Abstract
Background
Sitravatinib is a spectrum-selective tyrosine kinase inhibitor targeting TAM (TYRO3, AXL, MER), VEGFR-2, KIT, and MET. SAFFRON-104 (NCT03941873) was a multicohort phase Ib/II study investigating sitravatinib with/without tislelizumab, an anti-programmed cell death protein 1 (PD-1) antibody, in patients with advanced hepatocellular carcinoma (HCC) or gastric cancer/gastroesophageal junction cancer (GC/GEJC).
Methods
Eligible patients had histologically/cytologically confirmed advanced HCC or GC/GEJC. Phase I determined the recommended phase II dose (RP2D) of sitravatinib with/without tislelizumab. Phase II evaluated sitravatinib monotherapy in patients with pretreated HCC, and sitravatinib plus tislelizumab in anti-PD-(L)1–naïve or –treated HCC and anti-PD-(L)1–naïve GC/GEJC. Primary endpoints were safety/tolerability (phase I) and objective response rate (ORR) (phase II).
Results
At data cutoff (March 31, 2023), 111 patients were enrolled; 102 were efficacy-evaluable (median study follow-up 9.1 months [range: 0.7–36.9]). The RP2D of sitravatinib was determined as 120 mg orally once daily. In patients receiving sitravatinib monotherapy and sitravatinib in combination with tislelizumab, grade ≥ 3 treatment-related adverse events occurred in 14 (51.9%) and 42 (50.0%) patients, respectively. The ORR was 25% (95% confidence interval [CI]: 8.7–49.1) in patients with pretreated HCC receiving sitravatinib monotherapy. In patients receiving sitravatinib with tislelizumab, the ORR was 11.5% (95% CI 2.4–30.2) with anti-PD-(L)1–naïve HCC, 9.5% (95% CI 1.2–30.4) with anti-PD-(L)1–treated HCC, and 16.1% (95% CI 5.5–33.7) in patients with anti-PD-(L)1–naïve GC/GEJC.
Conclusions
Sitravatinib with/without tislelizumab was generally well tolerated and showed preliminary antitumor activity in patients with advanced HCC and GC/GEJC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03806-2.
Keywords: Clinical trials, Phase II, Drug therapy, Combination therapy, Immune checkpoint inhibitors, Immunotherapy
Introduction
Hepatocellular carcinoma (HCC) and gastric cancer/gastroesophageal junction cancer (GC/GEJC) are among the most common causes of cancer death worldwide; both diseases have an extremely poor prognosis and therefore, an unmet need for alternative treatment options [1–4].Therapies targeting programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) have been transformative in the treatment of multiple cancer types [5, 6]. Despite some success with monoclonal antibodies targeting the PD-1–PD-L1 (PD-[L]1) immune checkpoint in HCC and GC/GEJC [7, 8], most patients respond poorly to available monotherapies, and many tumors develop resistance to immune checkpoint inhibitors [9, 10]. One mechanism behind PD-(L)1 resistance is immune suppression in the tumor microenvironment (TME) [11]. It is hypothesized that combining anti-PD-(L)1 agents with immunomodulatory therapies can overcome these barriers and enhance antitumor activity; combination therapy has emerged as a key strategy to improve outcomes in patients with little or no response to single-agent immune checkpoint inhibitors [7, 8, 12].
Tislelizumab is a humanized immunoglobulin G4 monoclonal antibody engineered to have reduced Fc gamma receptor binding on macrophages to minimize antibody-dependent phagocytic resistance pathways [13].Tislelizumab has demonstrated efficacy as monotherapy versus sorafenib in a phase III trial for first-line treatment of unresectable HCC [14] and in combination with chemotherapy versus placebo plus chemotherapy in the phase III RATIONALE-305 study in first-line GC/GEJC, with an acceptable safety and tolerability profile [15, 16]. Sitravatinib is an oral selective receptor tyrosine kinase inhibitor (TKI) that targets TAM (TYRO3, AXL, MER) family members as well as vascular endothelial growth factor receptor 2 (VEGFR-2), KIT, and MET [17]. Targeting TAM receptors reduces TME immunosuppression by influencing macrophage phenotypes and promoting their immunostimulatory properties [18]. Additionally, targeting of VEGFR and KIT reduces the number of immunosuppressive cell subtypes, such as regulatory T cells and monocyte-derived suppressor cells [19, 20] and induces a more favorable TME for immune checkpoint blockade while helping to overcome resistance to immune checkpoint inhibition [21]. As such, sitravatinib is being tested in combination with immune checkpoint inhibitors and has recently demonstrated clinical activity in combination with tislelizumab in a phase I trial in advanced non–small cell lung cancer (NSCLC) [22]. Antitumor activity of the combination was seen in both treatment-naïve and treatment-experienced patients, as well as in those with PD-(L)1–treated disease, while no new safety signals were identified [22].
Here, we present the safety and preliminary clinical activity of sitravatinib with or without tislelizumab from the phase Ib/II SAFFRON-104 study in patients with advanced HCC or GC/GEJC.
Methods
Study design and treatment
SAFFRON-104 (NCT03941873) was an open-label, multicenter, multicohort, phase Ib/II study conducted in China to determine the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of sitravatinib as monotherapy or in combination with tislelizumab in patients with unresectable locally advanced or metastatic HCC, and in combination with tislelizumab in patients with unresectable locally advanced or metastatic GC/GEJC.
In phase I, a modified 3 + 3 dose-escalation design was used to investigate two dose levels of free base sitravatinib (80 mg orally once daily [QD] and 120 mg QD) either as monotherapy or in combination with tislelizumab (200 mg intravenously on Cycle 1 Day 1 and once every 3 weeks [Q3W] thereafter in both sitravatinib dose cohorts) (Supplementary Fig. S1). The upper limit of sitravatinib dose was set at 120 mg once daily based on findings from the first-in-human study 516–001 [23]. Dose escalation proceeded if none of the first three evaluable patients experienced a dose-limiting toxicity (DLT); if one of the first three evaluable patients experienced a DLT, additional patients (minimum of six) were enrolled to that cohort. If less than one-third of evaluable patients experienced a DLT, escalation proceeded to the next dose level; if more than one-third experienced a DLT, dose escalation was stopped. The safety monitoring committee confirmed the recommended phase II dose (RP2D) of sitravatinib as monotherapy and in combination with tislelizumab based on all available data (safety, efficacy, pharmacokinetic, and exploratory). Phase II (dose expansion) evaluated four cohorts (Supplementary Fig. S1): sitravatinib monotherapy in patients with anti-PD-(L)1–naïve or –treated HCC, and sitravatinib plus tislelizumab in anti-PD-(L)1–naïve HCC, anti-PD-(L)1–treated HCC, and anti-PD-(L)1–naïve GC/GEJC. All patients received study treatment until progressive disease (PD) or unacceptable toxicity, death, withdrawal of consent, or study termination.
Patients
Eligible patients were aged ≥ 18 years, had histologically or cytologically confirmed unresectable locally advanced or metastatic HCC or GC/GEJC, had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and adequate organ function. In addition, patients with HCC had Barcelona Clinic Liver Cancer (BCLC) Stage C or BCLC Stage B disease that was not amenable to curative treatment, as well as a Child–Pugh A liver function classification, and no loco-regional therapy to the liver within 28 days of the first dose of study drug(s). Patients with GC/GEJC had no history of gastrointestinal perforation and/or fistulae within 6 months or clinically significant bleeding within 3 months of first dose of study drug(s). Patients recruited to phase II had at least one measurable lesion per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and had received up to two lines of prior systemic treatment (patients with HCC only); patients with advanced GC/GEJC had failed current standard-of-care treatment (or it was not appropriate).
Key exclusion criteria for all patients included active leptomeningeal disease or uncontrolled brain metastasis, active autoimmune diseases or history of autoimmune diseases that may relapse, any active malignancy within 2 years of first dose of study drug(s) (except for the specific cancer under investigation), any systemic chemotherapy within 28 days from first dose of study drug(s), and any condition that required systemic treatment with either corticosteroids or other immunosuppressive medication within 14 days before the first dose of study drug(s).
