Abstract
The role of N-glycosylation in the function of human acetylcholinesterase (HuAChE) was examined by site-directed mutagenesis (Asn to Gln substitution) of the three potential N-glycosylation sites Asn-265, Asn-350 and Asn-464. Analysis of HuAChE mutants, defective in a single or multiple N-glycosylation sites, by expression in transiently or stably transfected human embryonal 293 kidney cells suggests the following. (a) All three AChE glycosylation signals are utilized, but not all the secreted molecules are fully glycosylated. (b) Glycosylation at all sites is important for effective biosynthesis and secretion; extracellular AChE levels in mutants defective in one, two or all three sites amounted to 20-30%, 2-4% and about 0.5% of wild-type level respectively. (c) Some glycosylation mutants display impaired stability, as reflected by increased susceptibility to heat inactivation; substitution of Asn-464 has the most pronounced effect on thermostability. (d) Abrogation of N-glycosylation has no detectable effect on the enzyme activity of HuAChE; all glycosylation mutants, including the triple mutant, hydrolyse acetylthiocholine efficiently, displaying Km, kcat. and kcat./Km values similar to those of the wild-type enzyme. (e) In most mutants, inhibition profiles with edrophonium and bisquaternary ammonium ligands are identical with those of wild-type enzyme; the Asn-350 mutants, however, exhibit a slight decrease in their affinity towards these ligands. (f) Elimination of oligosaccharide side chains has no detectable effect on the surface-related 'peripheral-site' functions; like the wild-type enzyme, all mutants were inhibited by propidium and by increased concentrations of acetylthiocholine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Sun D. P., Nye J. A., Muchmore D. C., Matthews B. W. Temperature-sensitive mutations of bacteriophage T4 lysozyme occur at sites with low mobility and low solvent accessibility in the folded protein. Biochemistry. 1987 Jun 30;26(13):3754–3758. doi: 10.1021/bi00387a002. [DOI] [PubMed] [Google Scholar]
- Amitay R., Bar-Nun S., Haimovich J., Rabinovich E., Shachar I. Post-translational regulation of IgM expression in B lymphocytes. Selective nonlysosomal degradation of assembled secretory IgM is temperature-dependent and occurs prior to the trans-Golgi. J Biol Chem. 1991 Jul 5;266(19):12568–12573. [PubMed] [Google Scholar]
- Bon S., Méflah K., Musset F., Grassi J., Massoulié J. An immunoglobulin M monoclonal antibody, recognizing a subset of acetylcholinesterase molecules from electric organs of Electrophorus and Torpedo, belongs to the HNK-1 anti-carbohydrate family. J Neurochem. 1987 Dec;49(6):1720–1731. doi: 10.1111/j.1471-4159.1987.tb02429.x. [DOI] [PubMed] [Google Scholar]
- Campoy F. J., Cabezas-Herrera J., Vidal C. J. Interaction of AChE with Lens culinaris agglutinin reveals differences in glycosylation of molecular forms in sarcoplasmic reticulum membrane subfractions. J Neurosci Res. 1992 Dec;33(4):568–578. doi: 10.1002/jnr.490330409. [DOI] [PubMed] [Google Scholar]
