Abstract
Purpose
Out-of-hospital cardiac arrest (OHCA) survivors face significant risks of complications and death from hypoxic–ischemic brain injury leading to withdrawal of life-sustaining treatment (WLST). Accurate multimodal neuroprognostication, including automated pupillometry, is essential to avoid inappropriate WLST. However, inconsistent study results hinder standardized threshold recommendations. We aimed to validate proposed pupillometry thresholds with no false predictions of unfavorable outcomes in comatose OHCA survivors.
Methods
In the multi-center BOX-trial, quantitative measurements of automated pupillometry (quantitatively assessed pupillary light reflex [qPLR] and Neurological Pupil index [NPi]) were obtained at admission (0 h) and after 24, 48, and 72 h in comatose patients resuscitated from OHCA. We aimed to validate qPLR < 4% and NPi ≤ 2, predicting unfavorable neurological conditions defined as Cerebral Performance Category 3–5 at follow-up. Combined with 48-h neuron-specific enolase (NSE) > 60 μg/L, pupillometry was evaluated for multimodal neuroprognostication in comatose patients with Glasgow Motor Score (M) ≤ 3 at ≥ 72 h.
Results
From March 2017 to December 2021, we consecutively enrolled 710 OHCA survivors (mean age: 63 ± 14 years; 82% males), and 266 (37%) patients had unfavorable neurological outcomes. An NPi ≤ 2 predicted outcome with 0% false-positive rate (FPR) at all time points (0–72 h), and qPLR < 4% at 24–72 h. In patients with M ≤ 3 at ≥ 72 h, pupillometry thresholds significantly increased the sensitivity of NSE, from 42% (35–51%) to 55% (47–63%) for qPLR and 50% (42–58%) for NPi, maintaining 0% (0–0%) FPR.
Conclusion
Quantitative pupillometry thresholds predict unfavorable neurological outcomes in comatose OHCA survivors and increase the sensitivity of NSE in a multimodal approach at ≥ 72 h.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-024-07574-6.
Keywords: Out-of-hospital cardiac arrest, Intensive cardiovascular care, Pupillometry, Neuroprognostication, Outcome prediction
Take-home message
| Prespecified thresholds of quantitatively assessed pupillary light reflex < 4% and the Neurological Pupil index ≤ 2 predict unfavorable neurological outcomes with zero-percent false-positive rate in comatose out-of-hospital cardiac arrest survivors. Combined with neuron-specific enolase, pupillometry thresholds offer a robust multimodal neuroprognostication strategy, especially relevant for centers facing challenges in obtaining neurophysiological data. |
Introduction
Despite hospitalization, out-of-hospital cardiac arrest (OHCA) in Europe averages a 26% survival-to-discharge rate (41% in Denmark, rising to 81% if bystander-witnessed with initial shockable rhythm), reaching 35% for those admitted with return of spontaneous circulation (ROSC) [1]. Most die from hypoxic–ischemic brain injury, leading to prolonged coma and active withdrawal of life-sustaining treatment (WLST) [2, 3].
European countries practicing WLST report over 90% favorable long-term neurological outcomes in survivors, contrasting a 50% unfavorable outcome, with 33% in a persistent vegetative state, in countries where WLST is not implemented [4]. To avoid futile treatment and prevent inappropriate WLST, the European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM) recommend a multimodal neuroprognostication strategy in survivors remaining comatose at ≥ 72 h after ROSC [5]. This strategy involves clinical, electrophysiological, biomarker, and neuroimaging predictors, with an unfavorable outcome being likely with at least two positive indicators.
Assessment of pupillary reflexes is a crucial predictor in patients with acute brain injury [6, 7]. Due to significant inter-observer variability when manually assessing pupillary reflexes with a penlight, guidelines recommend automated pupillometers for neuroprognostication [5]. The automated pupillometer quantitatively measures pupil reaction, including the percentage of pupillary constriction (quantitatively assessed pupillary light reflex, qPLR) and several other reactivity variables. These can be integrated into an algorithm-based composite risk score, such as the Neurological Pupil index (NPi), ranging from 0 to 5 [8, 9].
Several studies have confirmed the significant prognostic value of qPLR and NPi and proposed specific thresholds predicting neurological outcomes with no false predictions [10–18]. However, using different devices and unaligned assessment and analyzing methods, results on absolute values differ across studies. Further, as no studies have reproduced and validated findings in a uniform methodology, guidelines have been unable to provide standardized threshold recommendations [19].
We aimed to perform an external validation with a similar methodology of the previous studies proposing pupillometry thresholds of qPLR < 4% and NPi ≤ 2, shown to predict unfavorable outcomes from admission to 72 h with zero-percent false-positive rate (FPR) in comatose OHCA survivors [10, 13]. As the recommended multimodal approach demands at least two positive predictors in unconscious patients at ≥ 72 h, we further aimed to investigate the prognostic effect of pupillometry combined neuron-specific enolase (NSE) > 60 μg/L, a biochemical marker of neurologic damage (with higher levels associated with more extensive brain injury) [20].
Early preliminary results from this substudy have previously been presented [21, 22].
Methods
Study design and patients
We conducted a protocolized [23], prospective, multi-center prognostic substudy within the Blood Pressure and Oxygenation Targets after Cardiac Arrest (BOX) trial. The BOX-trial, an investigator-initiated, multi-center, randomized trial, compared blood pressure, oxygen, and temperature targets [24–26]. From March 2017 to December 2021, we consecutively included 789 comatose OHCA survivors (≥ 18 years) of presumed cardiac origin from two Danish tertiary cardiac arrest centers. The BOX-trial design, data collection, management, and informed consent handling have been previously published [27]. This substudy adhered to the overall protocolized parameters of the main BOX-trial [23, 24, 27].
Subjects from the BOX-trial were eligible (criteria outlined in supplementary Table S1), however, excluded if pre-existing ophthalmic conditions affecting pupil movement were present (e.g., cataract and eye surgery) [28, 29]. Pre-hospital data were systematically collected according to Utstein-style guidelines [30] and reported in accordance with the STARD and TRIPOD statements.
Post-cardiac arrest care and study procedures
All patients received protocolized guideline-suggested post-resuscitation care [23, 24, 27]. This included blood-pressure management with vasoactive agents (primarily with norepinephrine and dopamine) and oxygenation during mechanical ventilation (primarily by FiO2 and positive end-expiratory pressure [PEEP] adjustments). Sedation was achieved with propofol and fentanyl to a Richmond agitation sedation scale of -4 or less, and temperature control targets were managed with active cooling devices. After 24 h of temperature control, core temperature was gradually increased to normothermia at a rewarming rate of < 0.5 °C per hour, and sedation was tapered.
