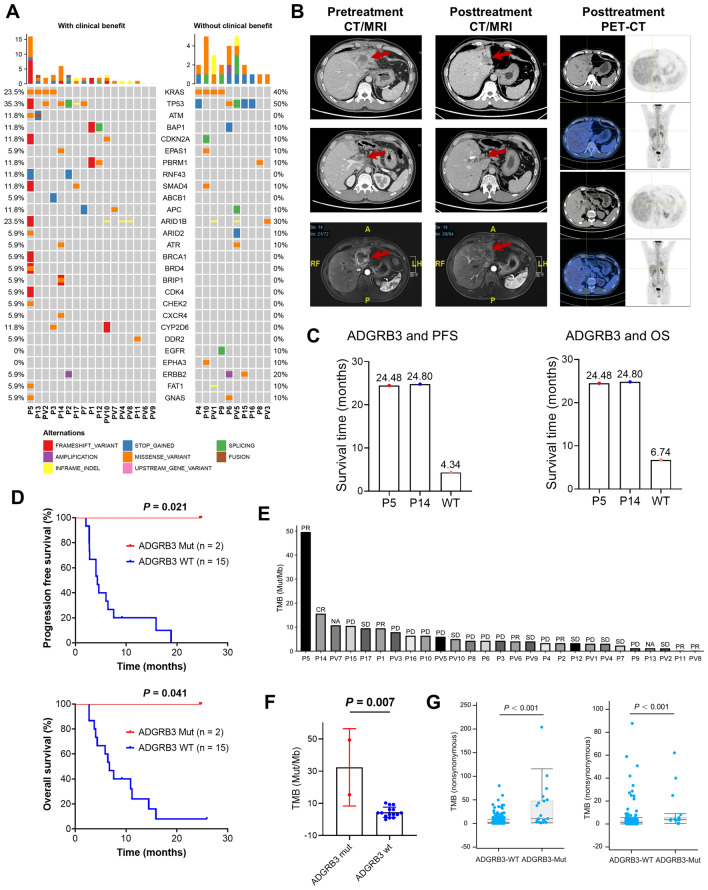
As shown in Fig. 5A, the most frequently mutated genes in ABC were TP53 (11/27, 40.7%) and KRAS (8/27, 29.6%). Moreover, the mutation frequencies of KRAS (23.5% vs. 40.0%) in patients who benefited from chemotherapy plus durvalumab were lower than those who did not. TP53 and KRAS were the top two mutated genes in the 17 patients undergone target sequencing of 437 genes and alterations in the PI3K, NOTCH and NRF2 pathways were observed only in patients who benefited from durvalumab (Supplementary Fig. S3A). Several gene mutations such as JUN, FAT1 and ADGRB3 were identified as prognosis predictors (Supplementary Fig. S3B and S3C). Specifically, mutations of ADGRB3 and RNF43 were only observed in patients with clinical benefit, occurring in two out of 17 patients (Fig. 5A and Supplementary Fig. S3A).
Fig. 5.
Genomic alterations in ABC patients receiving durvalumab. A Waterfall plots showing the frequency and types of top mutated genes found in 27 ABC patients who did or did not benefit from durvalumab. B Pre- and posttreatment CT/MRI scans as well as PET/CT images of patient P5. C, D PFS and OS of ADGRB3 Mut or WT patients when treated with chemotherapy plus durvalumab. E TMBs in 27 patients receiving durvalumab as well as the tumor response. F TMB degree of ADGRB3-mutated and WT patients. G TMB in solid tumor and cholangiocarcinoma patients with or without ADGRB3 mutations from the cBioPortal database. PFS, progression free survival; OS, overall survival; ABC, advanced biliary tract cancer; TMB, tumor mutational burden; ADGRB3, adhesion G protein-coupled receptor B3; Mut, mutation; WT, wild type
Through mining the cBioPortal database (https://www.cbioportal.org/), mutations in ADGRB3 were found in several cancers, including lung cancer (Supplementary Fig. S3D), cholangiocarcinoma and ampulla carcinoma (Supplementary Fig. S3E). Moreover, the cholangiocarcinoma patients with ADGRB3 mutation tended to have longer disease free survival (DFS) (Supplementary Fig. S3F) and OS (Supplementary Fig. S3G) than those without ADGRB3 mutations in this database.