- Chatonnet A., Lockridge O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J. 1989 Jun 15;260(3):625–634. doi: 10.1042/bj2600625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. K., Trimble R. B., Maley F. The effect of carbohydrate depletion on the properties of yeast external invertase. J Biol Chem. 1978 Dec 25;253(24):8691–8693. [PubMed] [Google Scholar]
- Doctor B. P., Chapman T. C., Christner C. E., Deal C. D., De La Hoz D. M., Gentry M. K., Ogert R. A., Rush R. S., Smyth K. K., Wolfe A. D. Complete amino acid sequence of fetal bovine serum acetylcholinesterase and its comparison in various regions with other cholinesterases. FEBS Lett. 1990 Jun 18;266(1-2):123–127. doi: 10.1016/0014-5793(90)81522-p. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé S., Fisher J. W., Powell J. S. Glycosylation at specific sites of erythropoietin is essential for biosynthesis, secretion, and biological function. J Biol Chem. 1988 Nov 25;263(33):17516–17521. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Edwards J. A., Brimijoin S. Thermal inactivation of the molecular forms of acetylcholinesterase and butyrylcholinesterase. Biochim Biophys Acta. 1983 Feb 15;742(3):509–516. doi: 10.1016/0167-4838(83)90268-6. [DOI] [PubMed] [Google Scholar]
- Fischer M., Ittah A., Liefer I., Gorecki M. Expression and reconstitution of biologically active human acetylcholinesterase from Escherichia coli. Cell Mol Neurobiol. 1993 Feb;13(1):25–38. doi: 10.1007/BF00712987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Spierer P. The Ace locus of Drosophila melanogaster: structural gene for acetylcholinesterase with an unusual 5' leader. EMBO J. 1986 Nov;5(11):2949–2954. doi: 10.1002/j.1460-2075.1986.tb04591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Järv J., Weise C. Substrate-binding sites in acetylcholinesterase. Trends Pharmacol Sci. 1991 Nov;12(11):422–426. doi: 10.1016/0165-6147(91)90621-x. [DOI] [PubMed] [Google Scholar]
- Kerem A., Kronman C., Bar-Nun S., Shafferman A., Velan B. Interrelations between assembly and secretion of recombinant human acetylcholinesterase. J Biol Chem. 1993 Jan 5;268(1):180–184. [PubMed] [Google Scholar]
- Kronman C., Velan B., Gozes Y., Leitner M., Flashner Y., Lazar A., Marcus D., Sery T., Papier Y., Grosfeld H. Production and secretion of high levels of recombinant human acetylcholinesterase in cultured cell lines: microheterogeneity of the catalytic subunit. Gene. 1992 Nov 16;121(2):295–304. doi: 10.1016/0378-1119(92)90134-b. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legay C., Bon S., Vernier P., Coussen F., Massoulié J. Cloning and expression of a rat acetylcholinesterase subunit: generation of multiple molecular forms and complementarity with a Torpedo collagenic subunit. J Neurochem. 1993 Jan;60(1):337–346. doi: 10.1111/j.1471-4159.1993.tb05856.x. [DOI] [PubMed] [Google Scholar]