Pupillometry assessments were conducted at admission (t0) and at 24 (t24), 48 (t48), and 72 h (t72), approximated to within ± 6 h as part of clinical practice. Clinical nursing staff used NPi®-200 pupillometers (NeurOptics®, Irvine, CA, USA) until awakening or death. Plasma NSE was measured at t48 using a COBAS analyzer system (Roche Diagnostics).
We included continuously comatose patients with Glasgow Motor Score (M) ≤ 3 at ≥ 72 h after admission in the analysis for the neuroprognostication strategy algorithm with combined predictors of 48-h plasma NSE > 60 μg/L and pupillometry [5].
Automatic pupillometry assessments were performed with the same device for each eye, and measurements were automatically imported into the electronic database through SmartGuard® devices, which were unviewed until post-trial analysis, blinded to outcome assessors. The lowest value of the two eyes defined threshold values associated with an unfavorable outcome, as in similar studies [10, 16, 18].
This study used qPLR (%) and NPi to predict outcomes. qPLR corresponds to the percentage of pupillary constriction to a calibrated light stimulus (1000 Lux) from the NPi-200. The NPi, a composite score from 0 to 5, integrates multiple pupillary variables from the device (size, latency, constriction, and dilation velocity), with lower values indicating less reactivity and greater pathology [14, 18, 31, 32]. NPi values ≥ 3 are considered normal, while < 3 indicates abnormal reactivity. An NPi value of 0 indicates a non-reactive/absent response.
Outcome measures
The primary outcome was neurological function defined by Cerebral Performance Category (CPC) score [33, 34] assessed at a follow-up visit no earlier than 90 days post-randomization. We invited patients to the follow-up that included neurological evaluation by CPC (ranging from 1 to 5, increasing with more significant disability; 3 or 4 being severe disability, coma, or vegetative state, and 5 being death), modified Rankin scale (mRS) score [35, 36] (range 0–6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death), and Montreal Cognitive Assessment (MOCA) score (range, 0 to 30, with a score of 26 or higher being normal), performed by trained personnel blinded to the pupillometry results [35–37]. A CPC or mRS score of ≥ 3 defines an unfavorable neurological outcome.
For non-participating outpatients, we conducted telephone interviews or reviewed medical records, excluding MOCA scoring. Some visits were delayed due to the coronavirus disease 2019 (COVID-19) pandemic hospital restrictions [24].
Secondary outcomes included death from any cause within 365 days, median 48-h plasma NSE, and follow-up mRS and MOCA. In addition to this, we evaluated the primary outcome, with all extracerebral deaths (no sign of severe brain injury in neuroprognostication and hemodynamic, multi-organ failure, sepsis, or other causes more likely) occurring during initial admission excluded.
Prognostication and withdrawal of life-sustaining treatment
The protocolized prognostication from the BOX-trial [24, 27] is referenced in the supplementary appendix. Decision on WLST was made no earlier than 72 h after ending sedation, based on criteria including brain death due to cerebral herniation, severe myoclonus status ≤ 24 h with bilateral absence of N20-peak on median nerve somatosensory-evoked potentials (SSEP), persisting M ≤ 3 with bilateral absence of N20-peak on median nerve SSEP < 72 h, and persisting M ≤ 3 with treatment-refractory status epilepticus < 72 h. Prior to prognostication, electroencephalographs (EEGs) were performed on all patients who were persistently comatose 24 h after sedation and analgesics had been tapered. If inconclusive, subsequent EEGs were performed.
Automated pupillometry was used for research only (unutilized by treating physicians or included in clinical neuroprognostication/WLST), as it was only recently recommended in guidelines [5].
Statistical methods
Categorical variables are expressed as counts (percentage), and continuous variables as mean (± standard deviation [SD]) or median (interquartile range [IQR]). We assessed differences using Chi-square or Fisher’s exact test for categorical variables and t tests for continuous variables. We assumed a linear mixed-effects model with an unstructured covariance for repeated measures to analyze mean value differences over time and between primary outcome groups of unfavorable outcomes at follow-up. We incorporated outcome group, time-point, and their interaction term as fixed effects, with random intercepts for each patient. We employed logistic regression models to estimate the performance of quantitative pupillometry in predicting the outcomes of CPC3-5 and mRS3-6 at follow-up after 90 days, and death from any cause within 365 days. The analysis involved evaluating the predictive accuracy using Receiver-Operating Characteristic (ROC) curves and Area Under the Curve (AUC) statistics. Difference in AUCs was tested with the De Long method [38]. Models were adjusted for age, sex, time-to-ROSC, lactate level at admission, witnessed arrest, NSE, bystander cardiopulmonary resuscitation (CPR), shockable primary rhythm, and randomization site.
We calculated specificity (%), sensitivity (%), FPR (%), positive predictive value (PPV, %), negative predictive value (NPV, %), Youden Index, and area under the curve (AUC) and presented results with 95% confidence intervals (95% CI) when appropriate. The combination of pupillometry and NSE thresholds was performed sequentially with a conditional subsequent retest (qPLR < 4 or NPi ≤ 2) if the first test was negative (NSE ≤ 60 μg/L). The overall test was positive if either test was positive. As a sensitivity analysis, we performed interaction analyses and assessed the characteristics of patients with missing quantitative pupillometry data. If at random, we conducted multiple imputations by chained equations.
As per protocol [23], we calculated sample size using the Jones et al.’s method, similar to that of Oddo et al. [10, 39]. Based on a specificity of 95%, a 95% CI of 3%, and a prevalence of unfavorable outcomes of 38%, we needed at least 534 patients. We used R statistical software (version 4.2.2) for all analyses, with a two-sided significance level of 5%.
This trial is registered with ClinicalTrials.gov, NCT03141099.
Results
Baseline demographic and characteristics of patients
Of the 789 comatose OHCA survivors in the BOX-trial [24], 71 lacked pupillometry data, and 8 were excluded due to a history of cataract surgery. The final study population included 710 patients (mean age: 63 ± 14 years; 82% males). The mean time-to-ROSC was 21 ± 14 min, 91% had a primary shockable rhythm, 85% had witnessed OHCA, and 89% received bystander CPR (Table 1). Patients with qPLR < 4% or NPi ≤ 2 at t48 had fewer occurrences of shockable rhythm, longer time-to-ROSC, significantly lower pH, and higher lactate levels on admission (supplementary Table S2). Patient flow and exclusions are summarized in supplementary Fig. S1.
Table 1.