In our cohort, the P5 and P14 with ADGRB3 mutation (Supplementary Fig. S5A and S5B) achieved PR (Fig. 5B) and CR (Supplementary Fig. S4A) after treatment with chemotherapy plus durvalumab, as evidenced by a remarkable reduction in tumor size. More importantly, no metabolic signals of any lesions were detected via PET/CT (Fig. 5B and Supplementary Fig. S4A), and the baseline elevated CA19-9 decreased to normal levels (Supplementary Fig. S4B). The PFS (P = 0.021) and OS (P = 0.041) in patients harboring ADGRB3 mutations were significantly longer than those in wild type (WT) patients (Fig. 5C and D). These two patients experienced no disease progression or death until last follow-up (Fig. 5C and D). The OS of P5 and P14 was 24.48 and 24.8 months, which was longer than those who were WT (Fig. 5C and D).
TMB was not associated with clinical benefit of chemotherapy plus durvalumab
We then pooled the TMB level in each patient and found that the patients P5 and P14, who were deemed as PR or CR showed a TMB of 49.40 and 15.40mut/Mb, respectively, which ranked in the first two positions (Fig. 5E). Moreover, the TMB of patients with ADGRB3 mutations was significantly higher than that of patients without ADGRB3 mutations (Fig. 5F), which was further validated in solid cancer and cholangiocarcinoma from the cBioPortal database (Fig. 5G). Our previous reports have indicated that RNF43 mutations were associated with better OS and PFS than WT colorectal cancer patients and gained clinical benefit and tumor shrinkage from immunotherapy [20], which was validated in our cohort study (Supplementary Fig. S5B–S5D). Moreover, RNF43-mutated patients tended to have a higher TMB than WT patients in our cohort (Supplementary Fig. S5E) and in the cBioPortal cholangiocarcinoma cohort (Supplementary Fig. S5F and S5G).
Discussion
GP has been regarded as the preferred standard first-line treatment regimen for locally advanced or metastatic cholangiocarcinoma for decades since ABC-02 [4]. This therapeutic strategy was revolutionized following release of the TOPAZ-1 trial, which demonstrated the survival as well as tumor response benefit from adding durvalumab to the standard GP regimen [17]. The efficacy and safety of combining durvalumab with GP was recently evidenced in a real-world experience in Italy, which yielded an mPFS of 8.9 months, a mOS of 12.9 months and a confirmed ORR of 34.5%, while the adverse events were comparable to those seen in the TOPAZ-1 trial [21].
In our retrospective study, a significant reduction in disease progression risk was observed for patients receiving durvalumab plus chemotherapy. However, the mPFS in these patients was 6.77 months, which was numerically shorter than that reported in TOPAZ-1 and the Italy study [17, 21]. Caution is warranted when comparing data from other studies because of differences in the study designs and enrolled patients. For example, the percentage of patients with ECOG = 0 in our study was 12.9%, less than that in the Italian cohort (58.6%) and the TOPAZ-1 trial (50.7%). Moreover, the percentage of patients diagnosed with intrahepatic cholangiocarcinoma was 46.2% in our study, while that in TOPAZ-1 global and China cohort was 55.7% and 72.3%, respectively [22]. These differences may inevitably lead to varying outcomes, as the ECOG status and primary origins were associated with clinical benefit to ICIs in ABC patients [21].
