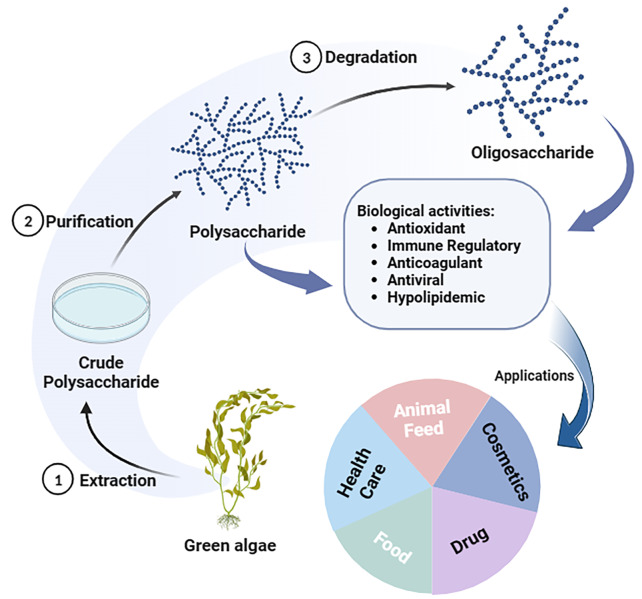
Abstract
Abstract
With the proceeding of global warming and water eutrophication, the phenomenon of green tide has garnered significant societal interest. Consequently, researchers had increasingly focused on the potential applications of green algae biomass, particularly its polysaccharides. The polysaccharide serves as the primary active constituent of green algae and has demonstrated numerous advantageous biological activities, including antioxidant, antiviral, anticoagulant, hypolipidemic and immuno-modulatory activities. The favorable bioavailability and solubility of green algae oligosaccharides are attributed to their low molecular weight. So there has been a growing interest in researching green algae polysaccharides and oligosaccharides for the utilization of marine biological resources. This review summarized the extraction, purification, chemical structure, composition, biological activity, and potential applications prospect of polysaccharides and oligosaccharides derived from green algae. The review could be helpful for expanding the applications of polysaccharides and oligosaccharides of green algae.
Graphical Abstract
Keywords: Green algae polysaccharide, Oligosaccharide, Structure, Extraction, Activity
Introduction
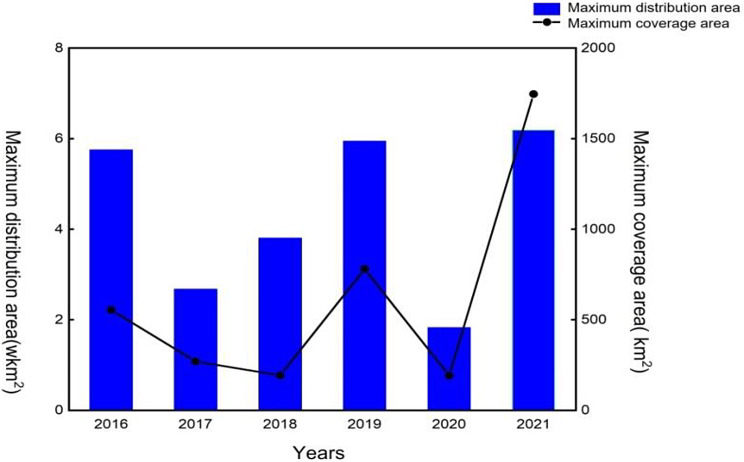
With over 70% of the Earth’s surface being covered by oceans and approximately half of total global biodiversity consisting of marine species, marine organisms serve as valuable sources of natural products possessing diverse biological activities and structures (Aneiros et al. 2004, Kim et al. 2010, Wijesekara et al. 2011). Common algae found in the sea include brown algae, red algae, cyanobacteria, and green algae, many of which are edible and offer high nutritional value. Examples of edible algae include Laminaria japonica (brown algae), Gelidium amansii Lamouroux and Chondrus ocellatus Aolmes (red algae) (Wijesekara et al. 2011), and Ulva and Enteromorpha (green algae). Brown and red algae, specifically carrageenan and alginate, are extensively utilized, whereas research on green algae has been comparatively limited. In recent years, occurrences of “green tides” along coastlines have been frequently observed, primarily attributed to the proliferation of Ulva and Enteromorpha (Li et al. 2016b, c). This ecological anomaly is characterized by the rapid growth or aggregation of large green algae, detached from their original substrate, forming floating clusters. If not treated in time, the consequences of this issue include the mortality of marine animals, depletion of oxygen in algae and seawater, and deterioration of seagrass habitats. Additionally, it will have significant impacts on the economic and ecological well-being of coastal cities (Fan et al. 2022). Against the backdrop of offshore eutrophication and global warming, the escalation of green tide occurrences is of great concern (Smetacek et al. 2013, Van Alstyne et al. 2015; Wang et al. 2015; Zhang et al. 2015; Coelho et al. 2016, Gao et al. 2017) (Fig. 1). Therefore, there is an urgent need to dig deep into the biological resource potential of green algae and effectively harness these resources.
Fig. 1.
Changes of green tide distribution area and coverage area in the Yellow Sea in recent years
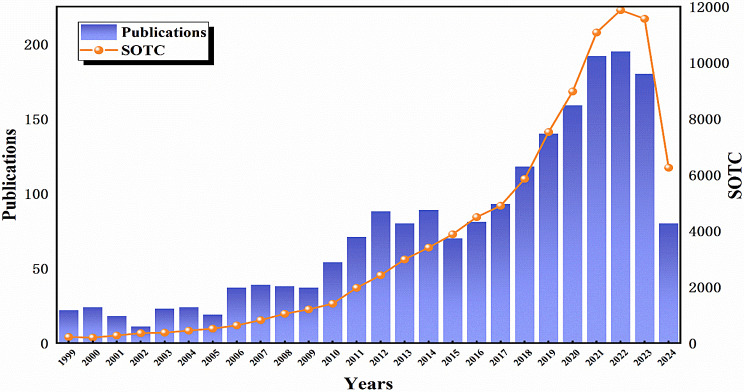
At present, brown algae and red algae have been extensively cultivated and industrialized among the four major types of seaweed. In contrast, green algae, despite being the most diverse type, has not been widely exploited and utilized, with only select high-yield varieties being utilized for purposes such as feed, bait, and fertilizer. Green algae are rich in nutritional value, boasting low fat content and abundant protein, cellulose, and trace elements. Additionally, it contains over ten types of polyunsaturated fatty acids, including linoleic acid, linolenic acid, palmitic acid, and arachidonic acid. As research on green algae continues to advance, its utilization across various industries, including industry, agriculture, medicine, and food, has become increasingly prevalent (Muhamad et al. 2019; Rial-Hermida et al. 2021; Srivastava et al. 2021; Vinchhi et al. 2021) (Fig. 2). Green algae, serving as a valuable source of raw materials for the development of feed, food, and pharmaceutical products, are primarily composed of green algae polysaccharides. The green algae polysaccharides have garnered significant attention due to their biocompatibility, low toxicity, mini-mal side effects, natural residue free properties, non-polluting nature, and lack of drug resistance (Wassie et al. 2021). A comprehensive search of the Web of Science database (www.webofscience.com, accessed on 31 July 2024) utilizing the keyword “green algae polysaccharides” resulted in the identification of 2,179 publications. Notably, the majority of these publications were released post-2010 (Fig. 3). Green algae polysaccharides are abundant in green algae, exhibiting diverse components and structures that contribute to various biological activities such as anti-coagulation, anti-viral, immune regulation, lipid-lowering, anti-radiation, anti-oxidation, and anti-tumor properties (Abd-Ellatef et al. 2017; Fournière et al. 2019; Kidgell et al. 2019; Chi et al. 2020b; Jiang et al. 2020; Klongklaew et al. 2020; Ponce et al. 2020). The green algae oligosaccharides, derived from the degradation of polysaccharides, not only retain the biological activities of polysaccharides but also enhances solubility and bioavailability. Currently, Ulva and Enteromorpha are the primary species of green algae that have been extensively studied for their polysaccharides. In recent years, there has been a growing interest among scholars in the study of green algae polysaccharides and oligosaccharides. This review examines the chemical composition, structure, separation and purification methods, and biological activities of green algae polysaccharides and oligosaccharides in nature.
Fig. 2.
The morphology of some representative species of green algae. (A). Ulva lactuca; (B). Enteromorpha prolifera; (C). Monostroma nitidum; (D). Chlorella vulgaris
Fig. 3.
Number of publications and SOTC on green algae polysaccharides from 1999
Green algae polysaccharide
Chemical composition
Green algae polysaccharides are predominantly found in the cell interstitium and cell wall (Bobin-Dubigeon et al. 1997), primarily consisting of water-soluble sulfated polysaccharides. The chemical composition of green algae polysaccharides is influenced by the species of green algae utilized for extraction, the environmental conditions in which the algae are grown, and the time of harvest, resulting in a complex composition (Li et al. 2017; Zhong et al. 2020). Ulvan is a polysaccharide derived from green algae, predominantly consisting of glucuronic acid (GlcA), iduronic acid (IdoA), 3-sulfated rhamnose (Rha3S), and minor amounts of xylose (Xyl) (Ulaganathan et al. 2017). It exhibits low concentrations of mannose, galactose, and arabinose (Guidara et al. 2019; Jmel et al. 2019). Kidgella et al. (Kidgell et al. 2021) conducted an analysis of the monosaccharide compositions of different sources of Ulva and observed significant differences in the polysaccharides derived from leafy and filamentous Ulva. Specifically, the polysaccharides from these sources were found to be primarily composed of rhamnose, with glucuronic acid also present. Notably, the proportion of iduronic acid in the polysaccharides of filamentous Ulva was generally lower (approximately 7 mol%) compared to leafy Ulva (approximately 14 mol%). In addition to this, the polysaccharides of U. ralfsii exhibited a galactose proportion of 16 mol%, a significantly higher value compared to other Ulva species. The harvest time of Ulva also affects the chemical composition of the polysaccharides. Samarasinghea et al. (Samarasinghe et al. 2021) analyzed the monosaccharide composition of Ulva at different harvest times, revealing consistent main components but varying content levels. For instance, the dry matter content ratios of rhamnose, xylose, galactose, glucose and uronic acid in ulvan collected in June and August were 3.65:0.43:0.41:0.32:0.62 and 0.84:0.33:0.22:0.75:1.92, respectively. It is evident that ulvan harvested in August exhibit higher levels of glucose and uronic acid compared to those harvested in June. Moreover, the growing conditions also have an impact on the chemical composition of ulvan. Olsson et al. (Olsson et al. 2020) studied the effects of cultivation conditions, such as temperature, irradiance, pCO2, nitrogen, and phosphate, on the monosaccharide composition of ulvan. Their findings revealed that lower sulfate concentrations and high temperatures could promote an increase in monosaccharide content, while increased irradiance and temperature levels were associated with higher concentrations of rhamnose and iduronic acid.