Demographic and clinical characteristics of the patients
| All, n = 710 |
Outcome at follow-up | P value | ||
|---|---|---|---|---|
| Favorable, n = 444 |
Unfavorable, n = 266 |
|||
| Demographic characteristics | ||||
| Age—years | 63 ± 14 | 60 ± 14 | 67 ± 12 | < 0.001 |
| Male sex—n (%) | 580 (82) | 372 (84) | 208 (78) | 0.062 |
| Medical history—n (%) | ||||
| Hypertension, medically treated | 320 (45) | 186 (42) | 134 (51) | 0.024 |
| Diabetes | 93 (13) | 46 (10) | 47 (18) | 0.005 |
| Myocardial infarction | 150 (21) | 89 (20) | 61 (23) | 0.332 |
| Atrial fibrillation | 117 (17) | 57 (13) | 60 (23) | < 0.001 |
| Heart failure | 126 (18) | 60 (14) | 66 (25) | < 0.001 |
| Chronic obstructive pulmonary disease | 55 (8) | 24 (5) | 31 (12) | 0.002 |
| Stroke | 51 (7) | 28 (6) | 23 (9) | 0.241 |
| Chronic kidney disease | 33 (5) | 14 (3) | 19 (7) | 0.014 |
| Characteristics of the cardiac arrest—n (%) | ||||
| Shockable rhythm | 643 (91) | 420 (95) | 223 (84) | < 0.001 |
| Pulseless electrical activity | 28 (4) | 7 (2) | 21 (8) | < 0.001 |
| Witnessed arrest | 604 (85) | 386 (87) | 218 (82) | 0.060 |
| Bystander cardiopulmonary resuscitation | 619 (89) | 406 (93) | 213 (81) | < 0.001 |
| First defibrillation by automated external defibrillator | 158 (23) | 110 (25) | 48 (18) | 0.033 |
| Time to return of spontaneous circulation—minutes | 21 ± 14 | 17 ± 11 | 27 ± 16 | < 0.001 |
| Findings and procedures on arrival at hospital | ||||
| ST-segment elevation ECG—n (%) | 316 (45) | 200 (46) | 116 (44) | 0.643 |
| Coronary angiogram obtained—n (%) | 644 (91) | 397 (89) | 247 (93) | 0.126 |
| PCI performed—n (%) | 298 (46) | 189 (48) | 109 (44) | 0.358 |
| pH level | 7.21 ± 0.13 | 7.23 ± 0.12 | 7.18 ± 0.13 | < 0.001 |
| Lactate level—mmol/liter | 5.8 ± 3.8 | 5.2 ± 3.7 | 6.9 ± 3.8 | < 0.001 |
Plus–minus values are means ± SD. Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 30 ml per minute per 1.73 m2 of body-surface area
N number, ECG electrocardiogram, PCI percutaneous coronary intervention
The 71 patients lacking pupillometry were older, had fewer primary shockable rhythms, and instances of bystander CPR. However, there were no differences in outcome data, and multiple imputations yielded prognostic results similar to the complete cases.
Outcomes
As the 710 (100%) patients at t0 gradually woke up or died, we obtained pupillometry at t24 (n = 682, 96%), t48 (n = 543, 76%), and t72 (n = 301, 42%). After 72 h, 39 (5%) patients had died, and 187 (26%) were still unconscious with M ≤ 3.
At follow-up (median time of 134 days), we estimated unfavorable neurological outcomes with median values (IQR) in 266 (37%) patients with 1 (1–5) for CPC, and in 280 (39%) patients with 1 (0–6) for mRS, similar to other Danish studies [13, 15]. Median MOCA was 27 (24–29), and plasma NSE level at 48 h was 18 (11–37) μg/L. With a median lifetime of 7 (4–21) days from admission, 257 (35%) patients died within 365 days (extracerebral deaths, n = 63). Patients with qPLR < 4% or NPi ≤ 2 at t48 had significantly higher incidents of primary and secondary outcomes than for thresholds above (Table 2).
Table 2.
Primary and secondary outcomes according to t48 pupillometry thresholds
| qPLR ≥ 4%, n = 494 |
qPLR < 4%, n = 46 |
P value | NPi > 2, n = 523 |
NPi ≤ 2, n = 20 |
P value | |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| CPC 3–5, unfavorable outcome at follow-up, n (%)a | 172/494 (35) | 45/46 (98) | < 0.001 | 200/523 (38) | 20/20 (100) | < 0.001 |
| Median CPC at follow-up (IQR)a | 1 (1–5) | 5 (5–5) | < 0.001 | 1 (1–5) | 5 (5–5) | < 0.001 |
| CPC 3–5, unfavorable outcome at follow-up (extracerebral deaths excluded), n (%)a | 136/451 (30) | 42/43 (98) | < 0.001 | 164/480 (34) | 17/17 (100) | < 0.001 |
| Secondary outcomes | ||||||
| Death from any cause within 365 days, n (%) | 160/494 (33) | 43/46 (93) | < 0.001 | 186/523 (36) | 20/20 (100) | < 0.001 |