Endpoints and assessments
The primary endpoint for phase I was sitravatinib safety and tolerability as monotherapy or in combination with tislelizumab throughout the study. Secondary endpoints of phase I included investigator-assessed objective response rate (ORR), duration of response (DoR), disease control rate (DCR), and progression-free survival (PFS), based on RECIST v1.1. The primary endpoint of phase II was investigator-assessed ORR per RECIST v1.1. Phase II secondary endpoints included investigator-assessed DoR, DCR, and PFS per RECIST v1.1, and sitravatinib safety and tolerability as monotherapy and in combination with tislelizumab.
Safety assessments included vital signs, physical examinations, 12-lead electrocardiograms, and laboratory safety tests (hematology, clinical chemistry, and urinalysis). Adverse events (AEs) were coded using Medical Dictionary for Regulatory Activities version 18.1 or higher and were assessed and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Treatment-emergent adverse events (TEAEs) were defined as AEs with an onset, or worsening in severity from baseline, on or after the first dose of study drug, and up to 30 days following study drug discontinuation or initiation of new anticancer therapy (whichever occurred first). Treatment-related TEAEs (TRAEs) were those considered by the investigator to be related to study treatment or with missing assessment of the causal relationship.
Tumor imaging was performed with computed tomography (preferred) or contrast-enhanced magnetic resonance imaging of the chest, abdomen, and pelvis; other known or suspected sites of disease were included in the assessments. Imaging took place within 28 days prior to the first dose of study drug(s), then every 6 weeks in the first year and every 9 weeks thereafter. ORR was defined as the proportion of patients achieving a confirmed (for at least 4 weeks) complete response (CR) or partial response (PR). DCR was defined as the proportion of patients with a best overall response of CR, PR, or stable disease (SD). Clinical benefit rate (CBR) was defined as the proportion of patients with a confirmed PR, confirmed CR, or durable (for at least 24 weeks) SD. DoR was assessed among responders (CR or PR) and defined as the time between the date of the earliest qualifying response and the date of PD or any-cause death, whichever occurred first. PFS was defined as the time from the date of the first dose of study drug(s) to the date of the first documentation of PD or death, whichever occurred first. Overall survival (OS) was defined as the time from the date of the first dose of study drug(s) to the date of death due to any cause.
Statistical analyses
The study planned to enroll approximately 98–116 patients, including ~ 18–36 DLT-evaluable patients to phase I and ~ 80 patients to phase II (~ 20 patients per cohort). Enrollment into, and evaluation of, these cohorts were independent. The sample size was not driven by statistical considerations.
The safety population included all patients from phase I and II receiving at least one dose of study drug (any component for the combination therapy). The efficacy-evaluable population included all patients from phase I and II receiving at least one dose of study drug with measurable disease at baseline (per RECIST v1.1) and at least one postbaseline tumor assessment (unless treatment was discontinued due to clinical PD or early death [within 13 weeks of the first dose date]). The DLT-evaluable analysis set for sitravatinib monotherapy included patients in phase I who received at least 75% of the assigned total dose for the DLT assessment window and had sufficient safety evaluation. For sitravatinib plus tislelizumab, the DLT-evaluable analysis set included patients in phase I who received at least 75% of the assigned total dose of sitravatinib and at least 67% of the assigned total dose for tislelizumab for the DLT assessment window. Patients not meeting these criteria but who had a DLT event were also included.
Patient demographics and baseline characteristics were summarized using descriptive statistics. Incidence of DLT events, TEAEs, and TRAEs were reported as number (percentage) of patients with TEAEs by system organ class and preferred term. Patients were counted only once by the highest severity grade, even if more than one TEAE was observed. Phase I and phase II data were pooled to provide safety and efficacy analyses based on tumor type, treatment received, and demographics. ORR was estimated along with Clopper–Pearson two-sided 95% confidence intervals (CIs) and presented by indication. DCR and CBR were analyzed similarly to ORR. Median DoR and median PFS (and 95% CIs) were estimated using Kaplan–Meier (KM) methodology with 95% CIs estimated using the Brookmeyer and Crowley method with log–log transformation. OS at different timepoints was estimated using KM methodology; patients who remained alive before data cutoff or discontinuation of study (other than from death) were censored at the time of data cutoff or last date known to be alive.
Results
Patient characteristics
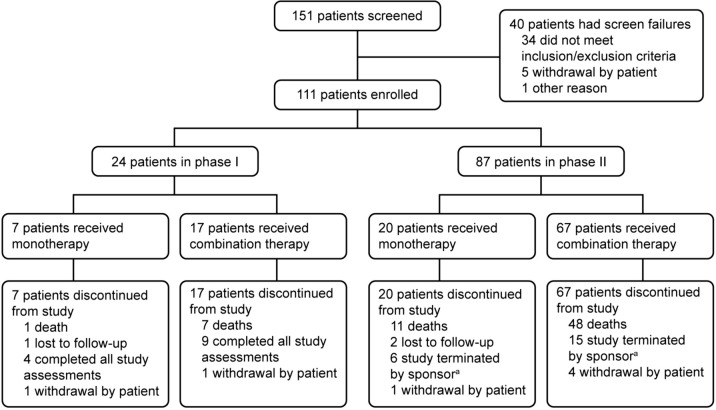
Between February 21, 2019, and April 19, 2021, 151 patients from 18 sites across China were screened, of whom 111 were enrolled (Fig. 1). At data cutoff (March 31, 2023), 24 patients had received treatment in phase I and 87 in phase II. No patients remained on the study; the main reason for discontinuation across all cohorts was PD (Fig. 1). The overall median study follow-up was 9.1 months (range: 0.7–36.9). Median study follow-up was 5.9 months (range: 0.8–15.4) in phase I and 12.6 months (range: 0.7–36.9) in phase II. Patient demographics and baseline disease characteristics are shown in Table 1. Further disease history for patients with HCC is shown in Supplementary Table S1.
Fig. 1.
Patient flow. aAfter the study was terminated by the sponsor, all patients who were still on treatment were transferred to the long-term extension study to continue treatment as assigned
Table 1.