Enteromorpha, as a natural resource, is abundant in nutrients, comprising essential components such as carbohydrates (43–51%), proteins (26–33%), fats (0.2–0.8%), total amino acids (20.26–23.32%), ash (13–14%), and iron (1.1–3.4 mg/g) (Li et al. 2018a; Zhang et al. 2019). Enteromorpha polysaccharides (EPs) primarily consist of water-soluble sulfate polysaccharides, comprising of rhamnose, xylose, mannose, galactose and glucose interconnected by glycosidic bonds (Tang et al. 2013; Li et al. 2017; Jiang et al. 2021). Qi et al. (Qi et al. 2012, 2013) studied the chemical composition of polysaccharides in different species of Enteromorpha (E. linza, E. prolifera and E. clathrata), revealing distinct differences in the chemical composition of these polysaccharides. Specifically, the polysaccharides of E. linza and E. prolifera were mainly composed of rhamnose, whereas those of E. clathrata contained primarily arabinose and galactose, along with minor quantities of rhamnose, fucose, and xylose. This indicated that significant variations in polysaccharide composition among different species of Enteromorpha. Ji et al. (Ji et al. 2009) analyzed the chemical composition of polysaccharides in E. clathrata, revealing the presence of rhamnose, glucuronic acid, and iduronic acid, but not mannose. This observation indicated a notable distinction in the polysaccharide components of E. clathrata during the outbreak stage compared to normal growth conditions. Additionally, Shi et al. investigated the monosaccharide composition of polysaccharides extracted from E. clathrata at different time points, finding consistent types of monosaccharides across samples, but differing levels of content. However, due to the complexity of their composition and structure, the conformational relationships of EPs are mostly unknown (Yu et al. 2017).
It could be seen that the chemical composition of green algae would vary with the different species of green algae, growth environment and harvest time, a phenomenon commonly observed in the analysis of algae polysaccharides. (Benslima et al. 2021).
Structure
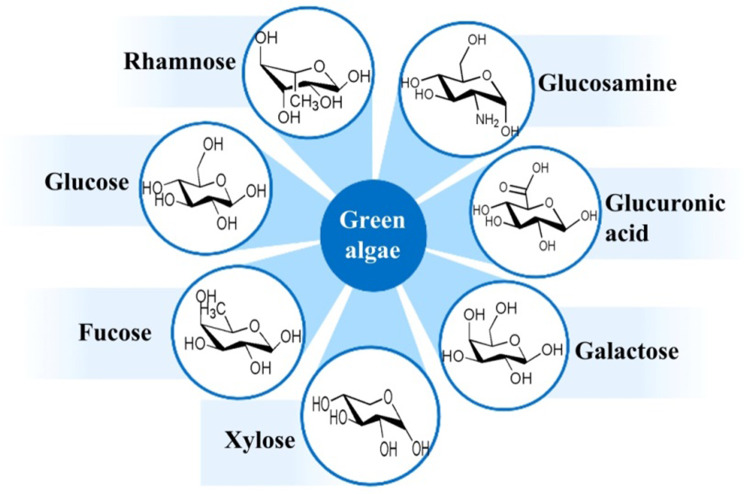
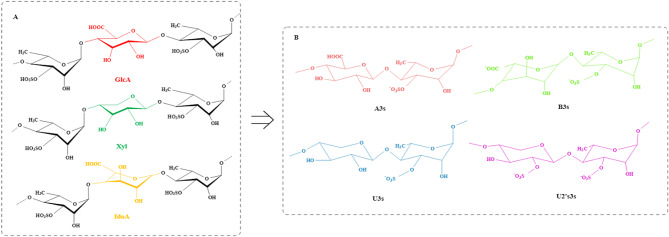
The structural complexity of green algae polysaccharides surpasses that of polysaccharides found in brown and red algae, attributable to the intricate composition of monosaccharides, the diverse glycosidic linkages between these monosaccharides, and the extensive array of structural and motif modifications within their branched configurations (Yang et al. 2011; Stender et al. 2019). The structure of polysaccharides differs among various algae species (Fig. 4) (Table 1), with ulvan being the most extensively studied polysaccharide structure in green algae. Lahaye et al. (Lahaye et al. 2007) studied the structure of ulvan derived from U. regida and founded the main disaccharide repeat structure of ulvan was A3s [→4)β-D-GlcA(1→4)-α-L-Rha3S(1→] and B3s [→4)α-L-IdoA(1→4)-α-L-Rha3S(1→]. In addition, structural analysis identified the presence of U3s [→4)β-D-Xyl(1→4)-α-L-Rha3S(1→] and U2’s,3s [→4)β-D-Xyl2S(1→4)-α-L-Rha3S(1→] as two repeat disaccharide units. The structural characterization of ulvan can be further elucidated through the application of ulvan lyase, an endonuclease isolated from marine bacteria. Ulvan lyases catalyze the cleavage of the β-(1→4)-glycosidic bond between Rha3S and GlcA or IdoA via a β-elimination mechanism, resulting in the formation of oligosaccharides that contain unsaturated uronic acid (∆GlcA) (Collén et al. 2011; Gao et al. 2019). These oligosaccharides exhibit repeating units such as -A3s-A3s-, -A3s-B3s-, -A3s-U3s-, and -A3s-GlcA-A3s. Chi et al. (2020a) employed ulvan lyase to degrade polysaccharides derived from U. clathrata, resulting in three distinct degradation products with varying molecular weights, designated as UO-1, UO-2, and UO-3. Structural analysis was conducted on the higher molecular weight component, UO-3, revealing that it predominantly consists of A3s-type and U3s-type disaccharide repeating units, with the presence of U2’s,3s-type disaccharide repeating units also identified. It can be seen that ulvan exhibits a complex structure predominantly comprising disaccharide repeating units of the A3s-type or B3s-type, with a minor presence of U3s-type or U2’s,3s-type disaccharide repeating units, as shown in Fig. 5. The main chain of ulvan consists primarily of residues connected by α-(1→4)- and β-(1→4)- linkages. The branch was situated at the O-2 position of rhamnose, while the sulfated group was positioned at the C-3 position of rhamnose (Lahaye et al. 2007, Thu et al. 2015; Tziveleka et al. 2019). Among them, the A3s type ulvan consisted of glucuronic acid linked to rhamnose via 1→4 glycosidic bonds, with rhamnose further connected to glucuronic acid through 1→4 glycosidic bonds to constitute the main chain. Modification at the C-3 position of rhamnose involved the addition of a sulfate group, with potential branches occurring at the C-2 position. This structural configuration represented the predominant disaccharide unit in ulvan. Substitution glucuronic acid for iduronic acid was another B3s type of ulvan. Physicochemical property analysis showed that ulvan was a semicrystalline polymer structure devoid of a triple helix configuration (Gao et al. 2020). The unique chemical composition of ulvan results in its disordered conformation, a characteristic that is heavily influenced by the species and growth environment of the green algae from which it is derived.
Fig. 4.
The main monosaccharide composition of green algae polysaccharides
Table 1.
The monosaccharide composition and structure of different types of green algae polysaccharides
| Species | Molecular mass/ Da | Structural features | Monosaccharide composition | Ref |
|---|---|---|---|---|
| Ulva pertusa | 23.6 × 104 | 8.3% sulfate | Rhamnose: Xylose: Arabinose: Galactose: Glucose = 5.5: 3.8: 0.7: 0.3: 0.3 | (Gao et al. 2020) |
| Ulva pertusa | 14.4 × 104 | 19.9% sulfate | Rhamnose: Xylose: Glucose: Glucuronic acid: Fucose = 31.3: 19.9: 6.7: 5.5: 0.5 | (Wan et al. 2022) |
| Ulva pertusa | 37.7 × 104 | 26.0% sulfate | Arabinose: Galactose: Glucuronic acid: Mannuronic acid = 0.80: 0.30: 1.80: 6.80 | (Han et al. 2021) |
| Ulva fasciata | 2.9 × 104 | 11.7% sulfate | Rhamnose: Xylose: Glucose = 17.1: 9.9: 10.7 | (Shao et al. 2013) |
| Ulva lactuca | 46.6 × 104 | 23.7% total sugar content | Glucose: Arabinose: Xylose: Mannose: Sorlose = 1.9: 0.5: 0.3: 6.7: 0.5 | (He et al. 2016) |
| Ulva armoricana | 14.0 × 104 ~ 50.0 × 104 | 14.3%~19.1% sulfate | Rhamnose: Galactose: Glucose: Xylose = 40.0: 6.7: 26.2: 4.4 | (Hardouin et al. 2016) |
| Enteromorpha prolifera | 0.4 × 104 | 9.0% sulfate | Mannose: Rhamnose: Glucuronic acid: Galacturonic acid: Glucose: Galactose = 0.6: 12.5: 30.6: 3.3: 1.7: 21.7 | (Lin et al. 2020) |
| Enteromorpha prolifera | 10.4 × 104 | 18.6% sulfate | Rhamnose: Xylose: Mannose: Galactose: Glucose = 3.6: 1.1: 0.2: 0.8: 0.3 | (Tang et al. 2013) |
| Enteromorpha prolifera | 1.1 × 104 | 95.8% total sugar content | Rhamnose: Glucose: Xylose = 3.6: 1.2: 1.0 | (Zhou et al. 2020) |
| Enteromorpha prolifera | 1.2 × 106 | 19.9% sulfate | Mannose: xylose: galactose: arabinose: glucuronic acid = 5.10: 2.80: 1.20: 0.30: 0.30 | (Liu et al. 2022) |
| Enteromorpha prolifera | 0.8 × 104 | 16.0% sulfate | Rhamnose: Glucuronic acid: Xylose = 1.00: 0.41: 0.12 | (Jin et al. 2020) |
| Enteromorpha prolifera | 4.4 × 104 | 12.3% sulfate | Rhamnose: Glucose: Xylose: Galactose = 6.8: 1.9: 0.8: 0.4 | (Shi et al. 2017) |
Fig. 5.
The structural characteristics (A) and the main disaccharide repeat units (B) of ulvan
EPs have garnered increased research attention in recent years. The monosaccharide composition, sulfate positioning, and sugar chain structure of EPs are influenced by the species, seasonal variations, and environmental conditions. Consequently, it is very difficult to elucidate the precise structure of EPs. Limited research has been conducted on the detailed chemical structures of EPs (Kim et al. 2011). Earlier studies indicated that polysaccharides from Enteromorpha consist of various linked Rha and Xyl units, including α-(1→4)-, α-(1→3)-, α-(1→3,4)-, and α-(1→2,3,4)-linked Rha units as well as β-(1→4)- and β-(1→2,4)-linked Xyl units (Ray et al. 1995). Yu et al. (Yu et al. 2017) extracted the polysaccharides from E. prolifera and analyzed the structure by MS and NMR, revealing that the backbone chain consisted of D-GlcUAp-α-(1→4)-3-sulfate-L-Rhap-β-(1→4)-D-Xylp-β-(1→4)-3-sulfate-L-Rhap units. Qi et al. (Qi et al. 2012) extracted sulfated polysaccharides from E.clathrata (FEP) and the backbone of FEP was composed of (1→4) glycosidically linked β-L-arabinopyranose residues, with a partial sulfate group at the C-3 position. Generally, green algae polysaccharides are composed of glucuronic acid, rhamnose, xylose, galactose, and fucose. Specifically, rhamnose is linked by 1→2,4 and 1→4 glycosidic bonds; glucose is linked by 1→4; galactose is linked by 1→3 and 1→6; glucuronic acid and xylose are linked by 1→4 and are located at the termini, with xylose being partially sulfated at the O-2 position (Chattopadhyay et al. 2007). The structure of EPs exhibit significant variation, evident not only in their chemical composition but also in the arrangement of glycosidic bond connections, the distribution of sulfuric acid groups, and the presence and location of branch points. Table 2 provides an overview of the monosaccharide composition and structural features of different types of EPs. It can be seen that Enteromorpha is a heteropolysaccharide containing a variety of structural units, complex and diverse connection modes, and branched chains.
Table 2.