| mRS 3–6, unfavorable outcome at follow-up, n (%)b | 182/494 (37) | 45/46 (98) | < 0.001 | 210/523 (40) | 20/20 (100) | < 0.001 |
| Median mRS score at follow-up (IQR)b | 1 (0–6) | 6 (6–6) | < 0.001 | 1 (0–6) | 6 (6–6) | < 0.001 |
| Median MOCA score at follow-up (IQR)c | 27 (24–29) | NAd | NAd | 27 (24–29) | NAd | NAd |
| Median 48-h plasma NSE level (IQR), μg/liter | 18 (11–34) | 56 (34–127) | < 0.001 | 19 (11–39) | 45 (29–70) | 0.004 |
Occurrences of primary and secondary outcomes according to the specific qPLR and NPi thresholds measured at t48. Follow-up was after 90 days according to the detailed description in the methods section
qPLR percentage pupillary constriction in response to a calibrated light stimulus indicated as quantitatively assessed pupillary light reflex, NPi Neurological Pupil index, AUC area under the curve, CPC Cerebral Performance Category, mRS modified Rankin scale score, MOCA Montreal Cognitive Assessment, NSE neuron-specific enolase, IQR Interquartile range
aCPC ranges from 1 (no symptoms) to 5 (death); a category of 3 or 4 indicates severe disability or a coma or vegetative state
bmRS scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death
cMOCA scores range from 0 to 30, with a score of 26 or higher being normal. For the score at 3 months (per protocol), the lowest score found in the trial population was assigned to patients unavailable for follow-up, including deceased patients
dOnly one patient with qPLR < 4, and no patients NPi ≤ 2, had MOCA score available
Pupillometry
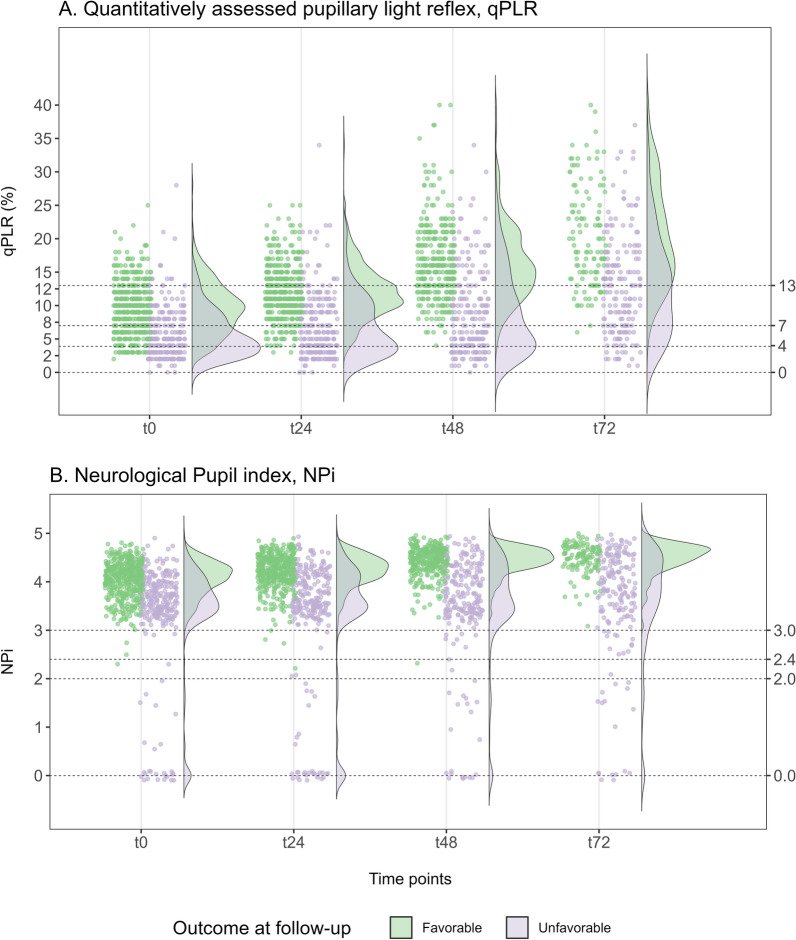
Values for favorable outcome patients were initially significantly lower, clustering tightly at admission and t24 compared to t48 and t72. Unfavorable outcome patients showed no significant NPi increase between time points, creating a wider gap to favorable outcome patients later. Even without statistical significance, we observed similar trends for qPLR. However, median values for both predictors remained significantly lower in patients with unfavorable outcomes at all time points. We have illustrated the distribution of qPLR and NPi according to time points by outcome at follow-up in Fig. 1.
Fig. 1.
Distribution of quantitative pupillometry measurements during the first 72 h (t0–t72) after cardiac arrest according to outcome at follow-up. The rainfall plots show the measurements of qPLR (A) and NPi (B) at time points, with the density and distribution depicted in the violin plot. Measures are divided by primary outcome group, dichotomized as favorable (green) and unfavorable (purple). qPLR quantitatively assessed pupillary light reflex, NPi Neurological Pupil index
Prognostic performance, assessed by AUC under the ROC curve, peaked at t48 for qPLR (0.81 [0.77–0.85]) and NPi (0.83 [0.80–0.87]) predicting primary outcome (supplementary Table S3), with consistent findings for secondary outcomes (supplementary Table S4). Adjusted for clinical predictors, the highest AUC occurred at t24 for qPLR (0.93 [0.91–0.96, p < 0.001]) and at t48 for NPi (0.93 [0.90–0.95, p < 0.001]). Both qPLR and NPi had similar significant contributions to AUC at all time points in the multivariable model. When excluding extracerebral causes of death, the increased AUC was insignificant in either predictor compared to the total population.
An NPi ≤ 2 from t0 to t72 predicted unfavorable neurological outcomes at follow-up and death within 365 days, with 0% (0–0%) FPR. Similarly, a qPLR < 4% from t24 to t72 predicted outcomes with a 0% (0–0% to 0–1%) FPR (Table 3). The sensitivity was highest at 26% for qPLR and 12% for NPi at t24. Exploring thresholds predicting outcome with maximum sensitivity and 0% FPR, qPLR < 3 (t0), < 4 (t24-t48), and < 6 (t72) showed a corresponding sensitivity of 16–26%. Similar NPi < 2.8 (t0), < 2.9 (t24), < 3.3 (t48), and < 3.1 (t72) resulted in a sensitivity of 10–18% (supplementary Table S5a + b).
Table 3.