Baseline characteristics (safety analysis set from phase I and phase II)
| Indication | HCC | GC/GEJC | |||
|---|---|---|---|---|---|
| Treatment | Sitravatinib monotherapy | Sitravatinib plus tislelizumab | Sitravatinib plus tislelizumab | ||
| Prior anti-PD-(L)1 treatment status | Naïve or treated (n = 24) | Naïve (n = 27) | Treated (n = 24) | Subtotal (n = 51) | Naïve (n = 32) |
| Median age, years (range) | 51.5 (31–70) | 61.0 (30–70) | 49.0 (29–71) | 55.0 (29–71) | 62.5 (44–74) |
| < 65 years, n (%) | 19 (79.2) | 21 (77.8) | 20 (83.3) | 41 (80.4) | 20 (62.5) |
| ≥ 65 years, n (%) | 5 (20.8) | 6 (22.2) | 4 (16.7) | 10 (19.6) | 12 (37.5) |
| Sex, n (%) | |||||
| Male | 23 (95.8) | 23 (85.2) | 22 (91.7) | 45 (88.2) | 27 (84.4) |
| Female | 1 (4.2) | 4 (14.8) | 2 (8.3) | 6 (11.8) | 5 (15.6) |
| ECOG performance status, n (%) | |||||
| 0 | 10 (41.7) | 14 (51.9) | 13 (54.2) | 27 (52.9) | 3 (9.4) |
| 1 | 14 (58.3) | 13 (48.1) | 11 (45.8) | 24 (47.1) | 29 (90.6) |
| PD-L1 TC score | |||||
| TC < 1% | 8 (33.3) | 10 (37.0) | 0 (0.0) | 10 (19.6) | 8 (25.0) |
| TC ≥ 1% | 1 (4.2) | 1 (3.7) | 0 (0.0) | 1 (2.0) | 2 (6.3) |
| Unknown | 15 (62.5) | 16 (59.3) | 24 (100.0) | 40 (78.4) | 22 (68.8) |
| Alcohol consumption, n (%) | |||||
| Never | 15 (62.5) | 19 (70.4) | 13 (54.2) | 32 (62.7) | 20 (62.5) |
| Current | 0 (0.0) | 0 (0.0) | 1 (4.2) | 1 (2.0) | 1 (3.1) |
| Former | 9 (37.5) | 8 (29.6) | 10 (41.7) | 18 (35.3) | 11 (34.4) |
| Disease stage,an (%) | |||||
| Unresectable locally advanced | 1 (4.2) | 5 (18.5) | 4 (16.7) | 9 (17.6) | 1 (3.1) |
| Metastatic | 23 (95.8) | 22 (81.5) | 20 (83.3) | 42 (82.4) | 31 (96.9) |
| Number of metastatic sites,an (%) | |||||
| 1 | 8 (33.3) | 10 (37.0) | 4 (16.7) | 14 (27.5) | 17 (53.1) |
| 2 | 8 (33.3) | 7 (25.9) | 8 (33.3) | 15 (29.4) | 8 (25.0) |
| ≥ 3 | 7 (29.2) | 5 (18.5) | 8 (33.3) | 13 (25.5) | 6 (18.8) |
| Prior anticancer therapy, n (%) | 23 (95.8) | 27 (100.0) | 24 (100.0) | 51 (100.0) | 32 (100.0) |
| Prior immunotherapy | 1 (4.2) | 0 (0.0) | 24 (100.0) | 24 (47.1) | 0 (0.0) |
| Prior TKI | 23 (95.8) | 26 (96.3) | 20 (83.3) | 46 (90.2) | 8 (25.0) |
| Number of prior treatment lines, n (%) | |||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) |
| 1 | 15 (62.5) | 18 (66.7) | 12 (50.0) | 30 (58.8) | 14 (43.8) |
| 2 | 8 (33.3) | 9 (33.3) | 12 (50.0) | 21 (41.2) | 14 (43.8) |
| ≥ 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (9.4) |
aAt study entry. Data from phase I and phase II were pooled per the tumor characteristics; four patients with GC/GEJC in phase I were excluded because they could not be mapped to any indication group, either due to unknown prior anti-PD-(L)1 treatment status (n = 1) or because they received sitravatinib monotherapy (n = 3)
ECOG Eastern Cooperative Oncology Group, GC/GEJC gastric cancer/gastroesophageal junction cancer, HCC hepatocellular carcinoma, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, TC tumor cell, TKI, tyrosine kinase inhibitor
Safety and tolerability
Median duration of exposure to sitravatinib was 4.8 months (range: 0.6–29.7) in sitravatinib monotherapy–treated patients and 3.4 months (range: 0.1–28.2) for patients treated with sitravatinib plus tislelizumab; median number of treatment cycles was 7.0 and 5.5, respectively. Median duration of exposure to tislelizumab was 4.1 months (range: 0.7–28.6) for patients treated with sitravatinib plus tislelizumab; median number of treatment cycles was 4.5. Of the DLT-evaluable set (n = 22), no DLTs were reported in patients receiving sitravatinib monotherapy at the 80 mg (n = 3) or 120 mg (n = 4) QD doses. Two DLTs were reported in patients receiving sitravatinib 120 mg QD plus tislelizumab 200 mg Q3W (n = 12): one (grade 3 palmar-plantar erythrodysesthesia syndrome) among six patients with HCC and one (grade 3 proteinuria) among six patients with GC/GEJC. No DLTs were observed among patients receiving sitravatinib 80 mg QD plus tislelizumab 200 mg Q3W (n = 3). RP2D of sitravatinib was determined as 120 mg QD, either as monotherapy or in combination with tislelizumab 200 mg Q3W.
All patients (100%) receiving sitravatinib monotherapy at either the 80 mg QD dose (n = 3) or the 120 mg QD dose (n = 24) in the safety analysis set (phases I and II combined) reported a TRAE (Table 2). Of patients receiving the sitravatinib 120 mg QD dose as monotherapy, grade ≥ 3 TRAEs were reported in 45.8% (n = 11/24), and 25.0% (n = 6/24) had serious TRAEs. One (4.2%) patient had a TRAE that led to treatment discontinuation and 17 (70.8%) patients had TRAEs that led to treatment modification.
Table 2.
Summary of treatment-related adverse events (safety analysis set from phase I and phase II)
| N (%) | Sitravatinib 80 mg monotherapy (n = 3) | Sitravatinib 120 mg monotherapy (n = 24) | Sitravatinib monotherapy combined (n = 27) | Sitravatinib 80 mg plus tislelizumab (n = 3) | Sitravatinib 120 mg plus tislelizumab (n = 81) | Sitravatinib plus tislelizumab combined (n = 84) |
|---|---|---|---|---|---|---|
| Any TRAE | 3 (100.0) | 24 (100.0) | 27 (100.0) | 3 (100.0) | 73 (90.1) | 76 (90.5) |
| Related to sitravatinib | 3 (100.0) | 24 (100.0) | 27 (100.0) | 3 (100.0) | 73 (90.1) | 76 (90.5) |
| Related to tislelizumab | – | – | – | 3 (100.0) | 67 (82.7) | 70 (83.3) |
| Grade ≥ 3 | 3 (100.0) | 11 (45.8) | 14 (51.9) | 2 (66.7) | 40 (49.4) | 42 (50.0) |
| Related to sitravatinib | 3 (100.0) | 11 (45.8) | 14 (51.9) | 2 (66.7) | 40 (49.4) | 42 (50.0) |
| Related to tislelizumab | – | – | – | 1 (33.3) | 23 (28.4) | 24 (28.6) |
| Serious | 0 (0.0) | 6 (25.0) | 6 (22.2) | 1 (33.3) | 17 (21.0) | 18 (21.4) |
| Related to sitravatinib | 0 (0.0) | 6 (25.0) | 6 (22.2) | 1 (33.3) | 15 (18.5) | 16 (19.0) |
| Related to tislelizumab | – | – | – | 1 (33.3) | 11 (13.6) | 12 (14.3) |
| Leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.9) | 4 (4.8) |
| Related to sitravatinib | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.9) | 4 (4.8) |
| Related to tislelizumab | – | – | 0 (0.0) | 0 (0.0) | 3 (3.7) | 3 (3.6) |
| Leading to treatment discontinuation | 0 (0.0) | 1 (4.2) | 1 (3.7) | 1 (33.3) | 8 (9.9) | 9 (10.7) |
| Leading to dose modificationa | 1 (33.3) | 17 (70.8) | 18 (66.7) | 2 (66.7) | 51 (63.0) | 53 (63.1) |
| TRAEs reported in ≥ 10% of patients | ||||||
| Proteinuria | 2 (66.7) | 13 (54.2) | 15 (55.6) | 2 (66.7) | 44 (54.3) | 46 (54.8) |