Structural compositions of different species Enteromorpha
| Species | Structural composition | Ref. |
|---|---|---|
| E. prolifera | D-GlcA-α-(1→4)-3-sulfate-L-Rha-β-(1→4)-D-Xyl-β-(1→4)-3-sulfate-L-Rha/(1→4)-β-L-rhamnose and (1→4)-linked xylose with sulfate groups linked on rhamnose at the C-3 position | (Jiao et al. 2009; Yu et al. 2017) |
| E. clathrata | (1→4)-linked β-L-Ara residues with partial sulfate groups at the C-3 position | (Qi et al. 2012) |
| E. compressa | (1→4)-and (1→2,4)-linked-Rha, (1→4)-linked Xyl, and (1→4)-and terminally linked-glucuronosyl residues/(1→4)-β-L-rhamnose and (1→4)-linked xylose with sulfate groups linked on rhamnose at the C-3 position | (Ray 2006; Jiao et al. 2009) |
| E. intestinalis | (1→4)-β-L-rhamnose and (1→4)-linked xylose with sulfate groups linked on rhamnose at the C-3 position | (Jiao et al. 2009) |
The study of the structure of green algae polysaccharide is helpful to strengthen the high-value development and utilization of green algae resources. However, The intricate and diverse nature of glycosidic bond compositions and linkages, coupled with the presence of sulfate groups at various positions and branching points, renders the investigation of the fine structure of green algae polysaccharides both difficult and challenging.
Extraction and purification of green algae polysaccharides
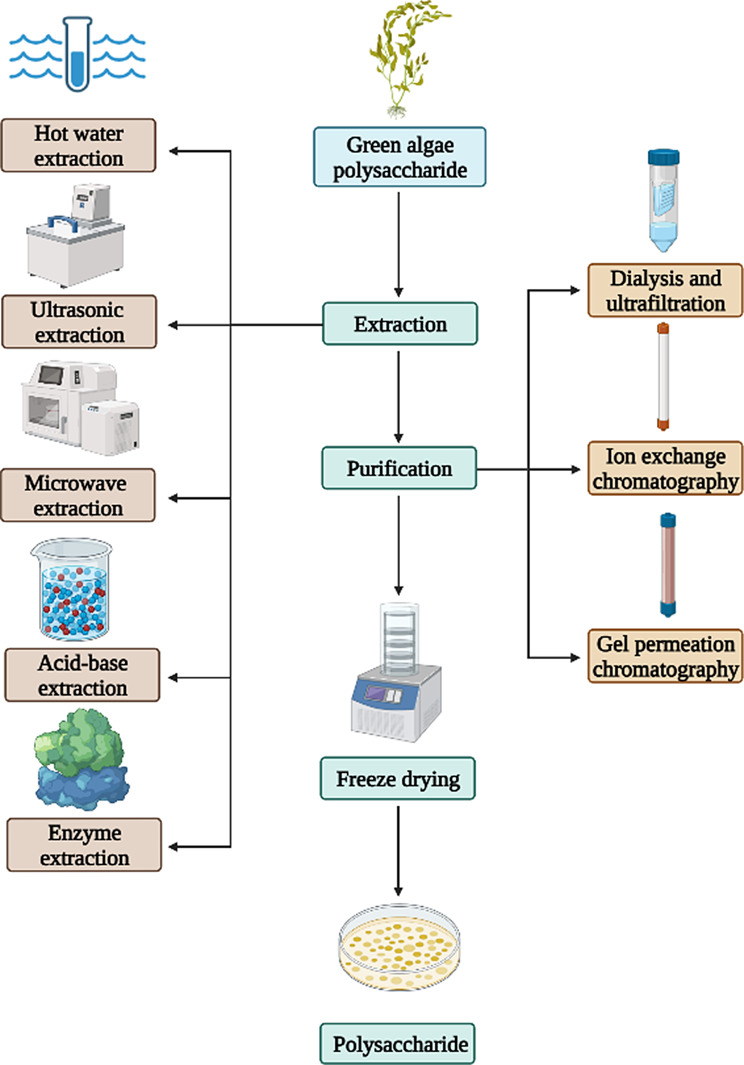
The distribution of green algae polysaccharides is predominantly in the interstitium and cell wall, with a minor presence in the cytoplasm. The water-soluble sulfated polysaccharides constitute 8–29% of the dry weight, and the yield of polysaccharides obtained varies depending on the extraction and purification methods employed. Currently, the most extensively studied method involves direct extraction of water-soluble polysaccharides using hot water (80–100 °C) (Chattopadhyay et al. 2007; de Carvalho et al. 2020), although enzyme-assisted extraction methods can also be utilized to enhance polysaccharide yield (Wahlström et al. 2020).
The hot water extraction method mainly uses water as a solvent to induce plasma wall separation of cells through thermal action. The intracellular or intercellular substances dissolved in water are exuded by diffusion. Pankiewicz et al. (Pankiewicz et al. 2016) extracted ulvan by stirring in hot water at 75–85 ℃ for 7 h with a solid to liquid of 1:30. The supernatant was concentrated after centrifugation, yielding a polysaccharide content of 16.23% after concentration of the supernatant post-centrifugation. Chen et al. (Chen et al. 2021a) extracted the ulvan by incubation in water at 90 ℃ for 3 h with the ratio of solid to liquid 1:20, resulting in the acquisition of 17.8 ± 0.6% of polysaccharide. The traditional hot water extraction method had the problems of long extraction time and diminished yield, with the elevated temperature leading to polysaccharides undergo the phenomenon of self-degradation. Therefore, in order to facilitate the subsequent research on polysaccharides, optimization of the hot water extraction process needs to be rationally optimized. Polysaccharides obtained through hot water extraction commonly harbor soluble impurities, necessitating the addition of alcohol for impurity removal and enhancement of polysaccharide purity. Xu et al. (Xu et al. 2015) extracted the EPs in hot water (90 ℃) for 4 h, obtaining 21.96% of polysaccharide after concentration and ethanol precipitation. Furthermore, it was suggested that the extraction process of green algae polysaccharides could be improved by adjusting the pH value of the extraction solution to improve the purity of the polysaccharide. For example, Glasson et al. (Glasson et al. 2017) extracted ulvan into 1 L of 0.05 M HCl for 1 h and then adjusted the pH value to 7 with 1 M NaOH. Finally, 8.1 ± 1.0% of polysaccharide was obtained. Song et al. (Song et al. 2010) treated the sample with 0.05 M HCl for 2 h and obtained 86.1% of EPs. The chemical extraction method is more efficient in terms of extraction time when compared to the hot water extraction method. However, it significantly raises the cost associated with the disposal of acid and alkali waste disposal.
The extraction methods for green algae polysaccharides have been continuously optimized and updated as research progresses. The physically assisted extraction method primarily involves the disruption of plant cell wall structure through physical mechanisms, facilitating the extraction of polysaccharides from the cells and thereby reducing extraction time and increasing the yield of polysaccharides. The ultrasonic extraction method used ultrasonic waves to disrupt the cell wall structure, facilitating the dissolution of polysaccharides and thereby shortening extraction time and improve the extraction efficiency. Guo et al. (Guo et al. 2010) extracted EP in 28 min using ultrasound and achieved an extraction of 25.84 mg/g of crude polysaccharide. Chen et al. (Chen et al. 2021a) obtained 20.6 ± 1.2% ulvan through ultrasonic treatment for 30 min followed by extraction in a water bath at 90 °C for 2.5 h with a material-liquid ratio of 1:20. However, the ultrasonic method exhibited variability in extraction efficiency despite its shorter extraction time. Microwave-assisted extraction could better maintain the biological activity of polysaccharides. Tsubaki et al. (Tsubaki et al. 2016) employed microwave-assisted technology to extract ulvan, achieving a polysaccharide yield of 40.4 ± 3.2%. The utilization of physical assistance in the extraction process has the potential to significantly enhance extraction efficiency. While microwave-assisted extraction offers the advantage of reduced extraction time, it may be susceptible to issues such as uneven heating.
The principle of enzyme-assisted extraction involves the enzymatic degradation of cell walls under mild reaction conditions. The specificity of enzymes allows for targeted substrate degradation while minimizing damage to non-targeted substances (Li et al. 2016a). This method can increase yield, decrease processing time and lower overall costs. In particular, enzymes such as cellulase and pectinase have been shown to effectively break down cell wall, leading to increased release of (Fernandes et al. 2019). Hardouin et al. (Hardouin et al. 2016) added six enzymes including protease and cellulase into Ulva extraction, and the polysaccharide yield reached 35.3 ± 0.3%. Lü et al. (Lu et al. 2014) introduced protease into the extraction solution and obtained an EP yield of 27.75%. These findings underscore the significant enhancement in polysaccharide yield that can be achieved through the efficient and mild action of enzymes.
In conclusion, there exist multiple extraction techniques for green algae polysaccharides, each presenting distinct advantages and limitations (Table 3). The amalgamation of different methods can greatly improve the efficiency of polysaccharide extraction. Chen et al. (Chen et al. 2021a) employed a combined approach of ultrasonic-assisted extraction and enzyme-assisted extraction to isolate ulvan, resulting in a yield of 26.7 ± 0.9%, surpassing that of individual methods such as hot water extraction, enzyme-assisted extraction, and ultrasonic-assisted extraction. This study suggests that the integration of diverse extraction methods can enhance the yield of the yield of green algae polysaccharides, reduce the production cost, and provide a basis for further research.
Table 3.
Advantages and disadvantages of different extraction methods
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hot water extraction |
A. Simple operation. B. Low cost and suitable for large-scale industrial extraction. |
A. Long time. B. Low extraction rate. C. High temperatures may cause degradation of polysaccharides. |
| Alkali solution extraction | A. Save time. |
A. May disrupt polysaccharide structure. B. Increased cost of waste liquid treatment. |
| Microwave assisted extraction | A. Save time and high extraction efficiency. | A. Can not improve polysaccharide yield and purity. |
| B. Maintain the structure and biological activity of polysaccharide. | B. Uneven heating. | |
| Ultrasonic assisted extraction | A. Save time. | A. The extraction efficiency is not stable. |
| B. Improve the homogeneity of raw material and solvent mixing degree. | ||
| Enzyme assisted extraction | A. Save time and high extraction efficiency. | A. Enzymes are easily inactivated. |
| B. Low reaction temperature. | B. Enzymes are more expensive. |
The crude polysaccharides obtained through the aforementioned method is found to contain proteins, small molecules, and other non-polysaccharide impurities, necessitating additional purification steps to ensure its suitability for subsequent structural analysis and investigation of biological activity. The protein can be removed by adding protease to the extract for hydrolysis and Savage method, followed by dialysis to remove small molecular impurities generated during hydrolysis (Lin et al. 2020). Further purification is performed through ion exchange chromatography (IEC) or gel permeation chromatography (GPC). The green algae polysaccharide exhibits varying charges at specific pH levels, allowing for the purification and separation of its components based on their charge differences. Glasson et al. (Glasson et al. 2022) used ion exchange chromatography with a Q Sepharose column and gradient elution to achieve purification, resulting in final polysaccharide yields of 1.45, 1.29 and 2.8%. Li et al. (Li et al. 2020a) utilized ion exchange chromatography with a DEAE-Sepharose column to purify ulvan from U. pertusa, successfully eluting three distinct polysaccharide components with 0 M, 0.5 M and 1 M NaCl. Pan et al. (Pan et al. 2019) used DEAE Sepharose Fast Flow with a NaCl concentration of 0.5-1 M for the purification of four polysaccharide components from E. prolifera. Chi et al. (2020a, 2021) used HiTrap Q FF gel for the purification of ulvan extracted from U. clathrata with a NaCl gradient of 0–2 M. Lv et al. (Lv et al. 2013) employed Sephadex G-100 gel with water as the mobile phase to isolate two EP components. In addition, Xu et al. (Xu et al. 2015) used the gel column SephacryTm S-300 h to isolate two components of EP. In summary, IEC relies on the different charges of polysaccharides to achieve the separation of different components of green algae polysaccharides, whereas GPC leverages the difference in molecular size of polysaccharides to achieve the separation of different components. Therefore, IEC may be more suitable for polysaccharide components with minimal disparity in molecular weight but different charges, whereas GPC may be more appropriate for components with similar charges but large differences in molecular weight (Table 4).