However, it is worth noting that patients receiving durvalumab plus chemotherapy in this study achieved similar mOS as reported in other phase II trials testing PD-1 inhibitors with gemcitabine-based doublet chemotherapy [13, 14]. Furthermore, their survival outcome was comparable to that observed in Japan’s FUGA-BT trial [5], and the Asian subgroup in TOPAZ-1 [17]. The imbalance of patients receiving salvage therapy upon progression on durvalumab (29/93, 31.1%) or chemotherapy (47/93, 50.5%) might influence the OS in our real-world study (Data not shown). These findings suggest potential geographic, immunogenic as well as therapeutic differences between Eastern and Western cholangiocarcinoma patients. On the other hand, pretreatment CA19-9 and NLR were found to be associated with survival of ABC patients receiving durvalumab, which is consistent with the post-hoc analysis of TOPAZ-1 [23].
Although several efforts have been made to predict the clinical benefit from anti-PD-1 therapy [12–15], biomarkers relating to durvalumab in ABC have not been reported. sPD-1 and sPD-L1 have long been recognized as easily accessible biomarkers that may help in pretreatment prognostication and in therapy monitoring of patients who have undergone chemotherapy or immune therapy [24, 25]. High sPD-L1 levels were associated with inferior outcomes in gastrointestinal malignancies receiving chemotherapy [25, 26], as well as in chemo- or ICIs-treated urothelial carcinoma and lung cancer patients [24]. In our retrospective cohort, the level of sPD-L1 but not sPD-1 was significantly negatively correlated with PFS and OS in ABC patients receiving durvalumab. It has been reported that sPD-L1 is released by mature dendritic cells (DCs) and tumor cells but not by activated T lymphocytes, macrophages, and monocytes [27]. Deletion of PD-L1 in DCs leads to robust suppression of tumor growth [28] and enhanced antitumor CD8+ T cell responses [29]. Therefore, it is reasonable to postulate that sPD-L1 may have predictive value for ABC patients receiving durvalumab; however, further investigation is necessary to elucidate the mechanisms underlying sPD-L1-mediated resistance to durvalumab and its implications for the future development of reversal strategies.
Cytokines reflect the immune status and can affect responses to ICI treatment [30]. Our study found that higher baseline levels of CSF1R, OPG and BMP-7 were significantly associated with worse OS and PFS in patients receiving chemotherapy plus durvalumab, albeit in a limited sample size. The median levels of these cytokines at baseline also differed significantly from those at disease progression, while the efficacy predictive roles of CSF1R, OPG and BMP-7 need to be explored in a larger cohort. Another study showed that treatment with anti-PD-L1 antibody (atezolizumab) combined with MEK inhibitors (cobimetinib) significantly decreased circulating PDGF-BB levels, while higher IL-23 and RANTES corresponded to improved OS and PFS following this combination regimen [30]. These differences could be explained by the enrolled patients as well as combination partners, as cytotoxic agents or target therapy may modulate the immune microenvironment (TME) and alter the antitumor immune response in a totally different manner [8].
The immunosuppressive and oncogenic roles of the CSF-1/CSF1R axis have been extensively explored in previous reports [31, 32] and heavily rely on tumorigenic cytokines released by tumor-associated macrophages (TAM) and subsequent deconditioning of the TME [33], which fueled interest in the development of therapeutic agents [31, 32]. In our study, circulating CSF1R was not only found to be significantly elevated when disease progressed but also correlated with worsened survival in ABC patients receiving chemotherapy plus durvalumab. Previous studies have shown that TAMs contribute to an immunosuppressive TME. Therefore, blocking the CSF-1/CSF1R pathway along with ICIs may function synergistically through improving intertumoral CD8+ T cell function [31, 33], which is currently being tested in a variety of clinical trials with pending results. TAM infiltration has been shown to foster the development of cholangiocarcinoma [34] and is associated with a special subtype known as the hepatic stem-like class in ABC [35]. Inhibiting CSF1/CSF-1R signaling in hepatocellular was shown to prevent TAM trafficking and induce CD8+ T cell infiltration, resulting in potent antitumor activity when combined with anti-PD-L1 antibody [36], as observed in a pancreatic adenocarcinoma mouse model [37]. Therefore, our data provide potential avenues for testing the antitumor potential of CSF1R inhibitors and durvalumab in ABC.