| ALT increased | 2 (66.7) | 12 (50.0) | 14 (51.9) | 2 (66.7) | 36 (44.4) | 38 (45.2) |
| AST increased | 2 (66.7) | 12 (50.0) | 14 (51.9) | 3 (100.0) | 35 (43.2) | 38 (45.2) |
| PPE syndrome | 2 (66.7) | 17 (70.8) | 19 (70.4) | 0 (0.0) | 30 (37.0) | 30 (35.7) |
| Diarrhea | 2 (66.7) | 14 (58.3) | 16 (59.3) | 0 (0.0) | 26 (32.1) | 26 (31.0) |
| Hypertension | 3 (100.0) | 4 (16.7) | 7 (25.9) | 2 (66.7) | 28 (34.6) | 30 (35.7) |
| Platelet count decreased | 2 (66.7) | 10 (41.7) | 12 (44.4) | 0 (0.0) | 22 (27.2) | 22 (26.2) |
| Blood CPK MB increased | 0 (0.0) | 2 (8.3) | 2 (7.4) | 1 (33.3) | 23 (28.4) | 24 (28.6) |
| Decreased appetite | 1 (33.3) | 3 (12.5) | 4 (14.8) | 1 (33.3) | 17 (21.0) | 18 (21.4) |
| Hypothyroidism | 2 (66.7) | 2 (8.3) | 4 (14.8) | 1 (33.3) | 15 (18.5) | 16 (19.0) |
| Anemia | 1 (33.3) | 5 (20.8) | 6 (22.2) | 1 (33.3) | 11 (13.6) | 12 (14.3) |
| Blood bilirubin increased | 1 (33.3) | 3 (12.5) | 4 (14.8) | 0 (0.0) | 13 (16.0) | 13 (15.5) |
| Blood thyroid stimulating hormone increased | 0 (0.0) | 2 (8.3) | 2 (7.4) | 0 (0.0) | 14 (17.3) | 14 (16.7) |
| Hypoalbuminemia | 0 (0.0) | 2 (8.3) | 2 (7.4) | 1 (33.3) | 13 (16.0) | 14 (16.7) |
| GGT increased | 0 (0.0) | 4 (16.7) | 4 (14.8) | 0 (0.0) | 12 (14.8) | 12 (14.3) |
| WBC count decreased | 1 (33.3) | 2 (8.3) | 3 (11.1) | 0 (0.0) | 13 (16.0) | 13 (15.5) |
| Blood CPK increased | 0 (0.0) | 1 (4.2) | 1 (3.7) | 0 (0.0) | 14 (7.3) | 14 (16.7) |
| Blood AP increased | 0 (0.0) | 2 (8.3) | 2 (7.4) | 0 (0.0) | 11 (13.6) | 11 (13.1) |
| Blood LDH increased | 0 (0.0) | 2 (8.3) | 2 (7.4) | 0 (0.0) | 11 (13.6) | 11 (13.1) |
| Abdominal pain upper | 0 (0.0) | 2 (8.3) | 2 (7.4) | 0 (0.0) | 11 (13.6) | 11 (13.1) |
| Nausea | 0 (0.0) | 2 (8.3) | 2 (7.4) | 1 (33.3) | 10 (12.3) | 11 (13.1) |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 11 (13.6) | 12 (14.3) |
| Dysphonia | 0 (0.0) | 5 (20.8) | 5 (18.5) | 0 (0.0) | 8 (9.9) | 8 (9.5) |
| Weight decreased | 1 (33.3) | 3 (12.5) | 4 (14.8) | 1 (33.3) | 7 (8.6) | 8 (9.5) |
| Fatigue | 0 (0.0) | 1 (4.2) | 1 (3.7) | 1 (3.33) | 6 (7.4) | 7 (8.3) |
| Hypokalemia | 0 (0.0) | 3 (12.5) | 3 (11.1) | 0 (0.0) | 6 (7.4) | 6 (7.1) |
| Neutrophil count decreased | 1 (33.3) | 1 (4.2) | 2 (7.4) | 0 (0.0) | 5 (6.2) | 5 (6.0) |
| Hypocalcemia | 0 (0.0) | 1 (4.2) | 1 (3.7) | 1 (3.33) | 3 (3.7) | 4 (4.8) |
| Hypercalcemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.33) | 2 (2.5) | 3 (3.6) |
| Stomatitis | 1 (33.3) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 3 (3.7) | 3 (3.6) |
| Coagulopathy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.33) | 1 (1.2) | 2 (2.4) |
| Hematuria | 1 (33.3) | 0 (0.0) | 1 (3.7) | 0 (0.0) | 2 (2.5) | 2 (2.4) |
| Blood pressure increased | 0 (0.0) | 3 (12.5) | 3 (11.1) | 0 (0.0) | 1 (1.2) | 1 (1.2) |
| Malnutrition | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (1.2) |
| Venous thrombosis limb | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (1.2) |
Data cutoff: March 31, 2023
Adverse events were graded based on National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0
aDose modification includes dose reduction and/or drug interruption for sitravatinib, and dose delay and/or interruption for tislelizumab
ALT alanine aminotransferase, AP alkaline phosphatase, AST aspartate aminotransferase, CPK creatine phosphokinase, GGT gamma glutamyltransferase, LDH lactate dehydrogenase, MB, myocardial band, PPE palmar-plantar erythrodysesthesia, TRAE treatment-related adverse event, WBC white blood cell
The majority (n = 81/84) of patients treated with sitravatinib plus tislelizumab received the sitravatinib 120 mg QD dose, of whom 90.1% (n = 73/81) experienced a TRAE; 90.1% of patients had events that were considered related to sitravatinib, and 82.7% had events considered related to tislelizumab (Table 2). Approximately half of all patients treated with sitravatinib 120 mg QD plus tislelizumab 200 mg Q3W (49.4%; n = 40/81) reported grade ≥ 3 TRAEs considered related to sitravatinib; 28.4% (n = 23/81) reported grade ≥ 3 TRAEs considered related to tislelizumab. Fifteen (18.5%) serious TRAEs considered related to sitravatinib and 11 (13.6%) serious TRAEs considered related to tislelizumab were reported. Eight (9.9%) TRAEs led to treatment discontinuation and 51 (63.0%) to treatment modification (Table 2).
Across all patients treated with sitravatinib plus tislelizumab, the most common TRAEs (occurring in > 30.0% of patients) were proteinuria, alanine aminotransferase increased, aspartate aminotransferase increased, palmar-plantar erythrodysesthesia syndrome, hypertension, and diarrhea (Table 2); the most common grade ≥ 3 TRAEs (occurring in > 5.0% of patients) were hypertension, palmar-plantar erythrodysesthesia syndrome, and platelet count decreased.
In total, three (3.7%) patients treated with sitravatinib 120 mg QD plus tislelizumab reported an infusion-related reaction (IRR), one of whom reported a grade ≥ 3 IRR (1.2%), leading to treatment discontinuation. Immune-mediated adverse events (imAEs) occurred in 36.9% (n = 31/84) of patients receiving sitravatinib plus tislelizumab (80 mg QD, 33.3% [n = 1/3]; 120 mg QD, 37.0% [n = 30/81]) (Supplementary Table S2). Overall, 29.8% (n = 25/84) and 35.7% (n = 30/84) of patients receiving sitravatinib plus tislelizumab reported imAEs that were considered related to sitravatinib and tislelizumab, respectively. Two patients (2.4%) treated with sitravatinib plus tislelizumab reported imAEs that led to treatment discontinuation, and six (7.1%) reported imAEs that led to treatment modification, comprising rash (n = 3), hyperthyroidism (n = 1), hypothyroidism (n = 1), and immune-mediated encephalitis (n = 1).
Twelve patients experienced TEAEs leading to death in the sitravatinib 120 mg QD plus tislelizumab group, fatal TEAEs that occurred in ≥ 2 patients included death (7 patients, 8.6%, reported terms were all unexplained death) and respiratory failure (2 patients, 2.5%). Four fatal TEAEs were considered to be treatment related; one TRAE of respiratory failure (related to both sitravatinib and tislelizumab) and three TRAEs of death due to unknown reasons (one fatal event with missing assessment of causality was reported as the preferred term of ‘death’ [reported term: unexplained death] and counted as treatment related). All TEAEs are summarized in Supplementary Table S3; all TRAEs are summarized in Supplementary Table S4.