Prognostic performance of pupillometry thresholds and NSE
| Sample size, n (%) |
FPR, % (95% CI) |
Sensitivity, % (95% CI) |
PPV, % (95% CI) |
NPV, % (95% CI) |
|
|---|---|---|---|---|---|
| qPLR < 4% | |||||
| t0 | 710 (100) | 5 (3–7) | 31 (25–36) | 78 (70–86) | 70 (68–71) |
| t24 | 677 (95) | 0 (0–1) | 26 (21–32) | 97 (92–100) | 71 (69–72) |
| t48 | 540 (76) | 0 (0–1) | 21 (16–26) | 98 (93–100) | 65 (64–67) |
| t72 | 298 (42) | 0 (0–0) | 8 (4–12) | 100 (100–100) | 43 (42–44) |
| NPi ≤ 2 | |||||
| t0 | 710 (100) | 0 (0–0) | 10 (6–13) | 100 (100–100) | 65 (64–66) |
| t24 | 682 (96) | 0 (0–0) | 12 (8–17) | 100 (100–100) | 67 (66–67) |
| t48 | 543 (76) | 0 (0–0) | 9 (5–13) | 100 (100–100) | 62 (61–63) |
| t72 | 301 (42) | 0 (0–0) | 9 (5–13) | 100 (100–100) | 43 (42–44) |
| NSE > 60 μg/L | |||||
| 48-h | 625 (88) | 2 (0–3) | 39 (33–46) | 95 (91–100) | 70 (67–72) |
| 48-h plasma NSE and pupillometry predicting outcome in unconscious patients, M < 3, at ≥ 72 h | |||||
| NSE > 60 μg/L | 187 (26) | 0 (0–0) | 42 (35–51) | 100 (100–100) | 76 (70–82) |
| NSE > 60 μg/L + qPLR < 4.0 | 187 (26) | 0 (0–0) | 55 (47–63) | 100 (100–100) | 73 (66–79) |
| NSE > 60 μg/L + NPi ≤ 2.0 | 187 (26) | 0 (0–0) | 50 (42–58) | 100 (100–100) | 74 (68–81) |
| 48-h plasma NSE and pupillometry predicting outcome in unconscious patients, M < 3, at ≥ 72 h (with extracerebral causes of death excluded) | |||||
| NSE > 60 μg/L | 172 (24) | 0 (0–0) | 45 (37–53) | 100 (100–100) | 76 (69–82) |
| NSE > 60 μg/L + qPLR < 4.0 | 172 (24) | 0 (0–0) | 58 (50–67) | 100 (100–100) | 72 (65–79) |
| NSE > 60 μg/L + NPi ≤ 2.0 | 172 (24) | 0 (0–0) | 51 (43–60) | 100 (100–100) | 75 (68–82) |
The prognostic probabilities of quantitative pupillometry thresholds, 48-h plasma neuron-specific enolase, and the combination in unconscious patients, M < 3, at ≥ 72 h, predicting unfavorable outcomes at follow-up
qPLR quantitatively assessed pupillary light reflex, NPi Neurological Pupil index, M Glasgow motor scale, FPR false-positive rate, PPV positive predictive value, NPV negative predictive value
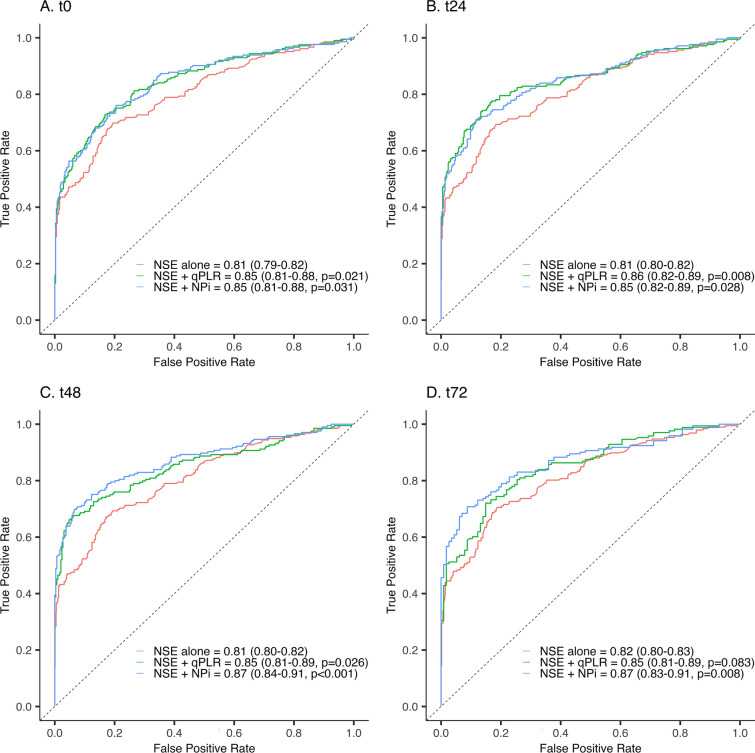
With an AUC of 0.81 (0.80–0.82), 48-h plasma NSE > 60 μg/L predicted the primary outcome with 2% (0–3%) FPR and 39% (33–46%) sensitivity. When combined, pupillometry significantly improved the prognostic performance of NSE (from t0 to t48 for qPLR and from t0 to t72 for NPi) (Fig. 2). In the multimodal neuroprognostication strategy for comatose patients, with M ≤ 3, at ≥ 72 h, NSE > 60 μg/L predicted an unfavorable outcome with 0% (0–0%) FPR and sensitivity of 42% (35–51%). When t48 pupillometry was implemented as the second predictor, it significantly increased sensitivity to 55% (47–63%) for qPLR and 50% (42–58%) for NPi. When exploring the performance of other pupillometry thresholds, qPLR < 9% and NPi < 3.3 maintained predictions with 0% FPR and improved sensitivity to 70% (62–77%) and 57% (49–65%), respectively.
Fig. 2.
Prognostic performance of pupillometry and 48-h NSE. Receiver-operating characteristic curves depicting the prognostic performance by the area under the curve of 48-h plasma neuron-specific enolase level alone and combined with quantitatively assessed pupillary light reflex and Neurological Pupil index, respectively, measured at t0 (A), t24 (B), t48 (C), and t72 (D). The P value depicts the significance of the contribution of pupillometry to neuron-specific enolase in predicting unfavorable outcomes at follow-up. NSE neuron-specific enolase, qPLR quantitatively assessed pupillary light reflex, NPi Neurological Pupil index
Discussion
In this prospective multi-center study of 710 OHCA survivors, we present the largest cohort to investigate the prognostic efficacy of automated quantitative pupillometry.
We successfully validated proposed pupillometry thresholds for predicting unfavorable neurological outcomes at follow-up with 0% FPR. Assessments from admission (NPi ≤ 2) and from day 1 (qPLR < 4%) were 100% specific for outcome at all remaining time points. Notably, both parameters remained robust as independent predictors, and thresholds of qPLR < 3 and NPi < 2.8 achieved a 0% false-positive rate immediately after admission.
These findings align with the previous studies [10, 13] and the recent ORANGE study [18], an extensive, international, multicentre, prospective, observational study that demonstrated clinically and statistically significant prognostic value of quantitative pupillometry for neurological outcomes and mortality in 514 patients with traumatic brain injury, aneurysmal subarachnoid hemorrhage, and intracerebral hemorrhage. This study emphasizes the importance of NPi as a very early predictor, as proposed by Oddo et al. [10].
Sedatives/analgesics (especially propofol and fentanyl used in this study) minimally affect NPi, whereas deep sedation may decrease qPLR [40, 41]. This may explain NPi's consistent predictability across all time points, with qPLR achieving 100% specificity only after day 1.
A recent systematic review and meta-analysis by Wang et al. [12] proposed thresholds of qPLR ≤ 7% and NPi ≤ 2 derived from pooled data median values. The NPi threshold was consistent with the validated data from this study, however, both Wang et al. and another recent systematic review [11, 12], identified variability in proposed qPLR thresholds ranging from 0% at 72 h to < 7% and < 13% at 48 h [10, 14, 17]. Studies using a Neurolight® (IDMED, Marseille, France) pupillometer reported qPLR thresholds of < 7–13% but also higher median values, with previous research indicating an approximately 10% variance between the Neurolight and the NPi-200 used in this study [42]. Oddo et al. [10], using an NPi-200, reported median qPLR values similar to this study (± 0–1%) but with a even more restricted threshold of qPLR = 0% for predicting outcomes with 0% FPR at day 3. Their population included cardiac and non-cardiac causes for arrest, and patients with favorable outcomes had longer time-to-ROSC and a higher rate of non-shockable primary rhythm compared to the general Danish OHCA population [1, 43]. This could have yielded more false-positive qPLR measurements due to extended “recovery period” from transient brain stem dysfunction after OHCA [44, 45]. As qPLR can be decreased when influenced by anesthetics [40], a lower qPLR threshold predicting outcome would be expected when pupillometry was performed regardless of sedation [10].