Table 4.
Advantages and disadvantages of different purification methods
| Methods | Advantages | Disadvantages |
|---|---|---|
| Dialysis |
A. Simple operation. B. Save time. C. Reduce the loss of polysaccharides. |
A. Incomplete purification. B. Requirements for molecular weight. |
| Ultrafiltration | ||
| Ion exchange chromatography |
A. High sensitivity. B. High separation efficiency. C. High reliability. |
A. Low productivity. B. Strict sample conditions. C. Requires large amounts of buffer and eluent. |
| Gel permeation chromatography | A. High sample recovery. |
A. Slower separation operation. B. The resolution is low, especially between molecules of similar relative molecular mass. |
|
B. Good repeatability of the experiment. C. No change in sample biological activity. |
The biological activities and application effects of green algae polysaccharides are intricately linked to their purity. Polysaccharides with high levels of purity exhibit enhanced biological activities, including antioxidant, immunomodulatory, anticoagulant, and antiviral properties, which play a significant role in the advancement of pharmaceuticals and health products. Nevertheless, the cost of commercial polysaccharides remains high due to the intricate purification process and low yield. Therefore, it is imperative to devise an appropriate method for the separation and purification of green algae polysaccharides. The process of extracting and purifying green algae polysaccharides is shown in Fig. 6.
Fig. 6.
The main methods in green algae polysaccharides extraction and purification
Activity of green algae polysaccharide
Antioxidant activity
When the body is in a state of oxidative stress, an abundance of free radicals accumulated and attack sugars, proteins, lipids and DNA within the body, leading to damage of cell membranes structures, oxidative harm to cells, and a decline in immune function. Oxidative stress can trigger a variety of related diseases, such as inflammation, cardiovascular disease, and tumors (Pisoschi et al. 2015, Huang et al. 2017). In vitro studies have shown antioxidant properties in polysaccharides extracted from green algae (Zhang et al. 2010). Green algae polysaccharides are an acidic polysaccharide containing sulfate. Higher sulfate content is associated with increased biological activity of this polysaccharide. Barakat et al.(Barakat et al. 2022) successfully extracted ulvan from U. fasciata, which contained 20.45% sulfate and demonstrated significant antioxidant activity, achieving 84.93% scavenging of DPPH radicals. Chen et al. (Chen et al. 2021a) employed enzyme-assisted extraction techniques to obtain ulvan, which exhibited superior and more pronounced DPPH free radical scavenging activity (SC 6.52 mg/mL) compared to ulvan extracted using hot water-assisted and ultrasound-assisted methods. It was observed that low molecular weight ulvan fractions possessed higher antioxidant capacities relative to their high molecular weight counterparts (Li et al. 2018b, c). Additionally, green algae polysaccharides can also significantly enhance the activity of endogenous antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD), while reducing levels of malondialdehyde (MDA). This mechanism may help alleviate oxidative stress caused by aging and hyperlipidemia (Xu et al. 2019; Li et al. 2020a; Yang et al. 2020). Wassie et al. (Wassie et al. 2022) demonstrated an increase in catalase (CAT) activity, total antioxidant capacity, and superoxide dismutase (SOD) activity, along with a decrease in malondialdehyde (MDA) levels in the serum of birds administered with EP.
This suggests that green algae polysaccharides have good free radical scavenging ability in vitro and affect the expression of antioxidant enzymes in vivo, thereby contributing to their antioxidant properties. However, the specific mechanism by which they modulate antioxidant molecular signaling pathways remain largely unexplored, necessitating further research in this area. In addition, the antioxidant capacity of these polysaccharides is dependent on their molecular weight, of polysaccharides, a topic that will be elaborated upon in the subsequent section on oligosaccharide activity (Qi et al. 2006).
Immune regulatory activity
Immunity serves as a defense mechanism for organisms against foreign pathogens, with numerous algae polysaccharides exhibiting immunomodulatory activity that can also regulate innate immune function. Specifically, green algae polysaccharides have been found to possess immunomodulatory activities that primarily involve the activation of immune responses and the modulation of immune cell activity (Zhao et al. 2016). Xu et al. (Xu et al. 2005) discovered that appropriate concentrations of EP had a notable impact on the proliferation of T and B lymphocytes, as well as on the increased production of interferon-γ (IFN-γ) triggered by the activation of antigen-presenting cells. Sulfated ulvan was found to regulate the levels of natural killers (NK) including tumor necrosis factor (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and interferon-c (IFN-c) (Meng et al. 2016; Wu et al. 2016). In S. senegalensis macrophages, ulvan has a stimulatory effect that is enhanced when delivered via nanoparticles (Fernández-Díaz et al. 2017). Furthermore, various molecular weight fractions (7、9、13、21 and 209 kDa) were derived from U. ohnoi, all of which exhibited immunomodulatory properties (Kidgell et al. 2020). An investigation into ulvan from U. intestinalis demonstrated that the immunomodulatory activity of the lower molecular weight fraction (28.7 kDa) was markedly superior to that of the higher molecular weight fraction (87.2 kDa) (Tabarsa et al. 2018). Additionally, treatment of the porcine intestinal epithelial cell line (IPEC-1) with purified low molecular weight (4.4 kDa) ulvan from U. armoricana (at concentrations ranging from 5 to 500 µg/ml) resulted in increased mRNA and protein expression levels of cytokines such as CCL20, IL-8, and TNF-α (Berri et al. 2017). These findings underscore the significance of interactions among the structural characteristics (e.g., molecular weight and degree of sulfation) of green algae polysaccharides in determining their biological activity. While a clear correlation exists between molecular weight, sulfation level, and the degree of immunomodulation exhibited by green algae polysaccharides, the intricate nature of their structural composition complicates the elucidation of the precise relationship between immunomodulation and specific structural features.
Hence, green algae polysaccharides can promote the production of cytokines and participate in the regulation of the body’s immune response, thereby serving as a potential immunomodulatory agent. Green algae polysaccharides can also be used as a nutritional supplement to enhance immune function.
Anticoagulant activity
The anticoagulant properties of seaweeds have been the subject of research for over six decades. Brown, red, and green algae all exhibit anticoagulant activity. The primary bioactive compounds responsible for this activity are various sulfated polysaccharides. Specifically, galactan sulfate and fucoidan sulfate are the active components in red and brown algae, respectively (Pozharitskaya et al. 2020; Ajarem et al. 2021; Li et al. 2021). In contrast, the anticoagulant activity in green algae is primarily attributed to arabinogalactan sulfate or rhamnogalactan sulfate (Wang et al. 2013). The intrinsic and extrinsic pathways culminate in the production of thrombin, which subsequently converts soluble fibrinogen into insoluble fibrin, leading to the formation of a blood clot. The mechanism of action of green algae polysaccharides primarily involves the enhancement of antithrombin III and heparin cofactor II, or the direct inhibition of thrombin activity and fibrin polymerization, both of which are critical endogenous inhibitors (Matsubara 2004; Adrien et al. 2017; Cui et al. 2018). Heparin is one of the main clinical drugs for the treatment and prevention of thrombosis, but it has some side effects such as bleeding, thrombosis syndrome and thrombocytopenia (Yu et al. 2018). The current research emphasis is on identifying alternative heparin analogs that can effectively prevent or treat cardiovascular diseases while possessing potent anticoagulant activity. Green algae polysaccharides have been identified as having anticoagulant effect, with its efficacy primarily assessed through measurements of thromboplastin time, thrombin time, and prothrombin time. It has been reported that the anticoagulant activity of green algae polysaccharides surpasses those of red algae and brown algae (Shanmugam et al. 2001; Athukorala et al. 2007).
The anticoagulant activity of green algae polysaccharides is typically associated with factors such as molecular size, monosaccharide composition, sulfate content, sulfate positioning, and attachment mode. For instance, ulvan derived from U. linza could extend the activated partial thromboplastin time (aPTT) by 3.3 to 6.2 times relative to the normal clotting time, contingent upon its degree of sulfation and molecular weight (Wang et al. 2013). Notably, lower concentrations of sulfated ulvan exhibit a prolonged anticoagulant effect compared to the commercially available anticoagulant Lovenox (Adrien et al. 2019). Cui et al. (Cui et al. 2018) conducted an extraction of polysaccharides from E. linza, subsequently obtaining five low molecular weight fractions through enzymatic hydrolysis. Their findings indicated a positive correlation between the degree of sulfation and anticoagulant activity, with the latter being retained until a molecular weight threshold of less than 200 kDa was reached. Mao et al. (Mao et al. 2006) studied polysaccharides from U. Conglobata and showed that the polysaccharide could prolong thrombin times which inhibited thrombin and modulated heparin cofactor II. Mao (Mao et al. 2009) isolated polysaccharides from Monostroma. Latissimum and revealed that its heightened anticoagulant activity was attributed to its high rhamnose content. Therefore, the findings suggest that green algae polysaccharides have the potential to be used as a novel anticoagulant agent. The anticoagulant activity of the sulfated polysaccharides was attributed, in part, to the strong interactions between the negatively charged sulfate esters and certain positively charged peptidic sequences of proteins involved in the coagulation process. Nevertheless, there is a paucity of research on the effect of monosaccharide composition on bioactivity, warranting further investigation.
Antiviral activities
Green algae polysaccharides are increasingly recognized as a new source of antiviral activity within the realm of natural compounds (Komatsu et al. 2013). Research has demonstrated that green algae polysaccharides exhibit inhibitory properties against many viruses, such as herpes simplex virus, cytomegalovirus, human immunodeficiency virus, and influenza virus. Lee et al. (Lee et al. 1999) successfully isolated sulfated rhamnosan from M. latissimum which displayed significant inhibitory effects on virus replication. Shefer S et al. (Shefer et al. 2021) evaluated the anti-SARS-CoV-2 activity of ulvan extracted using ammonium oxalate and hydrochloric acid. The results indicated that the AOX protocol was able to protect VERO E6 cells from SARS-CoV-2 cytopathy, with an inhibitory effect 11.3 times greater than that of the HCl protocol. These findings indicate that the more negatively charged sulfate groups facilitates interactions with viral envelope glycoproteins or surface receptors, leading to a reduction in viral entry into host cells. In vitro studies of ALV-J, ulvan have been found to effectively bind to viral particles, thereby preventing ALV-J from adsorbing to host cells and causing a notable decrease in the expression of ALV-J gene and gp85 protein (Sun et al. 2018). Furthermore, EPs have been shown to hinder the adsorption and penetration of herpes simplex virus (HSV) into laryngeal epithelial cancer cells, as well as inhibit HSV replication and transcription (Lopes et al. 2017). The potential antiviral natural active substances of green algae polysaccharides, particularly ulvan, have been extensively studied. Given the paucity of research on the antiviral properties of green algae polysaccharides, the comprehension of their conformational relationships remains constrained. Nonetheless, the conformational characteristics of green algae polysaccharides exhibiting antiviral activity appear to be analogous to those observed in other sulfated polysaccharides (Witvrouw et al. 1997, Ghosh et al. 2009).
