Abstract
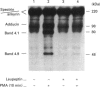
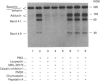
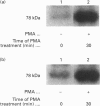
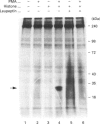
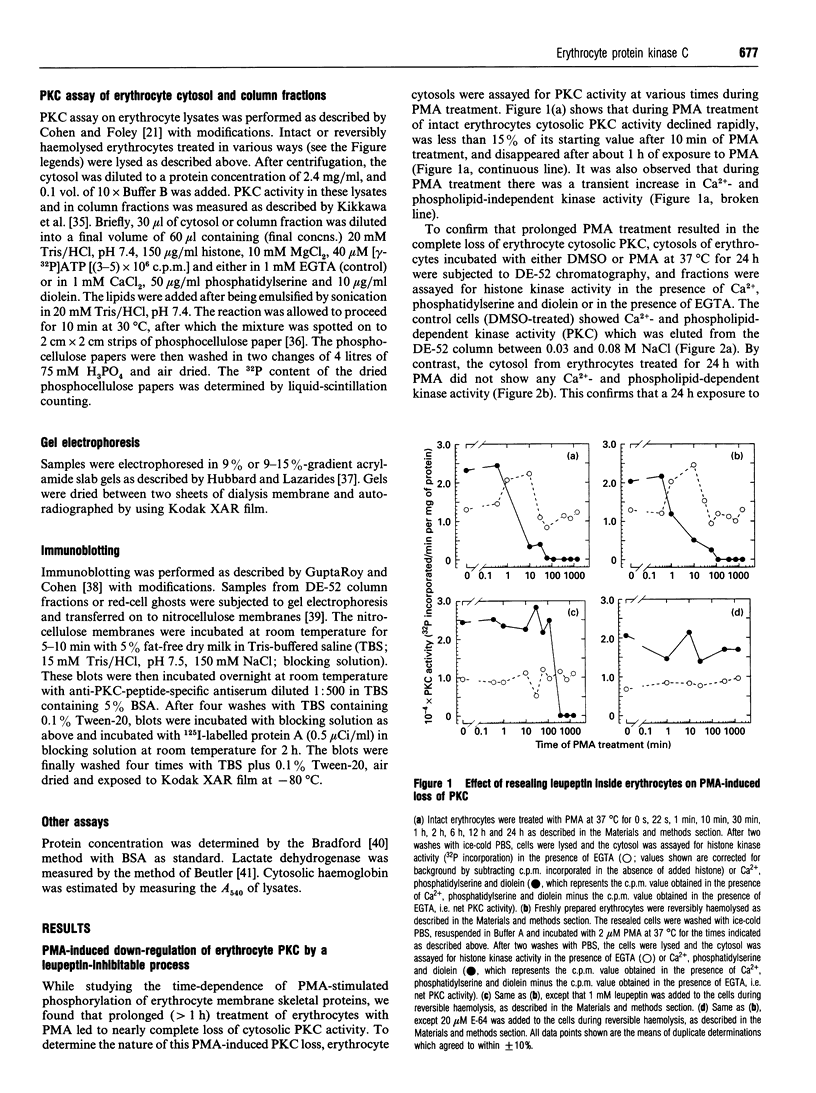
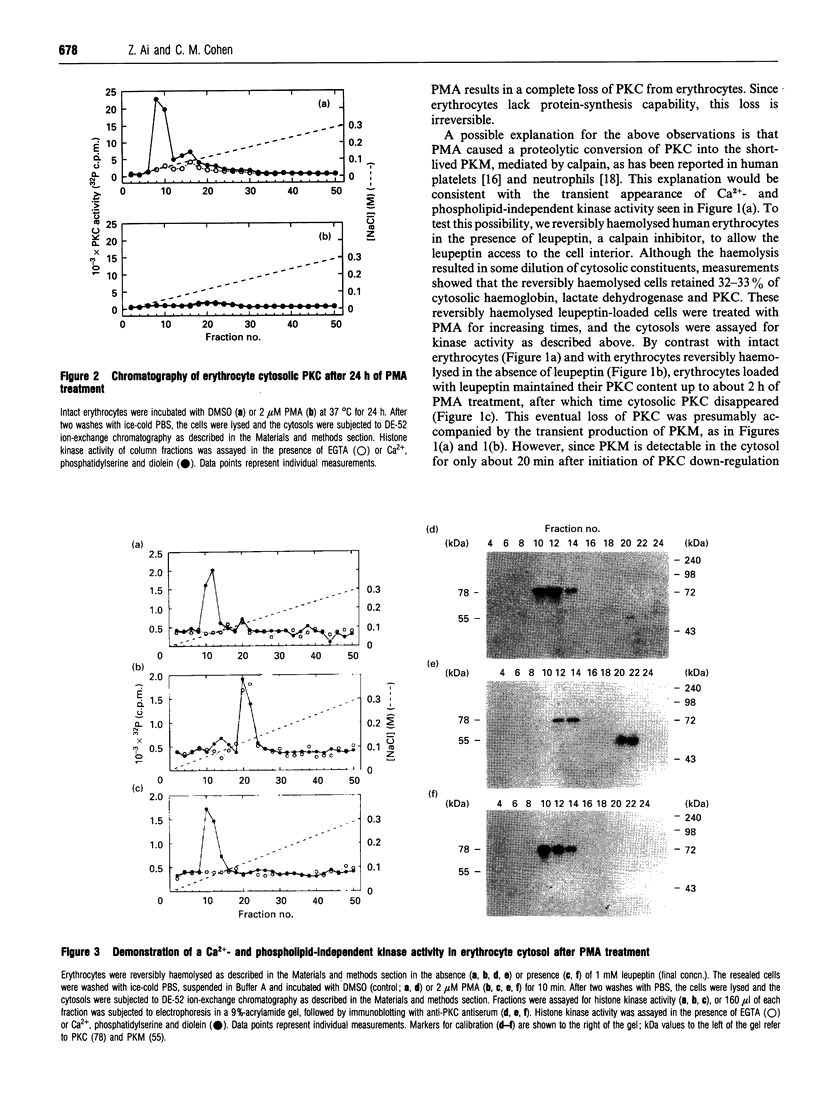
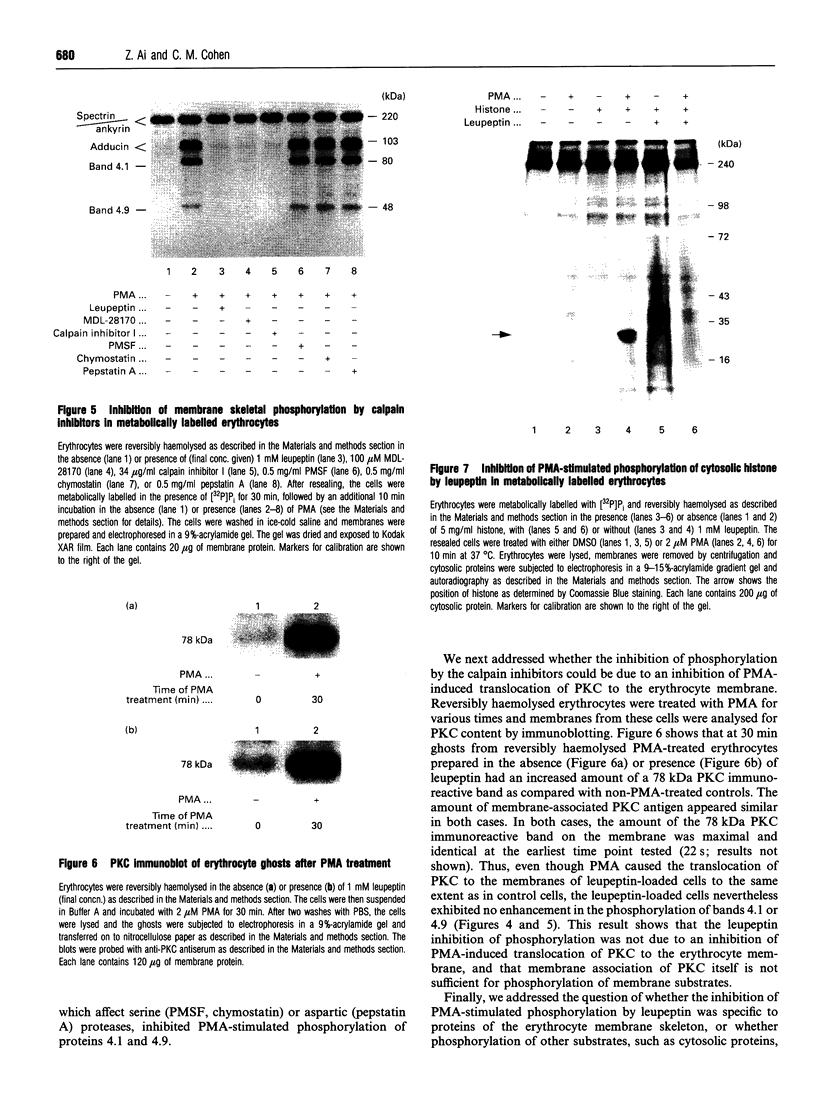
Human erythrocytes contain cytosolic protein kinase C (PKC) which, when activated by phorbol 12-myristate 13-acetate (PMA), induces the phosphorylation of the membrane skeletal proteins band 4.1, band 4.9 and adducin. We found that brief treatments of erythrocytes with PMA resulted in a decrease in cytosolic PKC content and in the transient appearance in the cytosol of a Ca(2+)- and phospholipid-independent 55 kDa fragment of PKC, called PKM. Prolonged treatment with PMA resulted in the complete and irreversible loss of erythrocyte PKC. To investigate the possible role of calpain in this process, the calpain inhibitors leupeptin and E-64 were sealed inside erythrocytes by reversible haemolysis. Both inhibitors prolonged the lifetime of PKC in PMA-treated cells, and leupeptin was shown to block the PMA-stimulated appearance of PKM in the cytosol. Significantly, leupeptin also completely blocked PMA-stimulated phosphorylation of membrane and cytosolic substrates. This effect was mimicked by other calpain inhibitors (MDL-28170 and calpain inhibitor I), but did not occur when other protease inhibitors such as phenylmethanesulphonyl fluoride, pepstatin A or chymostatin were used. In addition, the phosphorylation of exogenous histone sealed inside erythrocytes was also blocked by leupeptin. Immunoblotting showed that leupeptin did not prevent the PMA-induced translocation of PKC to the erythrocyte membrane. Thus inhibition of PKC phosphorylation of membrane skeletal proteins by calpain inhibitors was not due to inhibition of PKC translocation to the membrane. Our results suggest that PMA treatment of erythrocytes results in the translocation of PKC to the plasma membrane, followed by calpain-mediated cleavage of PKC to PKM. This cleavage, or some other leupeptin-inhibitable process, is a necessary step for the phosphorylation of membrane skeletal substrates, suggesting that the short-lived PKM may be responsible for membrane skeletal phosphorylation. Our results suggest a potential mechanism whereby erythrocyte PKC may be subject to continual down-regulation during the lifespan of the erythrocyte due to repeated activation events, possibly related to transient Ca2+ influx. Such