ADGRB3, a member of the adhesion G protein-coupled receptors family, plays a crucial role in synapse formation and maintenance. Previous studies have reported its involvement in small-cell lung cancer development [38], while certain variations in the intron of ADGRB3 gene have been associated with a reduced risk of primary sclerosing cholangitis in polish patients [39]. Additionally, a case report suggests that ADGRB3 mutations may contribute to intrahepatic mucinous cholangiocarcinoma [40]. We presented novel evidence linking ADGRB3 mutations to increased TMB and improved outcomes in ABC patients for the first time. Mutations of KRAS [19] and ARID1A [15] have been reported to be associated with clinical benefit of PD-1 inhibitors in ABC, which was not found in our study. However, the precise mechanisms of clinical benefit conferred by mutated ADGRB3 under the circumvent of immunotherapy remain unclear and warrant further investigation.
In conclusion, combining durvalumab with chemotherapy can enhance PFS for ABC patients; however, improvement in OS was not observed over chemotherapy alone within the real-world setting in Chinese cohort with the largest cases to date. This is the first report regarding circulating biomarkers in ABC patients receiving durvalumab, which might provide easily accessible and noninvasive methods to monitor the efficacy of this regimen. More importantly, CSF1R was found significantly elevated upon disease progression, providing possible strategy to reverse immune-resistance of durvalumab by combination with inhibitors targeting CSF1R. Third, mutation of ADGRB3 was associated with benefit of PD-L1 antibody. This is different from previous reports as KRAS [19] or ARID1A [15] mutations were stemming from trials evaluating efficacy of PD-1 with limited sample size. Altogether, we have discovered several prognosis predictive biomarkers in ABC patients receiving durvalumab, which would help patients to avoid the huge cost burden in China.
Limitations in our study include the following: First, this study was conducted at a single-center and has a retrospective design. Due to limited accessibility to durvalumab and availability of pretreatment samples, external validations of the circulating biomarkers was impossible, emphasizing the need for large-scale multicenter prospective verification studies. Secondly, biomarker analyses in our study were hypothesis-generating in a limited sample size, and therefore, the results should be interpreted with caution. Despite these limitations, our study provides valuable insights into the real-world effectiveness of PD-L1 inhibitors in ABC and for the first time, identifies potential noninvasive as well as genetic biomarkers for predicting treatment response and clinical outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ABC
Advanced biliary tract cancer
- ADGRB3
Adhesion G protein-coupled receptor B3
- BMP-7
Bone morphogenetic protein 7
- Chemo.
Chemotherapy
- CR
Complete response
- CSF1R
Colony-stimulating factor 1 receptor
- DCR
Disease control rate
- EGF
Epidermal growth factor
- GH
Growth factor
- HER2
Human epidermal growth factor receptor
- HR
Hazard ratio
- Mut
Mutation
- ORR
Objective response rate
- OPG
Osteoprotegerin
- OS
Overall survival
- PD-1
Programmed death 1
- PD-L1
Programmed death ligand 1
- PD
Progressive disease
- PFS
Progression free survival
- PR
Partial response
- SD
Stable disease
- sPD-1
Soluble programmed death 1
- sPD-L1
Soluble programmed death ligand 1
- TNFα
Tumor necrosis factor α
- TMB
Tumor mutational burden
- WT
Wild type
Author contributions
Conception and design were done by DS Zhang, PD Chi, YX Lu; Development of methodology was done by YX Lu, Y Jin, FR Liu, ZX Wang, Y Zhang; Data curation: YX Lu, Y Jin, W Zhou, B Bai, Y Wang, ZQ Wang, M Nie, HY Luo, GF Guo, MZ Qiu, SP Li, YH Li, FH Wang; Analysis and interpretation of data were done by W Zhou, CQ Liang, GF Guo, JW Chen, Y Liu; Writing–review and editing were done by YX Lu, Y Jin, FR Liu, ZX Wang, F Wang, DS Zhang; Study supervision was done by DS Zhang, PD Chi.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82002465; 82172711) and Natural Science Foundation of Guangdong Province (Grant No. 2019A1515011786).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethical Committee of Sun Yat-sen University Cancer Center. Written consent was obtained from all participants during their hospitalization.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunxin Lu, Yin Jin, Furong Liu, Zixian Wang and Wen Zhou have contributed equally to this work.