Hypolipidemic activity
Hyperlipidemia is associated with cerebrovascular and cardiovascular complications, as well as atherosclerosis, a prevalent endocrine disorder (Song et al. 2017, Chen et al. 2018; Zhao et al. 2019). In recent years, reports have confirmed the hypolipidemic effects of green algae polysaccharides. In a hyperlipidemia mouse model, ulvan significantly decreased serum total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) levels, while increasing high-density lipoprotein cholesterol (HDL-C) levels, suggesting its potential therapeutic efficacy (Li et al. 2020a). The molecular weight and degree of sulfation of green algae polysaccharides influence their hypolipidemic activity. Peng et al. (Pengzhan et al. 2003) incorporated two distinct molecular weights of ulvan (151.6 kDa and 28.2 kDa) into the diets of rats subjected to a high-cholesterol regimen to evaluate their antihyperlipidemic effects. The findings indicated that ulvan with a higher molecular weight significantly reduced serum total cholesterol and low-density lipoprotein (LDL) cholesterol levels, whereas the lower molecular weight ulvan fraction was more effective in decreasing triglycerides and increasing high-density lipoprotein (HDL) cholesterol levels. Teng et al. (Teng et al. 2013) also investigated which demonstrated that EPs presented high anti-hypolipidemic activities by suppressing body weight gain and reducing levels of TG, TC, and LDL-C levels in both plasma and liver. Furthermore, EPs were found to possess pancreatic lipase inhibition activities (Yuan et al. 2018). The hypolipidemic activity of green algae polysaccharides may be achieved by a combination of multiple mechanisms. However, there are few studies available on the specific antilipidemic mechanism, indicating a necessity for further investigation in this area.
Green algae oligosaccharide
Preparation of green algae oligosaccharide
The utilization of green algae polysaccharides in the food and pharmaceutical industries is constrained by its limited solubility and low bioavailability, as it is the main constituent of green algae. Green algae oligosaccharides, a degradation product of polysaccharides, have attracted more and more attentions due to its retention of various activities exhibited by polysaccharides, as well as its enhanced solubility and bioavailability (Liu et al. 2019). The preparation of green algae oligosaccharides can be categorized into three categories: chemical degradation, physical degradation, and enzymatic degradation. Yu et al. (Pengzhan et al. 2003) utilized microwave thermal degradation of ulvan to prepare Ulva oligosaccharides, resulting in the preservation of glycosidic linkages without destroying the important structural units. In a separate study, Zhang et al. (Zhang et al. 2013) employed ascorbic acid and H2O2 to degrade the EPs and obtain oligosaccharides. The limited studies on the degradation of polysaccharides by chemical and physical methods are attributed to the energy-intensive nature of physical degradation which necessitates significant energy input to break the glycosidic bonds, and the use of strong acids or strong oxidizing properties in chemical degradation to break glycosidic bonds.
Both methods suffer from long reaction times and difficult to control reaction conditions. However, enzymatic hydrolysis has attracted wide attention because of its mild reaction conditions and precise product specificity. Currently, the primary enzymes used in the degradation of ulvan are PL24, PL25, PL28 and PL40 ulvan lyases, which are specialized enzymes designed for ulvan degradation (Table 5). Ulvan lyases act by cleaving the β-(1→4)-glycosidic bond between Rha3S and GluA or IduA primarily by a β-elimination mechanism, resulting in the production of oligosaccharides with 2–4 degrees of polymerization (Dps) (Ulaganathan et al. 2017, 2018a, 2018b). This is the rationale behind the formation of even-odd oligosaccharides of DP2 and DP4 as the degradation products of most ulvan lyases targeting ulvan. The β-elimination mechanism effectively maintains the unique sugar structures present in ulvan, thereby facilitating the potential for valuable advancements in ulvan utilization. Despite this, there is a lack of literature documenting specific enzymes capable of degrading EPs. Only Li et al. (Li et al. 2013, 2016b, c) isolated Alteromonas sp. A321 as a novel EP-degrading strain, although the exact classification of the enzymes responsible as either polysaccharide lyases or glycoside hydrolases remains uncertain. Zhang et al. (Zhang et al. 2016) used enzymes that produced by Alteromonas sp. A321 to degrade the EPs and obtained 61.21% of the oligosaccharides. Therefore, it is also a problem worth further discussion whether there is a special degradation EPs enzyme.
Table 5.
The products of ulvan lyase from different sources
| Sources | PL family | Mw (kDa) | Products | Product Composition | Ref. |
|---|---|---|---|---|---|
| Alteromonas sp. KUL17 | PL24 | 55(110.85) | DP2, 4, 6 | - | (He et al. 2017) |
| Alteromonas sp. LOR_107 | PL24 | 59.64 | DP2, 4 | Δ-R3S, Δ-R3S-IdoA-R3S, Δ-R3S-Xyl-R3S | (Kopel et al. 2016) |
| Pseudoalteromonas sp. PLSV_3875 | PL24 | 59.62 | DP2, 4 | ΔUA-R3S, ΔUA-R3S-IdoA-R3S | (Qin et al. 2018) |
| Pseudoalteromonas sp. PLSV_3925 | PL24 | 111.4 | DP2, 4 | ΔUA-R3S, ΔUA-R3S-IdoA-R3S | (He et al. 2017) |
| Vibrio sp. FNV38 | PL24 | 54 | DP2, 4 | Δ-Rha3S, Δ-Rha3S-HexA-Rha3S, Δ-Rha3S-Xyl-Rha3S | (Rodrigues et al. 2024) |
| Alteromonas sp. LOR_29 | PL25 | 52 | DP2, 4 | Δ-R3S, Δ-R3S-Xyl-R3S | (Foran et al. 2017) |
| Pseudoalteromonas sp. PLSV_3936 | PL25 | 54.28 | DP2, 4 | ΔUA-Rha3S, ΔUA-Rha3S-Xyl-Rha3S | (Ulaganathan et al. 2017) |
| Alteromonas sp. A321. ALT3695 | PL25 | 53 | DP2, 4 | ∆GlcA-Rha3S, ∆GlcA-Rha3S-Xyl-Rha3S | (Gao et al. 2019) |
| Thalassomonas sp. LD5 | PL25 | 54.54 | DP2, 4 | ∆Rha3S, ∆Rha3S-Xyl-Rha3S | (Wang et al. 2022) |
| Alteromonas sp. TK-45 (2) | PL25 | 51.99 | DP2, 4 | ∆GlcA-Rha3S, ∆GlcA-Rha3S-Xyl-Rha3S | (Tang et al. 2022) |
| Alteromonas sp. KUL_42 | PL25 | 53.97 | DP2-4 | Rha3S-GlcA, Rha3S-Xyl-Rha, Rha3S-lduA-Rha, Rha3S-lduA-Rha3S-Xyl, Rha3S-lduA-Rha3S-Xyl2S | (Li et al. 2023) |
| Alteromonas sp. 76–1 | PL25 | 54.39 | DP2-4 | ∆Rha3S, Rha3S-GIcA-Rha, ∆Rha3S-IduA-Rha3S, ∆Rha3S-Xyl-Rha3S | (Tang et al. 2023) |
| Nonlabens Ulvanivorans NLR42 | PL28 | 46 | DP2, 4 | ∆-R3S, ∆-R3S-Xyl-R3S | (Collén et al. 2011) |
| Formosa agariphila KMM 3901T | PL28 | 54.73 | DP2,4 | Δ-Rha3S, Δ-Rha3S-GlcA-Rha3S, Δ-Rha3S-IdoA-Rha3S | (Reisky et al. 2018) |
| Tamlana fucoidanivorans CW2-9 | PL28 | 52 | DP2,4 | ΔRha3S, ΔRha3S-IduA-Rha3S, ΔRha3S-GlcA-Rha3S | (Xu et al. 2024) |
Activity of green algae oligosaccharide
At present, there is a growing body of literature on the activity of green algae oligosaccharides. However, these studies are fragmented. Moreover, the intricate structure of oligosaccharides has hindered the determination of the mechanism of their activity and the structure-activity relationship. Li et al. (Li et al. 2020b) studied the anti-inflammatory effects of Ulva oligosaccharides on bowel disease (IBD). The results indicated that a dosage of 50 mg/kg of Ulva oligosaccharides exhibited a protective effect on IBD, with the best protective effect observed at concentration between 100 and 120 mg/kg. Carvalho et al. (de Carvalho et al. 2020) found that the existence of Ulva oligosaccharide could improve the anti-cancer activity of ulvan. Liu et al. (Liu et al. 2010) observed that Enteromorpha oligosaccharides could significantly stimulate the secretion of nitric oxide (NO), upregulate the expression of cytokines such as IL-1β, IL-6 and TNF-α, and activate inflammatory agents such as iNOS, COX2, and NLRP3, thereby activating the immune system. In addition, Tabarsa et al. (Tabarsa et al. 2018) discovered that Ulva oligosaccharides containing low molecular weight and high sulfate group components could induce proliferation of RAW264.7 macrophages and prompt the release of significant amounts of nitric oxide, IL-1β, TNF-α, IL-6, IL-10, and IL-12 cytokines from RAW264.7 cells, suggesting a mild immunomodulatory activity that is closely related to molecular weight. In a related study, Qi et al. (Qi et al. 2006) prepared Ulva oligosaccharides with different molecular weights, and found that the degraded form exhibited greater antioxidant activity compared to the higher molecular weight ulvan. Wang et al. (Wang et al. 2013) discovered that Enteromorpha oligosaccharides possessed an anticoagulant activity which depended on their degree of acidification, the molecular and the distribution of sulfuric acid groups. Yu et al. (Pengzhan et al. 2003) studied the anti-hyperlipidemic activity of Ulva oligosaccharides in male Wistar rat models, revealing that Ulva oligosaccharides exhibited greater efficacy compared to polysaccharides in managing hyperlipidemia associated with diabetes. All these results indicate that low molecular weight green algae oligosaccharides possess superior bioavailability. Given the comparable activity of oligosaccharides to natural polysaccharides, they present a more advantageous option for the formulation of dietary supplements and pharmaceuticals.
To sum up, due to the complexity of the structure, the active mechanism of green algae oligosaccharides is not clear, but it can be hypothesized that it is related to some active groups produced by the degradation of polysaccharides. At present, researches on the activity of green algae oligosaccharides are relatively shallow, and there is no appropriate method to obtain oligosaccharide with a refined structure.
Conclusion and perspective
In this review, the progress of research on the composition, structure and biological activity of green algae polysaccharides have been investigated and summarized. Recent studies have proved the significant impact of green algae polysaccharides on human health and nutrition, showcasing their various physiological activities such as immune regulation, anticoagulant effects, and hypolipidemic activities. These attributes make green algae polysaccharides a promising candidate for the treatment of conditions including hyperlipidemia, hypertension, and other metabolic diseases, positioning them as an important source for the development of novel marine-based pharmaceuticals.
The green algae polysaccharides also can eliminate the oxidative radicals such as DPPH, OH− and O2−. The utilization of these biological activities in the development of new functional foods and pharmaceuticals for the management of diet-related chronic disorders holds promise. However, the exploration and utilization of green algae polysaccharides face numerous challenges due to its intricate structure, susceptibility to various influencing factors, high biological molecular weight, and low bioavailability. For instance, Ji et al. [103] isolated polysaccharides from E. clathrata at different harvesting times, demonstrating variations in the ratios of these polysaccharides. Moreover, while crude polysaccharides are easily extracted, their low purity hinders effective utilization. Thus, the purification of large-scale refined polysaccharides remains a key research focus. In conclusion, the myriad advantageous properties of green algae polysaccharides render them highly promising for applications in the realms of food, cosmetics, and biomedicine, thereby garnering considerable attention (Fig. 7). The escalating interest in green algae polysaccharides within the marine bioresources sector underscores their emergence as a focal point of discussion, accompanied by both opportunities and challenges coexist.
Fig. 7.

Potential application prospects of green algae polysaccharides
Acknowledgements
Dr. Zhu Benwei gratefully acknowledges the support of the Excellent Young Backbone Teachers of “Blue Project” in Jiangsu Province.
Author contributions
C.L. and H. W. wrote the manuscript; B.Z. was the supervisor for this work and revised the manuscript; Z.Y. and L. N. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National Natural Science Foundation of China (32372268) and the China Postdoctoral Science Foundation (2023M743532).