down-regulation may play an important role in erythrocyte survival or pathophysiology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apovo M., Gascard P., Rhoda M. D., Beuzard Y., Giraud F. Alteration in protein kinase C activity and subcellular distribution in sickle erythrocytes. Biochim Biophys Acta. 1989 Aug 21;984(1):26–32. doi: 10.1016/0005-2736(89)90338-6. [DOI] [PubMed] [Google Scholar]
- Boscá L., Márquez C., Martínez C. Lack of correlation between translocation and biological effects mediated by protein kinase C: an appraisal. Immunol Today. 1989 Jul;10(7):223–224. doi: 10.1016/0167-5699(89)90256-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chida K., Kato N., Kuroki T. Down regulation of phorbol diester receptors by proteolytic degradation of protein kinase C in a cultured cell line of fetal rat skin keratinocytes. J Biol Chem. 1986 Oct 5;261(28):13013–13018. [PubMed] [Google Scholar]
- Cohen C. M., Gascard P. Regulation and post-translational modification of erythrocyte membrane and membrane-skeletal proteins. Semin Hematol. 1992 Oct;29(4):244–292. [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Danilov Y. N., Fennell R., Ling E., Cohen C. M. Selective modulation of band 4.1 binding to erythrocyte membranes by protein kinase C. J Biol Chem. 1990 Feb 15;265(5):2556–2562. [PubMed] [Google Scholar]
- Darbon J. M., Oury F., Clamens S., Bayard F. TPA induces subcellular translocation and subsequent down-regulation of both phorbol ester binding and protein kinase C activities in MCF-7 cells. Biochem Biophys Res Commun. 1987 Jul 31;146(2):537–546. doi: 10.1016/0006-291x(87)90562-6. [DOI] [PubMed] [Google Scholar]
- Faquin W. C., Chahwala S. B., Cantley L. C., Branton D. Protein kinase C of human erythrocytes phosphorylates bands 4.1 and 4.9. Biochim Biophys Acta. 1986 Jul 11;887(2):142–149. doi: 10.1016/0167-4889(86)90048-0. [DOI] [PubMed] [Google Scholar]
- Fournier A., Murray A. W. Application of phorbol ester to mouse skin causes a rapid and sustained loss of protein kinase C. Nature. 1987 Dec 24;330(6150):767–769. doi: 10.1038/330767a0. [DOI] [PubMed] [Google Scholar]
- Grove D. S., Mastro A. M. Prevention of the TPA-mediated down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Feb 29;151(1):94–99. doi: 10.1016/0006-291x(88)90563-3. [DOI] [PubMed] [Google Scholar]
- GuptaRoy B., Cohen C. M. Maturation of murine erythroleukemia cells committed to differentiation requires protein kinase C. J Biol Chem. 1992 Aug 5;267(22):15326–15333. [PubMed] [Google Scholar]