Contributor Information
Peidong Chi, Email: chipd@sysucc.org.cn.
Dongsheng Zhang, Email: zhangdsh@sysucc.org.cn.
References
- 1.Banales JM, Marin JJG, Lamarca A et al (2020) Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 17:557–588. 10.1038/s41575-020-0310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX (2021) Biliary tract cancer. Lancet 397:428–444. 10.1016/S0140-6736(21)00153-7 [DOI] [PubMed] [Google Scholar]
- 3.Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME (2023) Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin 73:198–222. 10.3322/caac.21759 [DOI] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281. 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 5.Morizane C, Okusaka T, Mizusawa J et al (2019) Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol Off J Eur Soc Med Oncol 30:1950–1958. 10.1093/annonc/mdz402 [DOI] [PubMed] [Google Scholar]
- 6.Kim ST, Kang JH, Lee J et al (2019) Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial. Ann oncol Off J Eur Soc Med Oncol 30:788–795. 10.1093/annonc/mdz058 [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Kalyan Mohanti B, Pal Chaudhary S et al (2019) Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: results of a phase III randomised controlled trial. Eur J Cancer 123:162–170. 10.1016/j.ejca.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 8.Wang DR, Wu XL, Sun YL (2022) Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther 7:331. 10.1038/s41392-022-01136-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RD, Chung V, Alese OB et al (2020) A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol 6:888–894. 10.1001/jamaoncol.2020.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piha-Paul SA, Oh DY, Ueno M et al (2020) Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer 147:2190–2198. 10.1002/ijc.33013 [DOI] [PubMed] [Google Scholar]
- 11.Ueno M, Ikeda M, Morizane C et al (2019) Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 4:611–621. 10.1016/S2468-1253(19)30086-X [DOI] [PubMed] [Google Scholar]
- 12.Chiang NJ, Tan KT, Bai LY et al (2022) Impaired chromatin remodeling predicts better survival to modified gemcitabine and S-1 plus nivolumab in advanced biliary tract cancer: a phase II T1219 study. Clin Cancer Res Off J Am Ass Cancer Res 28:4248–4257. 10.1158/1078-0432.CCR-22-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng TM, Yang G, Lou C et al (2023) Clinical and biomarker analyses of sintilimab plus gemcitabine and cisplatin as first-line treatment for patients with advanced biliary tract cancer. Nat Commun 14:1340. 10.1038/s41467-023-37030-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Wang Y, Yu Y et al (2022) Toripalimab in advanced biliary tract cancer. Innovation 3:100255. 10.1016/j.xinn.2022.100255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Wu X, Wu H et al (2020) Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer. 10.1136/jitc-2020-001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley RK, Ueno M, Yoo C et al (2023) Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401:1853–1865. 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
- 17.Oh DY, He AR, Qin S, et al (2022) A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. NEJM Evidence. 1
- 18.Liddell SS, Chakrabarti S, Wintheiser GA et al (2022) Tumor mutational burden is a potential predictive biomarker for response to immune checkpoint inhibitors in patients with advanced biliary tract cancer. JCO Precis Oncol 6:e2200003. 10.1200/PO.22.00003 [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Wang D, Liu J et al (2021) Genomic alterations in biliary tract cancer predict prognosis and immunotherapy outcomes. J Immunother Cancer. 10.1136/jitc-2021-003214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye LF, Huang ZY, Chen XX et al (2022) Monitoring tumour resistance to the BRAF inhibitor combination regimen in colorectal cancer patients via circulating tumour DNA. Drug Resist Updates Rev Comm Antimicrob Anticancer Chemother 65:100883. 10.1016/j.drup.2022.100883 [DOI] [PubMed] [Google Scholar]
- 21.Rimini M, Fornaro L, Lonardi S et al (2023) Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: An early exploratory analysis of real-world data. Liver Int Off J Int Ass Study Liver 43:1803–1812. 10.1111/liv.15641 [DOI] [PubMed] [Google Scholar]