The sensitivity corresponding to the extreme FPR found in this study (NPi 9–12% and qPLR 8–26%) was lower than previously reported [11]. However, exploratory analyses for optimal thresholds showed increased sensitivity (NPi 10–18% and qPLR 16–26%). Slightly lower FPR at 1–2%, increased sensitivity significantly (NPi at 25–46% and qPLR at 41–50%). The lowest sensitivity was observed at t72, possibly influenced by the change in population composition (most patients with true-positive favorable outcomes were awake) and recovery from transient brain stem dysfunction. The ORANGE study confirmed that an abnormal NPi (< 3) is strongly associated with long-term mortality and poor neurological outcomes in acute brain injury patients [18]. While ORANGE included non-anoxic acute brain injury of a more heterogeneous composition, our results confirm the prognostic value of NPi < 3 in hypoxic–ischemic brain injury. At 48–72 h post-OHCA, NPi < 3 predicted unfavorable outcomes with 100% specificity. The pathology of non-anoxic brain injuries warrants repeated measurements for predicting outcomes. Dynamic changes in our results indicate that this could be beneficial for OHCA survivors as well and should be further investigated.
As pupillometry is still dichotomized in “present” versus “absent,” it was not included in the recent systematic review of predictors of a good outcome by Sandroni et al. [46]. Our prognostic performance results (Table 3) indicate that qPLR/NPi also has the potential to predict favorable outcomes. By accepting specific thresholds in prognostication guidelines, future studies could confirm this prognostic ability.
The ERC/ESICM neuroprognostication strategy incorporates a multimodal approach with at least two predictors to enhance sensitivity without increasing false predictions. Adding either qPLR < 4% or NPi ≤ 2 to NSE > 60 μg/L significantly increased performance (AUC under the ROC curve) and sensitivity to 50% (NPi) and 55% (qPLR) while maintaining a 0% (0–0%) FPR. The results of NSE combined with pupillometry at values above the proposed thresholds indicate that when used in a multimodal approach, even more, liberal cut-off values could achieve similar specificity with increased sensitivity.
Combining 48-h NSE and pupillometry with validated thresholds for evaluating persistingly comatose OHCA survivors with M ≤ 3 at ≥ 72 h provides a highly reliable (specificity) and useful (sensitivity) multimodal neuroprognostication strategy, especially for centers where neurophysiology is challenging to obtain.
Study limitations
The protocolized aim of this study was to validate and investigate the qPLR and NPi parameters, and therefore, the remaining quantitative parameters (CV, MCV, DV, and LAT) have not been included in this study. Due to the clinical design of the main trial, it was not possible to standardize ambient light conditions, diverge from the use of sedation or vasopressors in standard care, or test measurements in different conditions. Thus, the results of this study reflect the clinical setting for neuroprognostication.
Even though automated quantitative pupillometry was unavailable for personnel involved in outcome assessments and decision-making, clinicians involved in patient care were not blinded to the standard manual pupillometry performed. As there was no set order for the manual and quantitative pupillometry assessments, it is possible that manual measurements could have been recorded by the same personnel also performing automated assessments. Despite this, no formal training was provided for interpreting qPLR and NPi, and WLST decisions were never based on a single predictor.
Some patients were excluded if quantitative pupillometry data were missing. However, there was no difference in baseline characteristics or outcomes between the patients with missing and the present data, and we consider that missingness was at random and thus did not infer the generalizability of results.
The individual components and calculation of the NPi algorithm, developed by NeurOptics®, have never been fully published. However, the prognostic value of the index has been thoroughly verified through numerous studies [9, 10, 13, 15, 16, 18].
Though all analyses of the primary and secondary outcomes (Table 3) met the Bonferroni corrected α (0.05/7 = 0.0071), this was not protocolized and thus performed post hoc.
Conclusion
We successfully validated the robust prognostic value of quantitative pupillometry after OHCA, with specific thresholds of qPLR < 4% and the NPi ≤ 2 predicting unfavorable neurological outcomes with zero-percent FPR. Combining the thresholds with NSE > 60 μg/L increased sensitivity without raising false positives, suggesting its potential as a substitute for absent pupil reflex in multimodal neuroprognostication.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
First and foremost, the authors extend thanks to the patients who participated in the BOX-trial and their relatives. The authors would like to thank all the staff who participated in the inclusion process and the staff of the intensive care units at both participating sites. In particular, the authors would also like to thank the intensive care unit nursing staff for their overall commitment and meticulous performance of every pupillometry assessment included in this trial. The authors further thank Mie Christa Larsen and Áslaug Karlsdóttir from the Clinical Research Unit, Rigshospitalet; Mette Krefeld Bentzen (Department of Clinical Biochemistry, Copenhagen University Hospital Rigshospitalet) for the technical assistance in analyzing the biobank samples for neuron-specific enolase; Matilde Winther-Jensen and Sebastian Topalian for methodological and statistical guidance; and Jesper Nyvold Larsen for contributing a system for proxy consent that substantially reduced the time to randomization in the trial.
Author contributions
Benjamin Nyholm: conceptualization, methodology, writing—review & editing, visualization. Johannes Grand: investigation, methodology, review & editing, visualization. Laust E.R. Obling: investigation, methodology, review & editing, visualization. Christian Hassager: conceptualization, methodology, investigation, supervision, review & editing. Jacob E. Møller: investigation, methodology, review & editing, visualization. Henrik Schmidt: investigation, methodology, review & editing, visualization. Marwan Othman: investigation, review & editing, visualization. Daniel Kondziella: investigation, review & editing, visualization. Janneke Horn: investigation, methodology, review & editing, visualization. Jesper Kjaergaard: conceptualization, methodology, resources, investigation, supervision, writing original draft, review & editing.
Funding
Open access funding provided by National Hospital. The study was supported by funding from Novo Nordisk Foundation (NNF20OC0064043). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Data availability
The data supporting this study's findings are available upon reasonable request to the corresponding author.