Data availability
Not applicable.
Declarations
Institutional review board statement
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benwei Zhu, Email: zhubenwei@njtech.edu.cn.
Limin Ning, Email: ninglimin@njucm.edu.cn.
References
- Abd-Ellatef G-EF, Ahmed OM, Abdel-Reheim ES, Abdel-Hamid A-HZ (2017) Ulva lactuca polysaccharides prevent Wistar rat breast carcinogenesis through the augmentation of apoptosis, enhancement of antioxidant defense system, and suppression of inflammation. Targets Ther, Breast Cancer, pp 67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrien A, Dufour D, Baudouin S, Maugard T, Bridiau N (2017) Evaluation of the anticoagulant potential of polysaccharide-rich fractions extracted from macroalgae. Nat Prod Res 31(18):2126–2136 [DOI] [PubMed] [Google Scholar]
- Adrien A, Bonnet A, Dufour D, Baudouin S, Maugard T, Bridiau N (2019) Anticoagulant activity of sulfated ulvan isolated from the green macroalga Ulva rigida. Mar Drugs 17(5):291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajarem JS, Maodaa SN, Allam AA, Taher MM, Khalaf M (2021) Benign synthesis of cobalt oxide nanoparticles containing red algae extract: antioxidant, antimicrobial, anticancer, and anticoagulant activity. J Cluster Sci: 1–12
- Aneiros AA, Garateix (2004) Bioactive peptides from marine sources: pharmacological properties and isolation procedures. J Chromatogr B: Anal Technol Biomed Life Sci 803(1):41–53 [DOI] [PubMed] [Google Scholar]
- Athukorala Y, Lee KW, Kim SK, Jeon YJ (2007) Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour Technol 98(9):1711–1716 [DOI] [PubMed] [Google Scholar]
- Barakat KM, Ismail MM, Abou El Hassayeb HE, El Sersy NA, Elshobary ME (2022) Chemical characterization and biological activities of ulvan extracted from Ulva fasciata (Chlorophyta). Rend Lincei Scienze Fis E Naturali 33(4):829–841 [Google Scholar]
- Benslima A, Sellimi S, Hamdi M, Nasri R, Jridi M, Cot D, Li S, Nasri M, Zouari N (2021) The brown seaweed Cystoseira schiffneri as a source of sodium alginate: Chemical and structural characterization, and antioxidant activities. Food Biosci 40:100873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berri M, Olivier M, Holbert S, Dupont J, Demais H, Le Goff M, Collen PN (2017) Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Res 28:39–47 [Google Scholar]
- Bobin-Dubigeon C, Lahaye M, Guillon F, Barry JL, Gallant DJ (1997) Factors limiting the biodegradation of Ulva Sp cell‐wall polysaccharides. J Sci Food Agric 75(3):341–351 [Google Scholar]
- Chattopadhyay K, Mandal P, Lerouge P, Driouich A, Ghosal P, Ray B (2007) Sulphated polysaccharides from Indian samples of Enteromorpha compressa (Ulvales, Chlorophyta): isolation and structural features. Food Chem 104(3):928–935 [Google Scholar]
- Chen Y, Liu Y, Sarker MMR, Yan X, Yang C, Zhao L, Lv X, Liu B, Zhao C (2018) Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr Polym 198:452–461 [DOI] [PubMed] [Google Scholar]
- Chen J, Zeng W, Gan J, Li Y, Pan Y, Li J, Chen H (2021) Physicochemical properties and anti-oxidation activities of ulvan from Ulva pertusa Kjellm. Algal Res 55:102269 [Google Scholar]
- Chen J, Zeng W, Gan J, Li Y, Pan Y, Li J, Chen H (2021a) Physicochemical properties and anti-oxidation activities of ulvan from Ulva pertusa Kjellm. Algal Res 55
- Chi Y, Li H, Wang P, Du C, Ye H, Zuo S, Guan H, Wang P (2020a) Structural characterization of ulvan extracted from Ulva Clathrata assisted by an ulvan lyase. Carbohydr Polym 229:115497 [DOI] [PubMed] [Google Scholar]
- Chi Y, Zhang M, Wang X, Fu X, Guan H, Wang P (2020b) Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int J Biol Macromol 157:75–82 [DOI] [PubMed] [Google Scholar]
- Chi Y, Li H, Fan L, Du C, Zhang J, Guan H, Wang P, Li R (2021) Metal-ion-binding properties of ulvan extracted from Ulva Clathrata and structural characterization of its complexes. Carbohydr Polym 272:118508 [DOI] [PubMed] [Google Scholar]
- Coelho MS, Menezes BdS, Meza SLR, Gianasi BL, M. d. l. M., Salas-Mellado M, Copertino M. d. R. A. Z. de Souza (2016) Potential Utilization of Green Tide-Forming Macroalgae from Patos Lagoon, Rio Grande-RS, Brazil. J Aquat Food Prod Technol 25(7): 1096–1106
- Collén PN, Sassi J-F, Rogniaux H, Marfaing H, Helbert W (2011) Ulvan lyases isolated from the flavobacteria Persicivirga ulvanivorans are the first members of a new polysaccharide lyase family. J Biol Chem 286(49):42063–42071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Li Y, Wang S, Chi Y, Hwang H, Wang P (2018) Directional preparation of anticoagulant-active sulfated polysaccharides from Enteromorpha prolifera using artificial neural networks. Sci Rep 8(1):3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho MM, Noseda MD, Dallagnol JC, Ferreira LG, Ducatti DR, Gonçalves AG, de Freitas RA, Duarte MER (2020) Conformational analysis of ulvans from Ulva fasciata and their anticoagulant polycarboxylic derivatives. Int J Biol Macromol 162:599–608 [DOI] [PubMed] [Google Scholar]
- Fan Q, Shi K, Zhan M, Xu Q, Liu X, Li Z, Liu H, Xia Y, Chen Y, Shi X (2022) Acute damage from the degradation of Ulva prolifera on the environmental microbiota, intestinal microbiota and transcriptome of Japanese flounder Paralichthys olivaceus. Environ Pollut 302:119022 [DOI] [PubMed] [Google Scholar]
- Fernandes H, Salgado JM, Martins N, Peres H, Oliva-Teles A, Belo I (2019) Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour Technol 281:277–285 [DOI] [PubMed] [Google Scholar]
- Fernández-Díaz C, Coste O, Malta E-j (2017) Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immunostimulant effect in Solea senegalensis macrophages. Algal Res 26:135–142 [Google Scholar]
- Foran E, Buravenkov V, Kopel M, Mizrahi N, Shoshani S, Helbert W, Banin E (2017) Functional characterization of a novel ulvan utilization loci found in Alteromonas sp. LOR genome. Algal Res 25:39–46 [Google Scholar]
- Fournière M, Latire T, Lang M, Terme N, Bourgougnon N, Bedoux G (2019) Production of active poly-and oligosaccharidic fractions from Ulva sp. by combining enzyme-assisted extraction (EAE) and depolymerization. Metabolites 9(9):182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Clare AS, Rose C, Caldwell GS (2017) Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar Pollut Bull 114(1):439–447 [DOI] [PubMed] [Google Scholar]
- Gao J, Du C, Chi Y, Zuo S, Ye H, Wang P (2019) Cloning, expression, and characterization of a new PL25 family ulvan lyase from marine bacterium Alteromonas sp. A321. Mar Drugs 17(10):568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Qu H, Shan S, Song C, Baranenko D, Li Y, Lu W (2020) A novel polysaccharide isolated from Ulva Pertusa: structure and physicochemical property. Carbohydr Polym 233:115849 [DOI] [PubMed] [Google Scholar]
- Ghosh T, Chattopadhyay K, Marschall M, Karmakar P, Mandal P, Ray B (2009) Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiology 19(1):2–15 [DOI] [PubMed] [Google Scholar]
- Glasson CR, K. IM, Sims SM, Carnachan R, de Nys M, Magnusson (2017) A cascading biorefinery process targeting sulfated polysaccharides (ulvan) from Ulva Ohnoi. Algal Res 27:383–391 [Google Scholar]
- Glasson CRK, Luiten CA, Carnachan SM, Daines AM, Kidgell JT, Hinkley SFR, Praeger C, Andrade Martinez M, Sargison L, Magnusson M, de Nys R, Sims IM (2022) Structural characterization of ulvans extracted from blade (Ulva Ohnoi) and filamentous (Ulva Tepida and Ulva prolifera) species of cultivated Ulva. Int J Biol Macromol 194:571–579 [DOI] [PubMed] [Google Scholar]
- Guidara M, Yaich H, Richel A, Blecker C, Boufi S, Attia H, Garna H (2019) Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int J Biol Macromol 135:647–658 [DOI] [PubMed] [Google Scholar]
- Guo LY, Chen (2010) Optimization of ultrasonic-assisted extraction of polysaccharides from Enteromorpha Prolifera by response surface methodology. Food Sci 31:117–121 [Google Scholar]
- Han Y, Wu Y, Li G, Li M, Yan R, Xu Z, Lei H, Sun Y, Duan X, Hu L (2021) Structural characterization and transcript-metabolite correlation network of immunostimulatory effects of sulfated polysaccharides from green alga Ulva pertusa. Food Chem 342:128537 [DOI] [PubMed] [Google Scholar]
- Hardouin K, Bedoux G, Burlot A-S, Donnay-Moreno C, Bergé J-P, Nyvall-Collén P, Bourgougnon N (2016) Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res 16:233–239 [Google Scholar]
- He J, Xu Y, Chen H, Sun P (2016) Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int J Biol Macromol 17(12):1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Muramatsu H, Kato SI, Ohnishi K (2017) Characterization of an Alteromonas long-type ulvan lyase involved in the degradation of ulvan extracted from Ulva Ohnoi. Biosci Biotechnol Biochem 81(11):2145–2151 [DOI] [PubMed] [Google Scholar]
- Huang G, Mei X, Hu J (2017) The antioxidant activities of natural polysaccharides. Curr Drug Targets 18(11):1296–1300 [DOI] [PubMed] [Google Scholar]
- Ji G, Yu G, Wu J, Zhao X, Yang B, Wang L, Mei X (2009) Extraction, isolation and physiochemical character studies of polysaccharides from Enteromorpha clathrata in outbreak period. Chin J Mar Drugs 28:7–12 [Google Scholar]
- Jiang N, Li B, Wang X, Xu X, Liu X, Li W, Chang X, Li H, Qi H (2020) The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int J Biol Macromol 145:1059–1065 [DOI] [PubMed] [Google Scholar]
- Jiang F, Chi Z, Ding Y, Quan M, Tian Y, Shi J, Song F, Liu C (2021) Wound dressing hydrogel of enteromorpha prolifera polysaccharide–polyacrylamide composite: a facile transformation of marine blooming into biomedical material. ACS Appl Mater Interfaces 13(12):14530–14542 [DOI] [PubMed] [Google Scholar]
- Jiao L, Li X, Li T, Jiang P, Zhang L, Wu M, Zhang L (2009) Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int Immunopharmacol 9(3):324–329 [DOI] [PubMed] [Google Scholar]
- Jin W, He X, Long L, Fang Q, Wei B, Sun J, Zhang W, Wang H, Zhang F, Linhardt RJ (2020) Structural characterization and anti-lung cancer activity of a sulfated glucurono-xylo-rhamnan from Enteromorpha prolifera. Carbohydr Polym 237:116143 [DOI] [PubMed] [Google Scholar]
- Jmel MA, Anders N, Messaoud GB, Marzouki MN, Spiess A, Smaali I (2019) The stranded macroalga Ulva lactuca as a new alternative source of cellulose: extraction, physicochemical and rheological characterization. J Clean Prod 234:1421–1427 [Google Scholar]
- Kidgell JT, Magnusson M, de Nys R, Glasson CR (2019) Ulvan: a systematic review of extraction, composition and function. Algal Res 39:101422 [Google Scholar]
- Kidgell JT, Glasson CR, Magnusson M, Vamvounis G, Sims IM, Carnachan SM, Hinkley SF, Lopata AL, de Nys R, Taki AC (2020) The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. Int J Biol Macromol 150:839–848 [DOI] [PubMed] [Google Scholar]
- Kidgell JT, Carnachan SM, Magnusson M, Lawton RJ, Sims IM, Hinkley SF, de Nys R, Glasson CR (2021) Are all ulvans equal? A comparative assessment of the chemical and gelling properties of ulvan from blade and filamentous Ulva. Carbohydr Polym 264:118010 [DOI] [PubMed] [Google Scholar]
- Kim S-KI, Wijesekara (2010) Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods 2(1):1–9 [Google Scholar]
- Kim JK, Cho ML, Karnjanapratum S, Shin IS, You SG (2011) In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int J Biol Macromol 49(5):1051–1058 [DOI] [PubMed] [Google Scholar]
- Klongklaew N, Praiboon J, Tamtin M, Srisapoome P (2020) Antibacterial and antiviral activities of local Thai green macroalgae crude extracts in pacific white shrimp (Litopenaeus vannamei). Mar Drugs 18(3):140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Kido N, Sugiyama T, Yokochi T (2013) Antiviral activity of acidic polysaccharides from Coccomyxa Gloeobotrydiformi, a green alga, against an in vitro human influenza a virus infection. Immunopharmacol Immunotoxicol 35(1):1–7 [DOI] [PubMed] [Google Scholar]