Efficacy
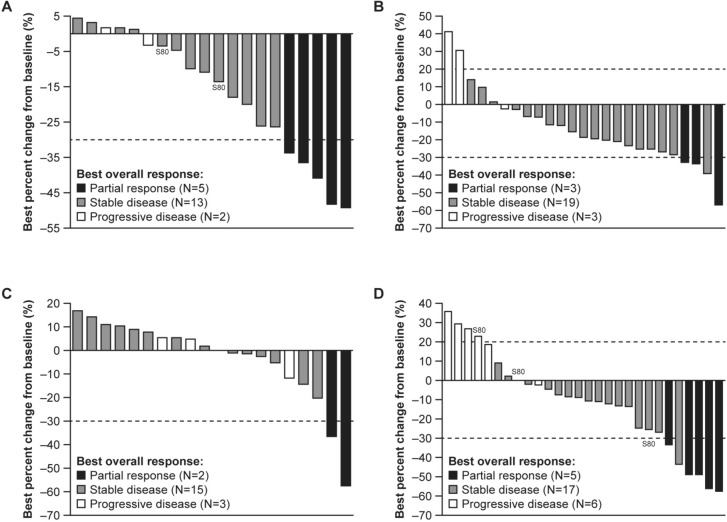
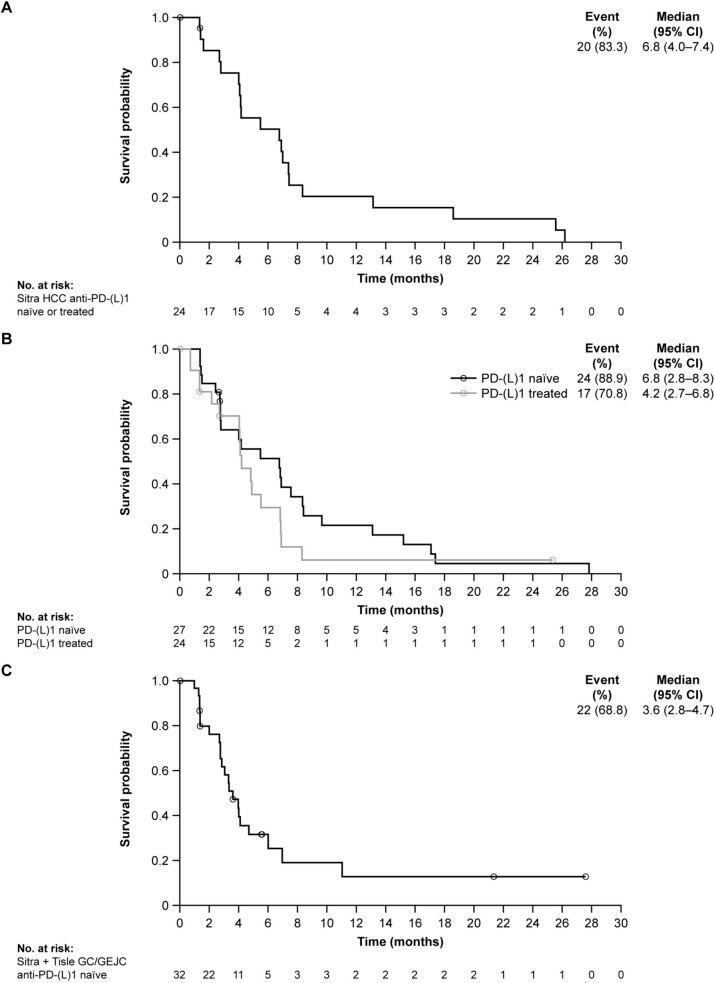
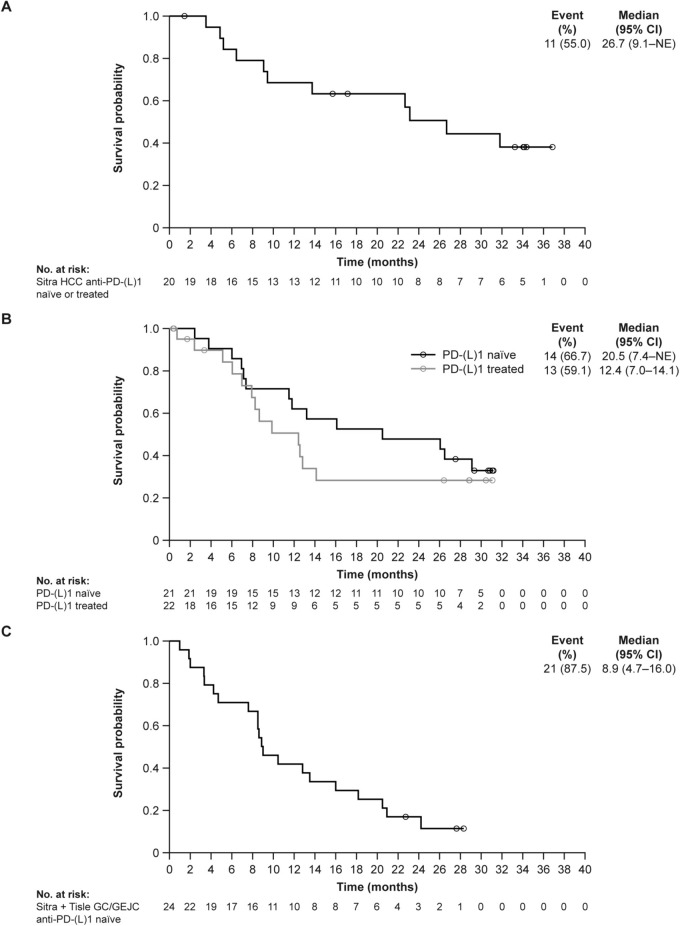
In the efficacy-evaluable set, the confirmed ORR with sitravatinib monotherapy was 25.0% (95% CI 8.7–49.1) in patients with anti-PD-(L)1–naïve or anti-PD-(L)1–treated advanced HCC (Table 3); all responses were PRs. Best percent change from baseline in target lesion sum of diameters is shown in Fig. 2A. Disease control was achieved in 90.0% (95% CI 68.3–98.8) of these patients, and median DoR was 7.7 months (95% CI 2.8–not estimable [NE]). In the safety analysis set (phases I and II combined), median PFS was 6.8 months (95% CI 4.0–7.4) (Fig. 3A). In the safety analysis set (phase II only, n = 20), during a median follow-up of 34.1 months, median OS was determined to be 26.7 months (95% CI 9.1–NE) (Fig. 4A).
Table 3.
Analysis of confirmed disease response based on investigator-assessment per RECIST v1.1 (efficacy evaluable analysis set from phase I and phase II)
| Indication | HCC | GC/GEJC | |||
|---|---|---|---|---|---|
| Treatment | Sitravatinib monotherapy | Sitravatinib plus tislelizumab | Sitravatinib plus tislelizumab | ||
| Prior anti-PD-(L)1 treatment status | Naïve or treated (n = 20) | Naïve (n = 26) | Treated (n = 21) | Subtotal (n = 47) | Naïve (n = 31) |
|
ORR, n (%) 95% CI |
5 (25.0) 8.7–49.1 |
3 (11.5) 2.4–30.2 |
2 (9.5) 1.2–30.4 |
5 (10.6) 3.5–23.1 |
5 (16.1) 5.5–33.7 |
| Best overall response, n (%) | |||||
| CR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PR | 5 (25.0) | 3 (11.5) | 2 (9.5) | 5 (10.6) | 5 (16.1) |
| SD | 13 (65.0) | 19 (73.1) | 15 (71.4) | 34 (72.3) | 17 (54.8) |
| PD | 2 (10.0) | 3 (11.5) | 3 (14.3) | 6 (12.8) | 6 (19.4) |
| Not determineda | 0 (0.0) | 1 (3.8) | 1 (4.8) | 2 (4.3) | 3 (9.7) |
|
DCR, n (%) 95% CI |
18 (90.0) 68.3–98.8 |
22 (84.6) 65.1–95.6 |
17 (81.0) 58.1–94.6 |
39 (83.0) 69.2–92.4 |
22 (71.0) 52.0–85.8 |
|
Median DoR, months 95% CI |
7.7 2.8–NE |
5.7 4.1–NE |
NR 5.4–NE |
5.7 4.1–NE |
5.5 2.7–NE |
|
CBR, n (%) 95% CI |
11 (55.0) 31.5–76.9 |
12 (46.2) 26.6–66.6 |
6 (28.6) 11.3–52.2 |
18 (38.3) 24.5–53.6 |
10 (32.3) 16.7–51.4 |
Data cutoff: March 31, 2023
aDue to no post-baseline response assessment. Data from phase I and phase II were pooled per the tumor characteristics; four patients with GC/GEJC in phase I were excluded because they could not be mapped to any indication group, either due to unknown prior anti-PD-(L)1 treatment status (n = 1) or because they received sitravatinib monotherapy (n = 3)
CBR clinical benefit rate, CI confidence interval, CR complete response, DCR disease control rate, DoR duration of response, GC/GEJC gastric cancer/gastroesophageal junction cancer, HCC hepatocellular carcinoma, NE not estimable, NR not reached, ORR objective response rate, PD progressive disease, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, SD stable disease
Fig. 2.