Declarations
Conflicts of interest
The study is supported by a grant from the Novo Nordisk Foundation (NNF20OC0064043) supporting the salary of BN and JK. JEM received institutional research grants from Abiomed and Novo Nordic Foundation outside submitted work. JG is supported by a research grant from the Danish Cardiovascular Academy, funded by the Novo Nordisk Foundation (NNF20SA0067242) and The Danish Heart Foundation. CH received grants from the Lundbeck Foundation (R186-2015–2132), Novo Nordisk Foundation (NNF20OC0064043), and The Danish Heart Foundation (21-R151-A10091-22200). On behalf of all authors, the corresponding author states that there is no conflict of interest for the remaining authors.
Ethical approval
Per protocol, permissions were obtained from the Regional Ethics Committee (ID: H-16033436) before initiating the main BOX-trial.
Consent to participate
The trial was conducted in accordance with the Declaration of Helsinki. Danish law allows for the immediate inclusion of patients in non-drug trials, requiring consent to be obtained as soon as possible after enrollment. Written informed consent was secured from a legal guardian and collected by a departmental doctor at the earliest opportunity following admission to the hospital. Once conscious, consent was obtained from patients through the same medical staff.
Role of the sponsor
This study is sponsor–investigator-initiated with no funding or involvement from pharmaceutical companies. The sponsor–investigator maintains authority over all aspects of the trial, including design, management, interpretation of results, and publication.
Name and contact information for the trial sponsor
Jesper Kjærgaard, consultant, MD, Ph.D., DMSc Department of Cardiology, The Heart Centre, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark. Jesper.kjaergaard.05@regionh.dk.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gräsner J-T, Wnent J, Herlitz J et al (2020) Survival after out-of-hospital cardiac arrest in Europe: results of the EuReCa TWO study. Resuscitation 148:218–226. 10.1016/j.resuscitation.2019.12.042 10.1016/j.resuscitation.2019.12.042 [DOI] [PubMed] [Google Scholar]
- 2.Laver S, Farrow C, Turner D, Nolan J (2004) Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 30:2126–2128. 10.1007/s00134-004-2425-z 10.1007/s00134-004-2425-z [DOI] [PubMed] [Google Scholar]
- 3.Schneider A, Böttiger BW, Popp E (2009) Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg 108:971–979. 10.1213/ane.0b013e318193ca99 10.1213/ane.0b013e318193ca99 [DOI] [PubMed] [Google Scholar]
- 4.Gräsner J-T, Herlitz J, Tjelmeland IBM et al (2021) European resuscitation council guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation 161:61–79. 10.1016/j.resuscitation.2021.02.007 10.1016/j.resuscitation.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Nolan JP, Sandroni C, Böttiger BW et al (2021) European resuscitation council and European society of intensive care medicine guidelines 2021: post-resuscitation care. Intensive Care Med 47:369–421. 10.1007/s00134-021-06368-4 10.1007/s00134-021-06368-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharshar T, Citerio G, Andrews PJD et al (2014) Neurological examination of critically ill patients: a pragmatic approach: report of an ESICM expert panel. Intensive Care Med 40:484–495. 10.1007/s00134-014-3214-y 10.1007/s00134-014-3214-y [DOI] [PubMed] [Google Scholar]
- 7.Marmarou A, Lu J, Butcher I et al (2007) Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma 24:270–280. 10.1089/neu.2006.0029 10.1089/neu.2006.0029 [DOI] [PubMed] [Google Scholar]
- 8.Chen JW, Vakil-Gilani K, Williamson KL, Cecil S (2014) Infrared pupillometry, the Neurological Pupil index and unilateral pupillary dilation after traumatic brain injury: implications for treatment paradigms. Springerplus 3:548. 10.1186/2193-1801-3-548 10.1186/2193-1801-3-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghauri MS, Ueno A, Mohammed S et al (2022) Evaluating the reliability of neurological pupillary index as a prognostic measurement of neurological function in critical care patients. Cureus 14:e28901. 10.7759/cureus.28901 10.7759/cureus.28901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oddo M, Sandroni C, Citerio G et al (2018) Quantitative versus standard pupillary light reflex for early prognostication in comatose cardiac arrest patients: an international prospective multi-center double-blinded study. Intensive Care Med 44:2102–2111. 10.1007/s00134-018-5448-6 10.1007/s00134-018-5448-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandroni C, D’Arrigo S, Cacciola S et al (2020) Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med 46:1803–1851. 10.1007/s00134-020-06198-w 10.1007/s00134-020-06198-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C-H, Wu C-Y, Liu CC-Y et al (2021) Neuroprognostic accuracy of quantitative versus standard pupillary light reflex for adult post-cardiac arrest patients: a systematic review and meta-analysis. Crit Care Med 49:1790–1799. 10.1097/CCM.0000000000005045 10.1097/CCM.0000000000005045 [DOI] [PubMed] [Google Scholar]
- 13.Nyholm B, Obling LER, Hassager C et al (2023) Specific thresholds of quantitative pupillometry parameters predict unfavorable outcome in comatose survivors early after cardiac arrest. Resusc Plus 14:100399. 10.1016/j.resplu.2023.100399 10.1016/j.resplu.2023.100399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solari D, Rossetti AO, Carteron L et al (2017) Early prediction of coma recovery after cardiac arrest with blinded pupillometry. Ann Neurol 81:804–810. 10.1002/ana.24943 10.1002/ana.24943 [DOI] [PubMed] [Google Scholar]
- 15.Obling L, Hassager C, Illum C et al (2019) Prognostic value of automated pupillometry: an unselected cohort from a cardiac intensive care unit. Eur Heart J: Acute Cardiovasc Care. 10.1177/2048872619842004 10.1177/2048872619842004 [DOI] [PubMed] [Google Scholar]
- 16.Riker RR, Sawyer ME, Fischman VG et al (2020) Neurological pupil index and pupillary light reflex by pupillometry predict outcome early after cardiac arrest. Neurocrit Care 32:152–161. 10.1007/s12028-019-00717-4 10.1007/s12028-019-00717-4 [DOI] [PubMed] [Google Scholar]
- 17.Heimburger D, Durand M, Gaide-Chevronnay L et al (2016) Quantitative pupillometry and transcranial Doppler measurements in patients treated with hypothermia after cardiac arrest. Resuscitation 103:88–93. 10.1016/j.resuscitation.2016.02.026 10.1016/j.resuscitation.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 18.Oddo M, Taccone FS, Petrosino M et al (2023) The Neurological Pupil index for outcome prognostication in people with acute brain injury (ORANGE): a prospective, observational, multicentre cohort study. Lancet Neurol 22:925–933. 10.1016/S1474-4422(23)00271-5 10.1016/S1474-4422(23)00271-5 [DOI] [PubMed] [Google Scholar]