- Kopel M, Helbert W, Belnik Y, Buravenkov V, Herman A, Banin E (2016) New family of ulvan lyases identified in three isolates from the Alteromonadales order. J Biol Chem 291(11):5871–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye MA, Robic (2007) Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 8(6):1765–1774 [DOI] [PubMed] [Google Scholar]
- Lee J-B, Hayashi K, Hayashi T, Sankawa U, Maeda M (1999) Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med 65(05):439–441 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang J, Yu Y, Li X, Jiang X, Hwang H, Wang P (2013) Production of enzymes by Alteromonas sp. A321 to degrade polysaccharides from Enteromorpha prolifera. Carbohydr Polym 98(1):988–994 [DOI] [PubMed] [Google Scholar]
- Li H, Li J, Zhao Z (2016a) Optimization of enzyme-assisted extraction of melanin from testae of wild apricots and evaluation of its stability. Food Sci 37(10):69–75 [Google Scholar]
- Li Y, Cui J, Zhang G, Liu Z, Guan H, Hwang H, Aker WG, Wang P (2016b) Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour Technol 214:144–149 [DOI] [PubMed] [Google Scholar]
- Li Y, Li W, Zhang G, Lu X, Hwang H, Aker WG, Guan H, Wang P (2016c) Purification and characterization of polysaccharides degradases produced by Alteromonas sp. A321. Int J Biol Macromol 86:96–104 [DOI] [PubMed] [Google Scholar]
- Li J, Chi Z, Yu L, Jiang F, Liu C (2017) Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int J Biol Macromol 105:1544–1553 [DOI] [PubMed] [Google Scholar]
- Li J-Y, Yang F, Jin L, Wang Q, Yin J, He P, Chen Y (2018a) Safety and quality of the green tide algal species Ulva prolifera for option of human consumption: a nutrition and contamination study. Chemosphere 210:1021–1028 [DOI] [PubMed] [Google Scholar]
- Li Q, Luo J, Wang C, Tai W, Wang H, Zhang X, Liu K, Jia Y, Lyv X, Wang L (2018b) Ulvan extracted from green seaweeds as new natural additives in diets for laying hens. J Appl Phycol 30:2017–2027 [Google Scholar]
- Li W, Jiang N, Li B, Wan M, Chang X, Liu H, Zhang L, Yin S, Qi H, Liu S (2018c) Antioxidant activity of purified ulvan in hyperlipidemic mice. Int J Biol Macromol 113:971–975 [DOI] [PubMed] [Google Scholar]
- Li B, Xu H, Wang X, Wan Y, Jiang N, Qi H, Liu X (2020a) Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int J Biol Macromol 146:756–762 [DOI] [PubMed] [Google Scholar]
- Li Y, Ye H, Wang T, Wang P, Liu R, Li Y, Tian Y, Zhang J (2020b) Characterization of low Molecular Weight Sulfate Ulva Polysaccharide and its protective effect against IBD in mice. Mar Drugs 18(10) [DOI] [PMC free article] [PubMed]
- Li Y, Zheng Y, Zhang Y, Yang Y, Wang P, Imre B, Wong AC, Hsieh YS, Wang D (2021) Brown algae carbohydrates: structures, pharmaceutical properties, and research challenges. Mar Drugs 19(11):620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Tang T, Jiang J, Yao Z, Zhu B (2023) Biochemical characterization of a new ulvan lyase and its applicability in utilization of ulvan and preparation of ulva oligosaccharides. Glycobiology 33(10):837–845 [DOI] [PubMed] [Google Scholar]
- Lin GP, Wu DS, Xiao XW, Huang QY, Chen HB, Liu D, Fu HQ, Chen XH, Zhao C (2020) Structural characterization and antioxidant effect of green alga Enteromorpha prolifera polysaccharide in Caenorhabditis elegans via modulation of microRNAs. Int J Biol Macromol 150:1084–1092 [DOI] [PubMed] [Google Scholar]
- Liu D, Keesing JK, Dong Z, Zhen Y, Di B, Shi Y, Fearns P, Shi P (2010) Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar Pollut Bull 60(9):1423–1432 [DOI] [PubMed] [Google Scholar]
- Liu XY, Liu D, Lin GP, Wu YJ, Gao LY, Ai C, Huang YF, Wang MF, El-Seedi HR, Chen XH, Zhao C (2019) Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int J Biol Macromol 139:342–351 [DOI] [PubMed] [Google Scholar]
- Liu G, Duan Y, Yang S, Yu M, Lv Z (2022) Simultaneous quantification of marine neutral neoagaro-oligosaccharides and agar-oligosaccharides by the UHPLC-MS/MS method: application to the intestinal transport study by using the Caco-2 cell monolayer. Anal Methods 14(22):2227–2234 [DOI] [PubMed] [Google Scholar]
- Lopes N, Ray S, Espada SF, Bomfim WA, Ray B, Faccin-Galhardi LC, Linhares REC, Nozawa C (2017) Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int J Biol Macromol 102:605–612 [DOI] [PubMed] [Google Scholar]
- Lu H, Gao Y, Shan H, Lin Y (2014) Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr Polym 107:98–102 [DOI] [PubMed] [Google Scholar]
- Lv H, Xiao B, Gao Y (2013) Study on the extraction, purification and structural characterization of polysaccharide from Enteromorpha. Food Res Dev 34(8):33–36 [Google Scholar]
- Mao W, Zang X, Li Y, Zhang H (2006) Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J Appl Phycol 18(1):9–14 [Google Scholar]
- Mao W, Li H, Li Y, Zhang H, Qi X, Sun H, Chen Y, Guo S (2009) Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int J Biol Macromol 44(1):70–74 [DOI] [PubMed] [Google Scholar]
- Matsubara K (2004) Recent advances in marine algal anticoagulants. Curr Med Chemistry-Cardiovascular Hematol Agents 2(1):13–19 [DOI] [PubMed] [Google Scholar]
- Meng X, Liang H, Luo L (2016) Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr Res 424:30–41 [DOI] [PubMed] [Google Scholar]
- Muhamad II, Zulkifli N, Lazim NAM (2019) Bioactive algal-derived polysaccharides: multi-functionalization, therapeutic potential and biomedical applications. Curr Pharm Des 25(11):1147–1162 [DOI] [PubMed] [Google Scholar]
- Olsson J, Toth GB, Oerbekke A, Cvijetinovic S, Wahlström N, Harrysson H, Steinhagen S, Kinnby A, White J, Edlund U (2020) Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. J Appl Phycol 32(5):3255–3263 [Google Scholar]
- Pan X, Wu H, Pan M, Zhang Y, Wei X, Cheng J (2019) Separation, purification and component analysis of Enteromorpha polysaccharides from Jiangsu. Chin J New Drugs 28(18):2274–2278 [Google Scholar]
- Pankiewicz R, Łęska B, Messyasz B, Fabrowska J, Sołoducha M, Pikosz M (2016) First isolation of polysaccharidic ulvans from the cell walls of freshwater algae. Algal Res 19:348–354 [Google Scholar]
- Pengzhan Y, Ning L, Xiguang L, Gefei Z, Quanbin Z, Pengcheng L (2003) Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol Res 48(6):543–549 [DOI] [PubMed] [Google Scholar]
- Pisoschi AMA, Pop (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74 [DOI] [PubMed] [Google Scholar]
- Ponce M, Zuasti E, Anguís V, Fernández-Díaz C (2020) Effects of the sulfated polysaccharide ulvan from Ulva ohnoi on the modulation of the immune response in Senegalese sole (Solea senegalensis). Fish Shellfish Immunol 100:27–40 [DOI] [PubMed] [Google Scholar]
- Pozharitskaya ON, Obluchinskaya ED, Shikov AN (2020) Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the Barents Sea. Mar Drugs 18(5):275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Zhao T, Zhang Q, Li Z, Zhao Z, Xing R (2006) Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J Appl Phycol 17(6):527–534 [Google Scholar]
- Qi X, Mao W, Gao Y, Chen Y, Chen Y, Zhao C, Li N, Wang C, Yan M, Lin C, Shan J (2012) Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydr Polym 90(4):1804–1810 [DOI] [PubMed] [Google Scholar]
- Qi X, Mao W, Chen Y, Chen Y, Zhao C, Li N, Wang C (2013) Chemical characteristics and anticoagulant activities of two sulfated polysaccharides from Enteromorpha linza (Chlorophyta). J Ocean Univ China 12:175–182 [Google Scholar]
- Qin H-M, Xu P, Guo Q, Cheng X, Gao D, Sun D, Zhu Z, Lu F (2018) Biochemical characterization of a novel ulvan lyase from Pseudoalteromonas sp. strain PLSV. RSC Adv 8(5):2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B (2006) Polysaccharides from Enteromorpha compressa: isolation, purification and structural features. Carbohydr Polym 66(3):408–416 [Google Scholar]
- Ray BM, Lahaye (1995) Cell-wall polysaccharides from the marine green alga Ulva rigida(Ulvales, Chlorophyta). Extraction and chemical composition. Carbohydr Res 274:251–261 [DOI] [PubMed] [Google Scholar]
- Reisky L, Stanetty C, Mihovilovic MD, Schweder T, Hehemann JH, Bornscheuer UT (2018) Biochemical characterization of an ulvan lyase from the marine flavobacterium Formosa Agariphila KMM 3901(T). Appl Microbiol Biotechnol 102(16):6987–6996 [DOI] [PubMed] [Google Scholar]
- Rial-Hermida MI, Rey-Rico A, Blanco-Fernandez B, Carballo-Pedrares N, Byrne EM, Mano JF (2021) Recent progress on polysaccharide-based hydrogels for controlled delivery of therapeutic biomolecules. ACS Biomater Sci Eng 7(9):4102–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues VJ, Jouanneau D, Fernandez-Fuentes N, Onime LA, Huws SA, Odaneth AA, Adams JM (2024) Biochemical characterisation of a PL24 ulvan lyase from seaweed-associated Vibrio sp. FNV38. J Appl Phycol 36(2):697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe M, van der Heide M, Weisbjerg M, Sehested J, Sloth JJ, Bruhn A, Vestergaard M, Nørgaard J, Hernández-Castellano LE (2021) A descriptive chemical analysis of seaweeds, Ulva sp., Saccharina latissima and Ascophyllum nodosum harvested from Danish and Icelandic waters. Anim Feed Sci Technol 278:115005 [Google Scholar]
- Shanmugam M, Ramavat B, Mody K, Oza R, Tewari A (2001) Distribution of heparinoid-active sulphated polysaccharides in some Indian marine green algae
- Shao P, Chen M, Pei Y, Sun P (2013) In intro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Int J Biol Macromol 59:295–300 [DOI] [PubMed] [Google Scholar]
- Shefer S, Robin A, Chemodanov A, Lebendiker M, Bostwick R, Rasmussen L, Lishner M, Gozin M, Golberg A (2021) Fighting SARS-CoV-2 with green seaweed Ulva sp. extract: extraction protocol predetermines crude ulvan extract anti-SARS-CoV-2 inhibition properties in in vitro Vero-E6 cells assay. PeerJ 9:e12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M-J, Wei X, Xu J, Chen B-J, Zhao D-Y, Cui S, Zhou T (2017) Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem 215:76–83 [DOI] [PubMed] [Google Scholar]
- Smetacek VA, Zingone (2013) Green and golden seaweed tides on the rise. Nature 504(7478):84–88 [DOI] [PubMed] [Google Scholar]
- Song D-x.,J.-g., Jiang (2017) Hypolipidemic components from medicine food homology species used in China: pharmacological and health effects. Arch Med Res 48(7): 569–581 [DOI] [PubMed]
- Song X, Guo X, Zhou W, Wen Y, Zhu C, Yang H (2010) Composition and Biological Activity of Water-Soluble Polysaccharide from Enteromorpha prolifera. LiShiZhen Med Materia Med Res 21(10):2448–2450 [Google Scholar]
- Srivastava R, Nedungadi S, Alharthi M, Ahamad M, Luqman M (2021) Nutraceutical products based on polysaccharides: sources, properties and applications. Food, Medical, and Environmental Applications of Polysaccharides, Elsevier, pp 531–554 [Google Scholar]
- Stender EG, Andersen CD, Fredslund F, Holck J, Solberg A, Teze D, Peters GH, Christensen BE, Aachmann FL, Welner DH (2019) Structural and functional aspects of mannuronic acid–specific PL6 alginate lyase from the human gut microbe Bacteroides cellulosilyticus. J Biol Chem 294(47):17915–17930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chen X, Song L, Liu S, Yu H, Wang X, Qin Y, Li P (2018) Antiviral activity against avian leucosis virus subgroup J of degraded polysaccharides from Ulva pertusa. BioMed Res Int 2018(3):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarsa M, You S, Dabaghian EH, Surayot U (2018) Water-soluble polysaccharides from Ulva intestinalis: molecular properties, structural elucidation and immunomodulatory activities. J Food Drug Anal 26(2):599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Gao H, Wang S, Wen S, Qin S (2013) Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int J Biol Macromol 58:186–189 [DOI] [PubMed] [Google Scholar]
- Tang T, Zhu B, Yao Z (2022) Biochemical characterization and elucidation the action mode of a new PL25 family ulvan lyase from marine bacterium Alteromonas Sp. TK-45 (2). Algal Research 67.