Best percent change from baseline in target lesion sum of diameters by best confirmed overall response (efficacy-evaluable population from phase I and phase II). Best percent change from baseline in target lesion sum of diameters in A sitravatinib-treated patients with anti-PD-(L)1–naïve or –treated HCC; B sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–naïve HCC; C sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–treated HCC; D sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–naïve GC/GEJC. Responses are investigator-assessed based on RECIST v1.1. GC/GEJC gastric cancer/gastroesophageal junction cancer, HCC hepatocellular carcinoma, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, RECIST Response Evaluation Criteria in Solid Tumors
Fig. 3.
Progression-free survival (safety analysis set from phase I and phase II). KM plot of PFS in A sitravatinib-treated patients with anti-PD-(L)1–naïve or –treated HCC; B sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–naïve and –treated HCC; C sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–naïve GC/GEJC. Four patients with GC/GEJC cancer in phase I were excluded, either due to unknown prior anti-PD-(L)1 treatment status or because they received sitravatinib monotherapy. CI confidence interval, GC/GEJC gastric cancer/gastroesophageal junction cancer, HCC hepatocellular carcinoma, KM Kaplan–Meier, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, PFS progression-free survival, Sitra sitravatinib, Tisle tislelizumab
Fig. 4.
Overall survival (safety analysis set from phase II only). KM plot of OS in A sitravatinib-treated patients with anti-PD-(L)1–naïve or –treated HCC; B sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–naïve and –treated HCC; C sitravatinib with tislelizumab–treated patients with anti-PD-(L)1–naïve GC/GEJC. CI confidence interval, GC/GEJC gastric cancer/gastroesophageal junction cancer, HCC hepatocellular carcinoma, KM Kaplan–Meier, NE not estimable, OS overall survival, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, Sitra sitravatinib, Tisle tislelizumab
The ORR with sitravatinib plus tislelizumab was 11.5% (95% CI 2.4–30.2) in patients with anti-PD-(L)1–naïve, pretreated HCC and 9.5% (95% CI 1.2–30.4) in patients with anti-PD-(L)1–treated HCC. Best percent change from baseline in target lesion sum of diameters is shown in Fig. 2B and C, respectively. Disease control was achieved in 84.6% (95% CI 65.1–95.6) and 81.0% (95% CI 58.1–94.6) of these patients, respectively. Median DoR was 5.7 months (95% CI 4.1–NE) in patients with anti-PD-(L)1–naïve HCC. Two patients with anti-PD-(L)1–treated HCC responded; one patient had a DoR of 5.4 months (95% CI 5.4–NE), while for the other patient, response was ongoing at up to 20 months of follow-up. Median PFS was 6.8 months (95% CI 2.8–8.3) and 4.2 months (95% CI 2.7–6.8) in patients with anti-PD-(L)1–naïve and anti-PD-(L)1–treated HCC, respectively (Fig. 3B). In the safety analysis set (phase II only), median OS was 20.5 months (95% CI 7.4–NE) and 12.4 months (95% CI 7.0–14.1), respectively (Fig. 4B), with a median follow-up of 30.9 months and 28.8 months, respectively.
In patients with anti-PD-(L)1–naïve, pretreated GC/GEJC, the ORR with sitravatinib plus tislelizumab was 16.1% (95% CI 5.5–33.7); the DCR was 71.0% (52.0–85.8); the median DoR was 5.5 months (Table 3); and the median PFS was 3.6 months (95% CI 2.8–4.7) (Fig. 3C). Best percent change from baseline in target lesion sum of diameters is shown in Fig. 2D. In the safety analysis set (phase II only, n = 24), median OS was 8.9 months (95% CI 4.7–16.0) (Fig. 4C), with a median follow-up of 27.6 months.
Discussion
Despite recent advances in immunotherapies, there remains unmet medical need for greater tumor control without significantly compromising treatment tolerability. Promising efficacy and manageable tolerability were observed in SAFFRON-104 with sitravatinib monotherapy in a predominantly pretreated, anti-PD-(L)1–naïve patient population with advanced HCC, and in combination with tislelizumab in pretreated patients with anti-PD-(L)1–naïve and anti-PD-(L)1–treated HCC, and pretreated patients with PD-(L)1–naïve GC/GEJC. Phase I determined the RP2D of sitravatinib to be 120 mg QD, both as monotherapy and in combination with tislelizumab. No new safety signals were identified. Of note, this study provides relatively more data for HCC than GC/GEJC.
Sitravatinib has been evaluated in multiple phase I–III clinical studies in advanced and metastatic solid tumors [22, 24–27]. Safety data from these studies have identified frequent but mild-moderate severity (grade < 3) AEs with sitravatinib as monotherapy or in combination with PD-1 inhibitors including tislelizumab [24–27]. In the current study, TRAEs were reported in all patients receiving sitravatinib monotherapy and 90.5% of patients receiving sitravatinib with tislelizumab. Most TRAEs were manageable by dose reduction or interruption and supportive treatment.
The safety profile for sitravatinib as monotherapy is generally consistent with the observed safety profiles of other TKI monotherapies [28, 29]. Furthermore, the safety profile of sitravatinib with tislelizumab in this study is consistent with that reported in the phase Ib SAFFRON-103 study of sitravatinib plus tislelizumab in patients with locally advanced or metastatic NSCLC [22]. Additionally, the safety findings of the combination are in accordance with those of other TKI and PD-1 inhibitor combinations [29, 30]. Common AEs reported here—hypertension, proteinuria, and palmar-plantar erythrodysesthesia syndrome—are also noted within the well-established AE profile of other VEGF inhibitors [31, 32].
In SAFFRON-104, grade ≥ 3 TRAEs occurred in approximately half of patients (51.9% of patients treated with monotherapy; 50.0% with combination therapy); however, few TRAEs led to treatment discontinuation (3.7% and 10.7%, respectively). In a phase II trial of sitravatinib plus nivolumab in patients with non-squamous NSCLC who progressed on or after checkpoint inhibitor therapy, 58.3% of patients experienced grade 3–4 TRAEs, with hypertension occurring in 16.7% of patients (vs. 10.7% in SAFFRON-104) and palmar-plantar erythrodysesthesia syndrome in 2.6% (vs. 6.0% in SAFFRON-104); 14.1% experienced any-grade TRAEs leading to treatment discontinuation (vs. 10.7% in SAFFRON–104) [33].
In the current study, sitravatinib monotherapy demonstrated promising antitumor activity in the second- or third-line setting in a primarily anti-PD-(L)1–naïve HCC population, with a confirmed ORR of 25.0%, the highest ORR with TKI single-agent therapy reported to date in this setting [28, 34]. The encouraging ORR was also successfully transformed into a promising median PFS (6.8 months, n = 24) and median OS (26.7 months, n = 20). Preliminary antitumor activity was also demonstrated with sitravatinib plus tislelizumab, with a moderate ORR and higher median OS observed in patients with anti-PD-(L)1–naïve HCC (11.5% and 20.5 months, respectively) compared with anti-PD-(L)1–treated HCC (9.5% and 12.4 months, respectively). Counterintuitively, the efficacy of sitravatinib monotherapy was comparable with or higher than that of the combination of sitravatinib plus tislelizumab, including a higher ORR and OS and a similar median PFS and DCR. This could possibly be attributed to the limited sample size in each group. Considering the non-randomized design of the current study, and the later initiation of phase II in patients with anti-PD-(L)1–naïve HCC treated with sitravatinib plus tislelizumab (almost 10 months later than for patients treated with sitravatinib monotherapy), there may have been an imbalance in the patient populations’ disease characteristics, despite enrollment under the same inclusion/exclusion criteria. These findings require confirmation in larger cohorts.