- Hubbard B. D., Lazarides E. Copurification of actin and desmin from chicken smooth muscle and their copolymerization in vitro to intermediate filaments. J Cell Biol. 1979 Jan;80(1):166–182. doi: 10.1083/jcb.80.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain-Chishti A., Levin A., Branton D. Abolition of actin-bundling by phosphorylation of human erythrocyte protein 4.9. Nature. 1988 Aug 25;334(6184):718–721. doi: 10.1038/334718a0. [DOI] [PubMed] [Google Scholar]
- Inomata M., Hayashi M., Nakamura M., Saito Y., Kawashima S. Properties of erythrocyte membrane binding and autolytic activation of calcium-activated neutral protease. J Biol Chem. 1989 Nov 5;264(31):18838–18843. [PubMed] [Google Scholar]
- Johnson R. M., Tang K. Induction of a Ca(2+)-activated K+ channel in human erythrocytes by mechanical stress. Biochim Biophys Acta. 1992 Jun 30;1107(2):314–318. doi: 10.1016/0005-2736(92)90418-l. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Larsen F. L., Katz S., Roufogalis B. D., Brooks D. E. Physiological shear stresses enhance the Ca2+ permeability of human erythrocytes. Nature. 1981 Dec 17;294(5842):667–668. doi: 10.1038/294667a0. [DOI] [PubMed] [Google Scholar]
- Ling E., Danilov Y. N., Cohen C. M. Modulation of red cell band 4.1 function by cAMP-dependent kinase and protein kinase C phosphorylation. J Biol Chem. 1988 Feb 15;263(5):2209–2216. [PubMed] [Google Scholar]
- Ling E., Gardner K., Bennett V. Protein kinase C phosphorylates a recently identified membrane skeleton-associated calmodulin-binding protein in human erythrocytes. J Biol Chem. 1986 Oct 25;261(30):13875–13878. [PubMed] [Google Scholar]
- Ling E., Sapirstein V. Phorbol ester stimulates the phosphorylation of rabbit erythrocyte band 4.1. Biochem Biophys Res Commun. 1984 Apr 16;120(1):291–298. doi: 10.1016/0006-291x(84)91447-5. [DOI] [PubMed] [Google Scholar]
- Mehdi S., Angelastro M. R., Wiseman J. S., Bey P. Inhibition of the proteolysis of rat erythrocyte membrane proteins by a synthetic inhibitor of calpain. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1117–1123. doi: 10.1016/s0006-291x(88)80989-6. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Cakiroglu A. G., Jackson J. F., Rifkind R. A., Marks P. A. Protein kinase C activity and hexamethylenebisacetamide-induced erythroleukemia cell differentiation. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5282–5286. doi: 10.1073/pnas.84.15.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Horecker B. L. The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myristic acid. J Biol Chem. 1986 Mar 25;261(9):4101–4105. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa K. Studies on proteolysis of protein kinase C with calpain I and II. Kobe J Med Sci. 1990 Apr;36(1-2):55–69. [PubMed] [Google Scholar]