- Liao J., Heider H., Sun M. C., Brodbeck U. Different glycosylation in acetylcholinesterases from mammalian brain and erythrocytes. J Neurochem. 1992 Apr;58(4):1230–1238. doi: 10.1111/j.1471-4159.1992.tb11333.x. [DOI] [PubMed] [Google Scholar]
- Liao J., Heider H., Sun M. C., Stieger S., Brodbeck U. The monoclonal antibody 2G8 is carbohydrate-specific and distinguishes between different forms of vertebrate cholinesterases. Eur J Biochem. 1991 May 23;198(1):59–65. doi: 10.1111/j.1432-1033.1991.tb15986.x. [DOI] [PubMed] [Google Scholar]
- Lim W. A., Farruggio D. C., Sauer R. T. Structural and energetic consequences of disruptive mutations in a protein core. Biochemistry. 1992 May 5;31(17):4324–4333. doi: 10.1021/bi00132a025. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J Biol Chem. 1988 Apr 25;263(12):5955–5960. [PubMed] [Google Scholar]
- Massoulié J., Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu Rev Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Boime I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol. 1988 Apr;106(4):1049–1059. doi: 10.1083/jcb.106.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Keene J. L., Boime I. Site specificity of the chorionic gonadotropin N-linked oligosaccharides in signal transduction. J Biol Chem. 1989 Feb 15;264(5):2409–2414. [PubMed] [Google Scholar]
- Mutero A., Fournier D. Post-translational modifications of Drosophila acetylcholinesterase. In vitro mutagenesis and expression in Xenopus oocytes. J Biol Chem. 1992 Jan 25;267(3):1695–1700. [PubMed] [Google Scholar]
- Méflah K., Bernard S., Massoulié J. Interactions with lectins indicate differences in the carbohydrate composition of the membrane-bound enzymes acetylcholinesterase and 5'-nucleotidase in different cell types. Biochimie. 1984 Jan;66(1):59–69. doi: 10.1016/0300-9084(84)90192-5. [DOI] [PubMed] [Google Scholar]
- Ordentlich A., Barak D., Kronman C., Flashner Y., Leitner M., Segall Y., Ariel N., Cohen S., Velan B., Shafferman A. Dissection of the human acetylcholinesterase active center determinants of substrate specificity. Identification of residues constituting the anionic site, the hydrophobic site, and the acyl pocket. J Biol Chem. 1993 Aug 15;268(23):17083–17095. [PubMed] [Google Scholar]
- Patinkin D., Seidman S., Eckstein F., Benseler F., Zakut H., Soreq H. Manipulations of cholinesterase gene expression modulate murine megakaryocytopoiesis in vitro. Mol Cell Biol. 1990 Nov;10(11):6046–6050. doi: 10.1128/mcb.10.11.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Zevin-Sonkin D., Gnatt A., Goldberg O., Soreq H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3555–3559. doi: 10.1073/pnas.84.11.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachinsky T. L., Camp S., Li Y., Ekström T. J., Newton M., Taylor P. Molecular cloning of mouse acetylcholinesterase: tissue distribution of alternatively spliced mRNA species. Neuron. 