While cross-trial comparisons are confounded by differences in factors such as study design, patient characteristics, and sample size, the meaningful and durable responses of sitravatinib monotherapy in patients with advanced HCC shown in SAFFRON-104 are superior to the responses in the phase III RESORCE study, which reported an ORR of 11% and median OS of 10.6 months following treatment with the multikinase inhibitor regorafenib in 379 patients with HCC who progressed during treatment with sorafenib [28]. Furthermore, the durable responses reported in patients with advanced anti-PD-(L)1–naïve HCC receiving combination treatment with sitravatinib plus tislelizumab complement the findings from the KEYNOTE-524 study of lenvatinib with pembrolizumab in patients with unresectable anti-PD-(L)1–naïve HCC [30]. Of 100 patients in KEYNOTE-524, disease control was reported in 86.0% (vs. 84.6% in SAFFRON-104) and median OS was 22.0 months (vs. 20.5 months in SAFFRON-104) [30].
While this study does not provide substantial data for patients with advanced.
anti-PD-(L)1–naïve GC/GEJC, preliminary antitumor activity is reported (ORR, 16.1%; median OS, 8.9 months; median PFS, 3.6 months). Treatment with tislelizumab plus chemotherapy has demonstrated efficacy in patients from a global population with treatment-naïve, advanced GC/GEJC in the phase III RATIONALE-305 study [15, 16], which reported an ORR of 47.3% and median OS of 15.0 months [15, 16]. As VEGFR2 inhibitors, both ramucirumab (anti-VEGFR2 monoclonal antibody) and apatinib (anti-VEGFR2 tyrosine kinase inhibitor) have demonstrated efficacy in patients with pretreated, advanced GC/GEJC, and have been approved either as monotherapy or in combination with paclitaxel in later line settings [35–37]. These data along with the results of the SAFFRON-104 study support the investigation of anti-PD-1 inhibitors and sitravatinib in this setting.
Current treatment options for patients with HCC or GC/GEJC are limited, and there is an urgent need for new therapies [1–4]. Following the approval of atezolizumab in combination with bevacizumab as the new standard of care, the treatment landscape has been rapidly evolving [38]; however, a broader range of therapies is essential to improve overall patient survival [39]. For patients with GC/GEJC, standard-of-care options are associated with low efficacy rates and toxicity with extended use [40]. The results from the SAFFRON-104 study add to the clinical evidence supporting the rationale for anti-PD-(L)1 and multi-targeted TKI combination therapies and further support continued investigation of these combinations in the clinical setting.
Conclusions
Sitravatinib as monotherapy or in combination with tislelizumab was generally well tolerated and demonstrated preliminary antitumor activity with durable responses in patients with unresectable, locally advanced, or metastatic HCC and GC/GEJC. The results from this phase Ib/II study support further investigation of sitravatinib as monotherapy and in combination with tislelizumab as a potential treatment option for patients with unresectable, locally advanced, or metastatic HCC and GC/GEJC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the study participants, investigators, and site staff for their contributions to the study. Medical writing support, under the direction of the authors, was provided by Gemma Walker, BSc, of Ashfield MedComms, an Inizio company, and Steven Moore, PhD, and Camile Semighini Grubor, PhD, of Envision Pharma Group and was funded by BeiGene, Ltd.
Abbreviations
- AE
Adverse event
- ALT
Alanine aminotransferase
- AP
Alkaline phosphatase
- AST
Aspartate aminotransferase;
- BCLC
Barcelona clinic liver cancer
- CBR
Clinical benefit rate
- CI
Confidence interval
- CPK
Creatine phosphokinase;
- CR
Complete response
- DCR
Disease control rate
- DLT
Dose-limiting toxicity
- DoR
Duration of response
- ECOG
Eastern cooperative oncology group
- Fc
Fragment crystallizable
- GC/GEJC
Gastric cancer/gastroesophageal junction cancer
- GGT
Gamma glutamyltransferase
- HCC
Hepatocellular carcinoma
- ImAE
Immune adverse event
- KM
Kaplan–Meier
- LDH
Lactate dehydrogenase
- MB
Myocardial band
- MET
Mesenchymal epithelial transition factor receptor
- NE
Not estimable
- NR
Not reached
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death-ligand 1
- PFS
Progression-free survival
- PPE
Palmar-plantar erythrodysesthesia
- PR
Partial response
- PS
Performance status
- Q3W
Once every 3 weeks
- QD
Once daily
- RECIST v1.1
Response evaluation criteria in solid tumors version 1.1
- RP2D
Recommended phase II dose
- SD
Stable disease
- TAM
TYRO3, AXL, MER
- TC
Tumor cell
- TEAE
Treatment-emergent adverse event
- TKI
Tyrosine kinase inhibitor
- TME
Tumor microenvironment
- TRAE
Treatment-related adverse event
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- WBC
White blood cell
Author contribution
Conceptualization and study design: Jin Li, Xin Li, Fan Yu, Juan Zhang, Zhang Zhang, Shukui Qin. Acquisition of data: Jin Li, Yuxian Bai, Zhendong Chen, Jieer Ying, Yabing Guo, Weijia Fang, Feng Zhang, Jianping Xiong, Tao Zheng, Zhiqiang Meng, Jingdong Zhang, Zhenggang Ren, Chunyi Hao, Yajin Chen, Xiaoyan Lin, Hongming Pan, Fuxiang Zhou, Shukui Qin. Data analysis: Xin Li, Fan Yu, Juan Zhang, Zhang Zhang. Data interpretation: Jin Li, Xin Li, Fan Yu, Juan Zhang, Zhang Zhang, Shukui Qin. Writing––original draft: All authors. Writing––review & editing: All authors. Funding acquisition: BeiGene, Ltd.
Funding
This study was sponsored by BeiGene, Ltd.
Data availability
On request, and subject to certain criteria, conditions, and exceptions, BeiGene, Ltd., will provide access to individual de-identified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. BeiGene will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data requests may be submitted to DataDisclosure@beigene.com.
Declarations
Conflict of interest
Jin Li reports payments or honoraria for consulting or speakers bureaus from ArteMed Hospital, Novartis, HengRui, BI, and Lilly; Jieer Ying and Hongming Pan report stocks or shares in BeiGene (institutional relationship with financial interest); Zhenggang Ren reports a consulting or advisory role with AstraZeneca, Roche, and Merck Sharp & Dohme; Zhang Zhang and Juan Zhang report financial relationships with BeiGene; Xin Li and Fan Yu report full-time employment by BeiGene; Yuxian Bai, Zhendong Chen, Yabing Guo, Weijia Fang, Feng Zhang, Jianping Xiong, Tao Zhang, Zhiqiang Meng, Jingdong Zhang, Chunyi Hao, Yajin Chen, Xiaoyan Lin, Fuxiang Zhou, and Shukui Qin have no potential conflict of interest to report.
Ethical approval and consent to participate
This study was performed in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and the principles of informed consent. Written informed consent was obtained from each patient prior to screening. The protocol was approved by an independent ethics committee prior to initiation.