- 19.Soar J, Berg KM, Andersen LW et al (2020) Adult advanced life support: 2020 International Consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 156:A80–A119. 10.1016/j.resuscitation.2020.09.012 10.1016/j.resuscitation.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stammet P, Collignon O, Hassager C et al (2015) Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. J Am Coll Cardiol 65:2104–2114. 10.1016/j.jacc.2015.03.538 10.1016/j.jacc.2015.03.538 [DOI] [PubMed] [Google Scholar]
- 21.Nyholm B, Grand J, Obling L et al (2023) Quantitative pupillometry thresholds for early neuroprognostication after cardiac arrest: A BOX-trial substudy. Eur Heart J Acute Cardiovasc Care 12(zuad036):141. 10.1093/ehjacc/zuad036.14136257652 10.1093/ehjacc/zuad036.141 [DOI] [Google Scholar]
- 22.Nyholm B, Grand J, Obling L et al (2023) Influence of blood-pressure and oxygen levels on the prognostic value of quantitative pupillometry for comatose cardiac arrest patients: a BOX-trial substudy. Eur Heart J Acute Cardiovasc Care 12:zuad036-142. 10.1093/ehjacc/zuad036.142 10.1093/ehjacc/zuad036.142 [DOI] [Google Scholar]
- 23.Nyholm B, Grand J, Obling LER et al (2023) Quantitative pupillometry for neuroprognostication in comatose post-cardiac arrest patients: a protocol for a predefined sub-study of the Blood pressure and Oxygenations Targets after Out-of-Hospital Cardiac Arrest (BOX)-trial. Resusc Plus 16:100475. 10.1016/j.resplu.2023.100475 10.1016/j.resplu.2023.100475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjaergaard J, Møller JE, Schmidt H et al (2022) Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med 387:1456–1466. 10.1056/NEJMoa2208687 10.1056/NEJMoa2208687 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt H, Kjaergaard J, Hassager C et al (2022) Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med 387:1467–1476. 10.1056/NEJMoa2208686 10.1056/NEJMoa2208686 [DOI] [PubMed] [Google Scholar]
- 26.Hassager C, Schmidt H, Møller JE et al (2022) Duration of device-based fever prevention after cardiac arrest. N Engl J Med. 10.1056/NEJMoa2212528 10.1056/NEJMoa2212528 [DOI] [PubMed] [Google Scholar]
- 27.Kjaergaard J, Schmidt H, Møller JE, Hassager C (2022) The “Blood pressure and oxygenation targets in post resuscitation care, a randomized clinical trial”: design and statistical analysis plan. Trials 23:177. 10.1186/s13063-022-06101-6 10.1186/s13063-022-06101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker M, Du R, Bacchetti P et al (2005) Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosci Nurs 37:34–40 10.1097/01376517-200502000-00006 [DOI] [PubMed] [Google Scholar]
- 29.Larson MD, Muhiudeen I (1995) Pupillometric analysis of the “absent light reflex.” Arch Neurol 52:369–372. 10.1001/archneur.1995.00540280051018 10.1001/archneur.1995.00540280051018 [DOI] [PubMed] [Google Scholar]
- 30.Perkins GD, Jacobs IG, Nadkarni VM et al (2015) Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the utstein resuscitation registry templates for out-of-hospital cardiac arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 96:328–340. 10.1016/j.resuscitation.2014.11.002 10.1016/j.resuscitation.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 31.Sandroni C, Cariou A, Cavallaro F et al (2014) Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med 40:1816–1831. 10.1007/s00134-014-3470-x 10.1007/s00134-014-3470-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JW, Gombart ZJ, Rogers S et al (2011) Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the Neurological Pupil index. Surg Neurol Int 2:82. 10.4103/2152-7806.82248 10.4103/2152-7806.82248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484. 10.1016/s0140-6736(75)92830-5 10.1016/s0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 34.Brain Resuscitation Clinical Trial I Study Group (1986) Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N Engl J Med 314:397–403. 10.1056/NEJM198602133140701 10.1056/NEJM198602133140701 [DOI] [PubMed] [Google Scholar]
- 35.Haywood KL, Whitehead L, Perkins GD (2019) An international, consensus-derived Core Outcome Set for Cardiac Arrest effectiveness trials: the COSCA initiative. Curr Opin Crit Care 25:226–233. 10.1097/MCC.0000000000000612 10.1097/MCC.0000000000000612 [DOI] [PubMed] [Google Scholar]
- 36.van Swieten JC, Koudstaal PJ, Visser MC et al (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19:604–607. 10.1161/01.str.19.5.604 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 37.Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. 10.1111/j.1532-5415.2005.53221.x 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 38.DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 39.Jones SR, Carley S, Harrison M (2003) An introduction to power and sample size estimation. Emerg Med J 20:453–458. 10.1136/emj.20.5.453 10.1136/emj.20.5.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirozu K, Setoguchi H, Tokuda K et al (2017) The effects of anesthetic agents on pupillary function during general anesthesia using the automated infrared quantitative pupillometer. J Clin Monit Comput 31:291–296. 10.1007/s10877-016-9839-3 10.1007/s10877-016-9839-3 [DOI] [PubMed] [Google Scholar]
- 41.Chan WP, Prescott BR, Barra ME et al (2022) Dexmedetomidine and other analgosedatives alter pupil characteristics in critically ill patients. Crit Care Explor 4:e0691. 10.1097/CCE.0000000000000691 10.1097/CCE.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robba C, Moro Salihovic B, Pozzebon S et al (2020) Comparison of 2 automated pupillometry devices in critically iii patients. J Neurosurg Anesthesiol 32:323–329. 10.1097/ANA.0000000000000604 10.1097/ANA.0000000000000604 [DOI] [PubMed] [Google Scholar]
- 43.Gräsner J-T, Lefering R, Koster RW et al (2016) EuReCa ONE-27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation 105:188–195. 10.1016/j.resuscitation.2016.06.004 10.1016/j.resuscitation.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 44.Jørgensen EO (1997) Course of neurological recovery and cerebral prognostic signs during cardiopulmonary resuscitation. Resuscitation 35:9–16. 10.1016/s0300-9572(97)00022-1 10.1016/s0300-9572(97)00022-1 [DOI] [PubMed] [Google Scholar]
- 45.Nolan JP, Neumar RW, Adrie C et al (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication—a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 79:350–379. 10.1016/j.resuscitation.2008.09.017 10.1016/j.resuscitation.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 46.Sandroni C, D’Arrigo S, Cacciola S et al (2022) Prediction of good neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med 48:389–413. 10.1007/s00134-022-06618-z 10.1007/s00134-022-06618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study's findings are available upon reasonable request to the corresponding author.