1990 Sep;5(3):317–327. doi: 10.1016/0896-6273(90)90168-f. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L. Biogenesis of acetylcholinesterase molecular forms in muscle. Evidence for a rapidly turning over, catalytically inactive precursor pool. J Biol Chem. 1988 Dec 25;263(36):19398–19406. [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Semenkovich C. F., Luo C. C., Nakanishi M. K., Chen S. H., Smith L. C., Chan L. In vitro expression and site-specific mutagenesis of the cloned human lipoprotein lipase gene. Potential N-linked glycosylation site asparagine 43 is important for both enzyme activity and secretion. J Biol Chem. 1990 Apr 5;265(10):5429–5433. [PubMed] [Google Scholar]
- Shafferman A., Kronman C., Flashner Y., Leitner M., Grosfeld H., Ordentlich A., Gozes Y., Cohen S., Ariel N., Barak D. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J Biol Chem. 1992 Sep 5;267(25):17640–17648. [PubMed] [Google Scholar]
- Shafferman A., Velan B., Ordentlich A., Kronman C., Grosfeld H., Leitner M., Flashner Y., Cohen S., Barak D., Ariel N. Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center. EMBO J. 1992 Oct;11(10):3561–3568. doi: 10.1002/j.1460-2075.1992.tb05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
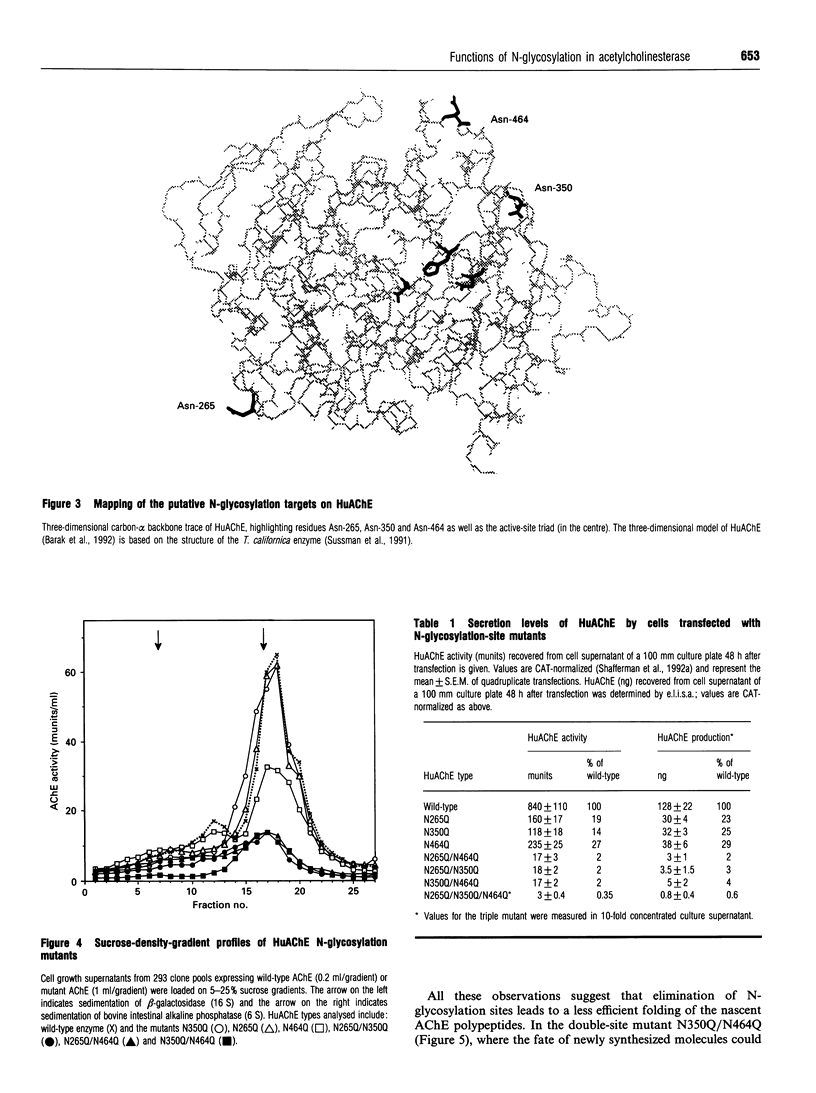
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Taylor P. The cholinesterases. J Biol Chem. 1991 Mar 5;266(7):4025–4028. [PubMed] [Google Scholar]
- Tifft C. J., Proia R. L., Camerini-Otero R. D. The folding and cell surface expression of CD4 requires glycosylation. J Biol Chem. 1992 Feb 15;267(5):3268–3273. [PubMed] [Google Scholar]
- Treskatis S., Ebert C., Layer P. G. Butyrylcholinesterase from chicken brain is smaller than that from serum: its purification, glycosylation, and membrane association. J Neurochem. 1992 Jun;58(6):2236–2247. doi: 10.1111/j.1471-4159.1992.tb10969.x. [DOI] [PubMed] [Google Scholar]
- Velan B., Grosfeld H., Kronman C., Leitner M., Gozes Y., Lazar A., Flashner Y., Marcus D., Cohen S., Shafferman A. The effect of elimination of intersubunit disulfide bonds on the activity, assembly, and secretion of recombinant human acetylcholinesterase. Expression of acetylcholinesterase Cys-580----Ala mutant. J Biol Chem. 1991 Dec 15;266(35):23977–23984. [PubMed] [Google Scholar]
- Velan B., Kronman C., Grosfeld H., Leitner M., Gozes Y., Flashner Y., Sery T., Cohen S., Ben-Aziz R., Seidman S. Recombinant human acetylcholinesterase is secreted from transiently transfected 293 cells as a soluble globular enzyme. Cell Mol Neurobiol. 1991 Feb;11(1):143–156. doi: 10.1007/BF00712806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz G., Proia R. L. Analysis of the glycosylation and phosphorylation of the alpha-subunit of the lysosomal enzyme, beta-hexosaminidase A, by site-directed mutagenesis. J Biol Chem. 1992 May 15;267(14):10039–10044. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]