- Mischak H., Bodenteich A., Kolch W., Goodnight J., Hofer F., Mushinski J. F. Mouse protein kinase C-delta, the major isoform expressed in mouse hemopoietic cells: sequence of the cDNA, expression patterns, and characterization of the protein. Biochemistry. 1991 Aug 13;30(32):7925–7931. doi: 10.1021/bi00246a008. [DOI] [PubMed] [Google Scholar]
- Mizuta K., Hashimoto E., Yamamura H. Proteolytic activation of protein kinase C by membrane-bound protease in rat liver plasma membrane. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1262–1268. doi: 10.1016/0006-291x(85)90227-x. [DOI] [PubMed] [Google Scholar]
- Murachi T. Intracellular regulatory system involving calpain and calpastatin. Biochem Int. 1989 Feb;18(2):263–294. [PubMed] [Google Scholar]
- Murakami T., Hatanaka M., Murachi T. The cytosol of human erythrocytes contains a highly Ca2+-sensitive thiol protease (calpain I) and its specific inhibitor protein (calpastatin). J Biochem. 1981 Dec;90(6):1809–1816. doi: 10.1093/oxfordjournals.jbchem.a133659. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Bazzi M. D. Activation and regulation of protein kinase C enzymes. J Bioenerg Biomembr. 1991 Feb;23(1):43–61. doi: 10.1007/BF00768838. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990 Dec 25;265(36):22434–22440. [PubMed] [Google Scholar]
- Palfrey H. C., Waseem A. Protein kinase C in the human erythrocyte. Translocation to the plasma membrane and phosphorylation of bands 4.1 and 4.9 and other membrane proteins. J Biol Chem. 1985 Dec 15;260(29):16021–16029. [PubMed] [Google Scholar]
- Pears C., Schaap D., Parker P. J. The regulatory domain of protein kinase C-epsilon restricts the catalytic-domain-specificity. Biochem J. 1991 May 15;276(Pt 1):257–260. doi: 10.1042/bj2760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Damiani G., Salamino F., Sparatore B., Michetti M., Horecker B. L. Effects of a monoclonal anti-calpain antibody on responses of stimulated human neutrophils. Evidence for a role for proteolytically modified protein kinase C. J Biol Chem. 1988 Feb 5;263(4):1915–1919. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Michetti M., Sparatore B., Salamino F., Sacco O., Horecker B. L. Phosphorylation and proteolytic modification of specific cytoskeletal proteins in human neutrophils stimulated by phorbol 12-myristate 13-acetate. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3604–3608. doi: 10.1073/pnas.84.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Michetti M., Salamino F., Horecker B. L. Isozymes of protein kinase C in human neutrophils and their modification by two endogenous proteinases. J Biol Chem. 1990 Jan 15;265(2):706–712. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Salamino F., Michetti M., Sacco O., Horecker B. L. Binding to erythrocyte membrane is the physiological mechanism for activation of Ca2+-dependent neutral proteinase. Biochem Biophys Res Commun. 1985 Apr 16;128(1):331–338. doi: 10.1016/0006-291x(85)91683-3. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Michetti M., Melloni E., Sparatore B., Salamino F., Horecker B. L. Identification of the proteolytically activated form of protein kinase C in stimulated human neutrophils. Proc Natl Acad Sci U S A. 1990 May;87(10):3705–3707. doi: 10.1073/pnas.87.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran M., Abraham E. C. Age-dependent variation in the cytosol/membrane distribution of red cell protein kinase-C. Am J Hematol. 1989 May;31(1):69–70. doi: 10.1002/ajh.2830310116. [DOI] [PubMed] [Google Scholar]
- Raval P. J., Allan D. The effects of phorbol ester, diacylglycerol, phospholipase C and Ca2+ ionophore on protein phosphorylation in human and sheep erythrocytes. Biochem J. 1985 Nov 15;232(1):43–47. doi: 10.1042/bj2320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropars C., Chassaigne M., Villereal M. C., Avenard G., Hurel C., Nicolau C. Resealed red blood cells as a new blood transfusion product. Bibl Haematol. 1985;(51):82–91. doi: 10.1159/000410231. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J. Expression, purification, and characterization of protein kinase C-epsilon. J Biol Chem. 1990 May 5;265(13):7301–7307. [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Modulation of Ca2+-activated, phospholipid-dependent protein kinase in platelets treated with a tumor-promoting phorbol ester. Biochem Biophys Res Commun. 1984 Jul 18;122(1):158–164. doi: 10.1016/0006-291x(84)90453-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trilivas I., McDonough P. M., Brown J. H. Dissociation of protein kinase C redistribution from the phosphorylation of its substrates. J Biol Chem. 1991 May 5;266(13):8431–8438. [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]