Consent for publication
Not applicable.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akinyemiju T, Abera S et al (2017) The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease study 2015. JAMA Oncol 3:1683–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, Wei W, Lemmens V, Soerjomataram I (2022) Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer 161:108–118. 10.1016/j.ejca.2021.11.023 [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S (2017) Gastric adenocarcinoma. Nat Rev Dis Primers 3:17036. 10.1038/nrdp.2017.36 [DOI] [PubMed] [Google Scholar]
- 4.Casamayor M, Morlock R, Maeda H, Ajani J (2018) Targeted literature review of the global burden of gastric cancer. ecancer 12:883. 10.3332/ecancer.2018.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma W, Xue R, Zhu Z, Farrukh H, Song W, Li T, Zheng L, Pan CX (2023) Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp Hematol Oncol 12:10. 10.1186/s40164-023-00372-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q, Hong Z, Zhang C, Wang L, Han Z, Ma D (2023) Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther 8:320. 10.1038/s41392-023-01522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Zhang Y, Zhang M et al (2023) Combined immunotherapy for hepatocellular carcinoma: how to maximize immune checkpoint blockade synergic anti-tumor effect. Crit Rev Oncol Hematol 189:104070. 10.1016/j.critrevonc.2023.104070 [DOI] [PubMed] [Google Scholar]
- 8.Karim F, Amin A, Liu M, Vishnuvardhan N, Amin S, Shabbir R, Swed B, Khan U (2023) Role of checkpoint inhibitors in the management of gastroesophageal cancers. Cancers 15:4099. 10.3390/cancers15164099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P et al (2019) β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov 9:1124–1141. 10.1158/2159-8290.CD-19-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxter MA, Middleton F, Cagney HP, Petty RD (2021) Resistance to immune checkpoint inhibitors in advanced gastro-oesophageal cancers. Br J Cancer 125:1068–1079. 10.1038/s41416-021-01425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minchom A, Popat S (2023) Sitravatinib and acquired immune checkpoint inhibitor resistance: a gem for the future? J Thorac Oncol 18:830–833. 10.1016/j.jtho.2023.03.023 [DOI] [PubMed] [Google Scholar]
- 12.Lu L, Zhan M, Li XY et al (2022) Clinically approved combination immunotherapy: current status, limitations, and future perspective. Curr Res Immunol 3:118–127. 10.1016/j.crimmu.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang T, Song X, Xu L et al (2018) The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 67:1079–1090. 10.1007/s00262-018-2160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S, Kudo M, Meyer T et al (2023) Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a phase 3 randomized clinical trial. JAMA Oncol 9:1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moehler MH, Kato K, Arkenau H-T et al (2023) Rationale 305: Phase 3 study of tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. 10.1200/JCO.2023.41.4_suppl.28637713657 [Google Scholar]
- 16.Xu RH, Oh DY, Kato K et al (2023) LBA80 Tislelizumab (TIS) plus chemotherapy (chemo) vs placebo (PBO) plus chemo as first-line (1L) treatment of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC): final analysis results of the RATIONALE-305 study. Ann Oncol 34:S1320–S1321. 10.1016/j.annonc.2023.10.081 [Google Scholar]
- 17.Du W, Huang H, Sorrelle N, Brekken RA (2018) Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight 3:e124184. 10.1172/jci.insight.124184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akalu YT, Rothlin CV, Ghosh S (2017) TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev 276:165–177. 10.1111/imr.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garton AJ, Seibel S, Lopresti-Morrow L et al (2017) Anti-KIT monoclonal antibody treatment enhances the antitumor activity of immune checkpoint inhibitors by reversing tumor-induced immunosuppression. Mol Cancer Ther 16:671–680. 10.1158/1535-7163.MCT-16-0676 [DOI] [PubMed] [Google Scholar]
- 20.Pircher A, Wolf D, Heidenreich A, Hilbe W, Pichler R, Heidegger I (2017) Synergies of targeting tumor angiogenesis and immune checkpoints in non-small cell lung cancer and renal cell cancer: from basic concepts to clinical reality. Int J Mol Sci 18:2291. 10.3390/ijms18112291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliva M, Chepeha D, Araujo DV et al (2021) Antitumor immune effects of preoperative sitravatinib and nivolumab in oral cavity cancer: SNOW window-of-opportunity study. J Immunother Cancer 9:e003476. 10.1136/jitc-2021-003476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Yu X, Huang D et al (2023) SAFFRON-103: a phase 1b study of the safety and efficacy of sitravatinib combined with tislelizumab in patients with locally advanced or metastatic non-small cell lung cancer. J Immunother Cancer 11:e006055. 10.1136/jitc-2022-006055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer T, Cho BC, Heist R et al (2022) First-in-human phase 1/1b study to evaluate sitravatinib in patients with advanced solid tumors. Invest New Drugs 40:990–1000. 10.1007/s10637-022-01274-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Msaouel P, Siefker-Radtke AO, Sweis R et al (2020) 705MO Sitravatinib (sitra) in combination with nivolumab (nivo) demonstrates clinical activity in checkpoint inhibitor (CPI) naïve, platinum-experienced patients (pts) with advanced or metastatic urothelial carcinoma (UC). Ann Oncol 31:S556. 10.1016/j.annonc.2020.08.777 [Google Scholar]
- 25.Doshi GK, Vogelzang NJ, Richards DA et al (2019) Phase II study of sitravatinib in combination with nivolumab in patients with advanced or metastatic urothelial carcinoma (UC) after checkpoint inhibitor therapy (CIT). J Clin Oncol 37:TPS498. 10.1200/JCO.2019.37.7_suppl.TPS498 [Google Scholar]
- 26.Goh J, Coward J, Gao B et al (2021) 153P Safety/tolerability and antitumor activity of sitravatinib plus tislelizumab (TIS) in patients with advanced platinum-resistant ovarian cancer (PROC). Ann Oncol 32:S1446–S1447. 10.1016/j.annonc.2021.10.172 [Google Scholar]
- 27.Liu X, Peng T, Liu C, Wang J, Zhu G, Zhang X (2022) 39TiP A phase II study of tislelizumab (TIS) plus sitravatinib as adjuvant therapy in patients with hepatocellular carcinoma (HCC) at high risk of recurrence after surgical resection. Ann Oncol 33:S1444. 10.1016/j.annonc.2022.10.049 [Google Scholar]
- 28.Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 29.Kelley RK, Rimassa L, Cheng AL et al (2022) Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 23:995–1008. 10.1016/S1470-2045(22)00326-6 [DOI] [PubMed] [Google Scholar]
- 30.Finn RS, Ikeda M, Zhu AX et al (2020) Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 38:2960–2970. 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidinger M (2013) Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl 11:172–191. 10.1016/j.ejcsup.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadows KL, Rushing C, Honeycutt W, Latta K, Howard L, Arrowood CA, Niedzwiecki D, Hurwitz HI (2015) Treatment of palmar-plantar erythrodysesthesia (PPE) with topical sildenafil: a pilot study. Support Care Cancer 23:1311–1319. 10.1007/s00520-014-2465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He K, Berz D, Gadgeel SM et al (2023) MRTX-500 phase 2 trial: sitravatinib with nivolumab in patients with nonsquamous NSCLC progressing on or after checkpoint inhibitor therapy or chemotherapy. J Thorac Oncol. 18:907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S, Li Q, Gu S et al (2021) Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 6:559–568. 10.1016/S2468-1253(21)00109-6 [DOI] [PubMed] [Google Scholar]
- 35.Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 36.Fuchs CS, Tomasek J, Yong CJ et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Qin S, Xu J et al (2016) Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 34:1448–1454. 10.1200/JCO.2015.63.5995 [DOI] [PubMed] [Google Scholar]
- 38.Zhu AX, Abbas AR, de Galarreta MR et al (2022) Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med 28:1599–1611. 10.1038/s41591-022-01868-2 [DOI] [PubMed] [Google Scholar]
- 39.Galle PR, Dufour JF, Peck-Radosavljevic M, Trojan J, Vogel A (2021) Systemic therapy of advanced hepatocellular carcinoma. Future Oncol 17:1237–1251. 10.2217/fon-2020-0758 [DOI] [PubMed] [Google Scholar]
- 40.Taieb J, Moehler M, Boku N, Ajani JA, Yañez Ruiz E, Ryu MH, Guenther S, Chand V, Bang YJ (2018) Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: current status and future perspectives. Cancer Treat Rev 66:104–113. 10.1016/j.ctrv.2018.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
On request, and subject to certain criteria, conditions, and exceptions, BeiGene, Ltd., will provide access to individual de-identified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. BeiGene will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data requests may be submitted to DataDisclosure@beigene.com.