- Tang T, Li C, Zhu B, Yao Z (2023) Efficient preparation and production of ulvan oligosaccharides by using a new PL25 family ulvan lyase from Alteromonas Sp. J Appl Phycol 35(5):76–71 [Google Scholar]
- Teng Z, Qian L, Zhou Y (2013) Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int J Biol Macromol 62:254–256 [DOI] [PubMed] [Google Scholar]
- Thu QTM, Bang TH, Nu NT, Luong ĐV, Ly BM, Van TTT, Thuy TTT (2015) Structural determination of ulvan from green seaweed Ulva reticulata collected at central coast of Vietnam. Chem Lett 44(6):788–790 [Google Scholar]
- Tsubaki S, Oono K, Hiraoka M, Onda A, Mitani T (2016) Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem 210:311–316 [DOI] [PubMed] [Google Scholar]
- Tziveleka L-A, Ioannou E, Roussis V (2019) Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: a review. Carbohydr Polym 218:355–370 [DOI] [PubMed] [Google Scholar]
- Ulaganathan T, Boniecki MT, Foran E, Buravenkov V, Mizrachi N, Banin E, Helbert W, Cygler M (2017) New Ulvan-degrading polysaccharide lyase family: structure and catalytic mechanism suggests convergent evolution of active site architecture. ACS Chem Biol 12(5):1269–1280 [DOI] [PubMed] [Google Scholar]
- Ulaganathan T, Banin E, Helbert W, Cygler M (2018a) Structural and functional characterization of PL28 family ulvan lyase NLR48 from Nonlabens ulvanivorans. J Biol Chem 293(29):11564–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaganathan T, Helbert W, Kopel M, Banin E, Cygler M (2018b) Structure–function analyses of a PL24 family ulvan lyase reveal key features and suggest its catalytic mechanism. J Biol Chem 293(11):4026–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alstyne KL, Nelson TA, Ridgway RL (2015) Environmental Chemistry and Chemical Ecology of Green Tide Seaweed blooms. Integr Comp Biol 55(3):518–532 [DOI] [PubMed] [Google Scholar]
- Vinchhi P, Rawal SU, Patel MM (2021) Biodegradable hydrogels. Drug delivery devices and therapeutic systems, Elsevier: 395–419
- Wahlström N, Nylander F, Malmhäll-Bah E, Sjövold K, Edlund U, Westman G, Albers E (2020) Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr Polym 233:115852 [DOI] [PubMed] [Google Scholar]
- Wan Y, Liu L, Zhang B, Wang S, Wang X, Chen K, Li Y, Zhao T, Qi H (2022) Structural characterization and anti-nonalcoholic fatty liver effect of high-sulfated Ulva pertusa Polysaccharide. Pharmaceuticals 16(1):62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Z, Yao Z, Zhao M, Qi H (2013) Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int J Biol Macromol 58:225–230 [DOI] [PubMed] [Google Scholar]
- Wang Z, Xiao J, Fan S, Li Y, Liu X, Liu D (2015) Who made the world’s largest green tide in China?-an integrated study on the initiation and early development of the green tide in Yellow Sea. Limnol Oceanogr 60(4):1105–1117 [Google Scholar]
- Wang D, Li Y, Han L, Yin C, Fu Y, Zhang Q, Zhao X, Li G, Han F, Yu W (2022) Biochemical properties of a new polysaccharide lyase family 25 ulvan lyase TsUly25B from marine bacterium Thalassomonas sp. LD5. Mar Drugs 20(3):168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie T, Niu K, Xie C, Wang H, Xin W (2021) Extraction techniques, biological activities and health benefits of marine algae Enteromorpha prolifera polysaccharide. Front Nutr 8:747928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie T, Cheng B, Zhou T, Gao L, Lu Z, Wang J, Mulu B, Taye M, Wu X (2022) Enteromorpha polysaccharide and yeast glycoprotein mixture improves growth, antioxidant activity, serum lipid profile and regulates lipid metabolism in broiler chickens. Poult Sci 101(10):102064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara I, Pangestuti R, Kim S-K (2011) Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym 84(1):14–21 [Google Scholar]
- Witvrouw ME, De Clercq (1997) Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacology: Vascular Syst 29(4):497–511 [DOI] [PubMed] [Google Scholar]
- Wu L, Sun J, Su X, Yu Q, Yu Q, Zhang P (2016) A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr Polym 154:96–111 [DOI] [PubMed] [Google Scholar]
- Xu D, Huang X, Ou C, Xue C, Yang W, Haihong W (2005) In Vitro Study on polysaccharides in Enteromorpha with non-specific immunity. Food Sci 26(10):232–235 [Google Scholar]
- Xu J, Xu L-L, Zhou Q-W, Hao S-X, Zhou T, Xie H-J (2015) Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int J Biol Macromol 81:1026–1030 [DOI] [PubMed] [Google Scholar]
- Xu Y, Mao W, Gao W, Chi Z, Chi Z, Liu G (2019) Efficient production of a recombinant ι-carrageenase in Brevibacillus choshinensis using a new integrative vector for the preparation of ι-carrageenan oligosaccharides. Process Biochem 76:68–76 [Google Scholar]
- Xu Y, Li J, An L, Qiu Y, Mao A, He Z, Guo J, Yan H, Li H, Hu Z (2024) Biochemical characterization of a Novel Thermostable Ulvan Lyase from Tamlana fucoidanivorans CW2-9. J Agric Food Chem. [DOI] [PubMed]
- Yang B, Yu G, Zhao X, Ren W, Jiao G, Fang L, Wang Y, Du G, Tiller C, Girouard G (2011) Structural characterisation and bioactivities of hybrid carrageenan-like sulphated galactan from red alga Furcellaria Lumbricalis. Food Chem 124(1):50–57 [Google Scholar]
- Yang Y, Zhang P, Liu G, Yang S, Wang Y, Jiang T, Lv Z, Yu M (2020) Simultaneous quantification of κ-Carrageenan oligosaccharides of DP 3, 5 and 7 by LC-MS/MS: application to an in vitro absorption study. J Ocean Univ China 19(5):1177–1182 [Google Scholar]
- Yu Y, Li Y, Du C, Mou H, Wang P (2017) Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr Polym 165:221–228 [DOI] [PubMed] [Google Scholar]
- Yu Y, Shen M, Song Q, Xie J (2018) Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym 183:91–101 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Xu X, Jing C, Zou P, Zhang C, Li Y (2018) Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: functional properties and bioactivities. Carbohydr Polym 181:902–910 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang F, Wang X, Liu X, Hou Y, Zhang Q (2010) Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr Polym 82(1):118–121 [Google Scholar]
- Zhang Z, Wang X, Mo X, Qi H (2013) Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr Polym 92(2):2084–2087 [DOI] [PubMed] [Google Scholar]
- Zhang W, Oda T, Yu Q, Jin JO (2015) Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar Drugs 13(3):1084–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Han X, Xu Y, Li J, Li Y, Hu Z (2016) Biodegradation of Enteromorpha polysaccharides by intestinal micro-community from Siganus oramin. J Ocean Univ China 15(6):1034–1038 [Google Scholar]
- Zhang R, Chen Y, Zhou Y, Tong D, Hu C (2019) Selective conversion of hemicellulose in macroalgae Enteromorpha prolifera to rhamnose. ACS Omega 4(4):7023–7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun W, Zhang S, Meng G, Qi C, Fan W, Wang Y, Liu J (2016) The immune adjuvant response of polysaccharides from Atractylodis macrocephalae Koidz in chickens vaccinated against Newcastle disease (ND). Carbohydr Polym 141:190–196 [DOI] [PubMed] [Google Scholar]
- Zhao C, Yang C, Wai STC, Zhang Y, M PP, Paoli P, Wu Y, San Cheang W, Liu B, Carpene C, Xiao J, Cao H (2019) Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit Rev Food Sci Nutr 59(6):830–847 [DOI] [PubMed] [Google Scholar]
- Zhong R, Wan X, Wang D, Zhao C, Liu D, Gao L, Wang M, Wu C, Nabavid SM, Daglia M (2020) Polysaccharides from marine Enteromorpha: structure and function. Trends Food Sci Technol 99:11–20 [Google Scholar]
- Zhou Z, Pan S, Wu S (2020) Modulation of the growth performance, body composition and nonspecific immunity of crucian carp Carassius auratus upon Enteromorpha prolifera polysaccharide. Int J Biol Macromol 147:29–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.