- 22.Qin S, Cai JQ, Li E et al (2023) 98P Efficacy and safety of durvalumab plus gemcitabine and cisplatin in Chinese participants with advanced biliary tract cancer: extension cohort of the phase III, randomised, double-blind, placebo-controlled, global TOPAZ-1 study. Ann Oncol 34:S216–S217. 10.1016/j.annonc.2023.09.1391 [Google Scholar]
- 23.He AR, Bouattour M, Gupta VG et al (2023) 102P Potentially prognostic factors of overall survival in advanced biliary tract cancer in the randomised phase III TOPAZ-1 study. Ann Oncol 34:S220. 10.1016/j.annonc.2023.09.1395 [Google Scholar]
- 24.Szeles A, Fazekas T, Vancsa S et al (2023) Pre-treatment soluble PD-L1 as a predictor of overall survival for immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Cancer Immunol Immunother CII 72:1061–1073. 10.1007/s00262-022-03328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shigemori T, Toiyama Y, Okugawa Y et al (2019) Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol 26:876–883. 10.1245/s10434-018-07112-x [DOI] [PubMed] [Google Scholar]
- 26.Tominaga T, Akiyoshi T, Yamamoto N, Taguchi S, Mori S, Nagasaki T, Fukunaga Y, Ueno M (2019) Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE 14:e0212978. 10.1371/journal.pone.0212978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, Dietz AB, Dong H, Kwon ED (2012) Soluble B7–H1: differences in production between dendritic cells and T cells. Immunol Lett 142:78–82. 10.1016/j.imlet.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh SA, Wu DC, Cheung J et al (2020) PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nature cancer 1:681–691. 10.1038/s43018-020-0075-x [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Velez-Delgado A, Mathew E et al (2017) Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66:124–136. 10.1136/gutjnl-2016-312078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggieri AN, Yarchoan M, Goyal S et al (2022) Combined MEK/PD-L1 inhibition alters peripheral cytokines and lymphocyte populations correlating with improved clinical outcomes in advanced biliary tract cancer. Clin Cancer Res 28:4336–4345. 10.1158/1078-0432.CCR-22-1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen J, Wang S, Guo R, Liu D (2023) CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment. Eur J Med Chem 245:114884. 10.1016/j.ejmech.2022.114884 [DOI] [PubMed] [Google Scholar]
- 32.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D (2017) Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5:53. 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan Z, Luo Y (2021) Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther 6:127. 10.1038/s41392-021-00506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo C, Xin H, Zhou Z et al (2022) Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 76:982–999. 10.1002/hep.32387 [DOI] [PubMed] [Google Scholar]
- 35.Martin-Serrano MA, Kepecs B, Torres-Martin M et al (2023) Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 72:736–748. 10.1136/gutjnl-2021-326514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Yang J, Xu D et al (2019) Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 68:1653–1666. 10.1136/gutjnl-2019-318419 [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Knolhoff BL, Meyer MA et al (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Can Res 74:5057–5069. 10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas M, Snead D, Mitchell D (2017) An investigation into the potential role of brain angiogenesis inhibitor protein 3 (BAI3) in the tumorigenesis of small-cell carcinoma: a review of the surrounding literature. J Recept Signal Transduct Res 37:325–334. 10.1080/10799893.2017.1328441 [DOI] [PubMed] [Google Scholar]
- 39.Paziewska A, Habior A, Rogowska A et al (2017) A novel approach to genome-wide association analysis identifies genetic associations with primary biliary cholangitis and primary sclerosing cholangitis in Polish patients. BMC Med Genomics 10:2. 10.1186/s12920-016-0239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masaki Y, Akutsu N, Adachi Y et al (2022) Genomic analysis of an aggressive case with metastatic intrahepatic mucinous cholangiocarcinoma. Clin J Gastroenterol 15:809–817. 10.1007/s12328-022-01649-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.