Abstract
Opioid use disorder (OUD) has been linked to macroscopic structural alterations in the brain. The monthly injectable, extended-release formulation of μ-opioid antagonist naltrexone (XR-NTX) is highly effective in reducing opioid craving and preventing opioid relapse. Here, we investigated the neuroanatomical effects of XR-NTX by examining changes in cortical thickness during treatment for OUD. Forty-seven OUD patients underwent structural magnetic resonance imaging and subjectively rated their opioid craving ≤1 day before (pre-treatment) and 11 ± 3 days after (on-treatment) the first XR-NTX injection. A sample of fifty-six non-OUD individuals completed a single imaging session and served as the comparison group. A publicly available [¹¹C]carfentanil positron emission tomography dataset was used to assess the relationship between changes in cortical thickness and μ-opioid receptor (MOR) binding potential across brain regions. We found that the thickness of the medial prefrontal and anterior cingulate cortices (mPFC/aCC; regions with high MOR binding potential) was comparable between the non-OUD individuals and the OUD patients at pre-treatment. However, among the OUD patients, mPFC/aCC thickness significantly decreased from pre-treatment to on-treatment. A greater reduction in mPFC/aCC thickness was associated with a greater reduction in opioid craving. Taken together, our study suggests XR-NTX-induced cortical thickness reduction in the mPFC/aCC regions in OUD patients. The reduction in thickness does not appear to indicate a restoration to the non-OUD level but rather reflects XR-NTX’s distinct therapeutic impact on an MOR-rich brain structure. Our findings highlight the neuroplastic effects of XR-NTX that may inform the development of novel OUD interventions.
Subject terms: Addiction, Neuroscience
Introduction
Opioid use disorder (OUD) is a major contributor to the overall morbidity and mortality in the US. Medication-assisted treatments have emerged as a highly successful approach to treating OUD and include full opioid agonist methadone, partial agonist buprenorphine, and antagonist naltrexone [1]. Unlike the agonist agents, naltrexone works by blocking μ-opioid receptors (MORs) in the brain and does not produce opioid-like effects or physiological dependence. The monthly injectable, extended-release formulation of naltrexone (XR-NTX) is effective in reducing cravings and preventing relapse in OUD patients and has demonstrated clinical effectiveness comparable to agonist treatments [2–6].
OUD is characterized by altered brain functioning as revealed by functional magnetic resonance imaging (MRI) [7]. Individuals with OUD show heightened activity in the brain’s reward circuits (e.g., striatum, prefrontal cortex) in response to drug-related stimuli (i.e., drug cues) [8], and such activation is linked to elevated opioid craving [9] and risk for relapse [10]. OUD patients also exhibit behavioral impulsivity [11–13] and decreased prefrontal engagement in inhibitory control, as compared to non-dependent individuals [14–16]. There is some evidence that prolonged abstinence and pharmacotherapy aid in reducing opioid cue-reactivity [17–20], enhancing inhibitory control [21], and improving decision-making [22]. Specifically, XR-NTX has been shown to reduce frontostriatal reactivity to opioid cues [19, 20]. However, full restoration of normal neurocognitive functioning remains challenging (e.g., cue-reactivity [23]; impulsivity [24]; decision-making deficits [22, 25]), potentially due to lasting structural [24, 26] and functional [27, 28] alterations in the brain as a result of chronic opioid misuse.
Structurally, OUD patients show lower gray matter volumes and/or lower cortical thickness compared to non-dependent controls, particularly in the prefrontal and temporal cortices [29–31]. Cortical thickness is a well-established and clinically relevant metric of brain structural integrity [32–34] and has been linked to OUD severity and treatment outcomes [29, 35]. However, research on the effect of abstinence and treatment on brain morphology remains limited and has yielded inconsistent results [35–37]. Notably, no studies to date have examined changes in brain structure during naltrexone/XR-NTX treatment for OUD. Elucidating the neuroplastic effect of XR-NTX will offer crucial mechanistic insights into its clinical efficacy and may inform on the long-term brain health of treated patients.
In this longitudinal observational study, we used structural MRI to examine changes in regional cortical thickness in patients receiving XR-NTX treatment for OUD. To examine whether such changes represented reversal of impairments caused by OUD, we compared the cortical thickness of non-OUD individuals to that of OUD patients before and during XR-NTX treatment. We then investigated the association between the change in cortical thickness and the clinical efficacy of XR-NTX as indexed by the reduction in opioid craving in OUD patients. Lastly, given the MOR antagonism property of XR-NTX, we examined whether XR-NTX-induced cortical thickness changes were related to interregional variation in MOR binding potential. We hypothesized that cortical regions with higher MOR binding potential (e.g., the prefrontal, anterior cingulate, and anterior insular cortices [38]) would show more pronounced cortical thickness change during XR-NTX treatment in OUD patients and that the change in cortical thickness would be associated with reductions in opioid craving.
Methods
Participants
Detoxified OUD patients were recruited from the greater Philadelphia region between 2012–2014 and were offered up to 3 monthly XR-NTX injections. Of the 113 individuals that were initially enrolled, we excluded fifty-two who dropped out before receiving XR-NTX, fourteen who did not complete MRI, and two with poor MRI data quality, leaving a total of forty-seven participants in the final analysis that completed at least one XR-NTX injection as well as MRI assessments immediately before (pre-treatment) and 11 ± 3 (mean ± SD) days after (on-treatment) the first injection [19, 20]. A subset of 25 participants completed post-treatment MRI 44 ± 17 (mean ± SD) days after the third injection (see Procedure). The DSM-IV-TR diagnosis of opioid dependence was established with history and physical exam and the Mini International Neuropsychiatric Interview (MINI) for DSM-IV [39]. Inclusion criteria were ages between 18 and 60 years; a DSM-IV-TR diagnosis of opioid dependence confirmed by self-report and medical records documenting daily opioid use for more than 2 weeks in the past 3 months; evidence of detoxification from opioids before XR-NTX injections as established by urine drug screen (UDS) (Redwood Toxicology Laboratory) and a negative naloxone challenge test; and good physical health ascertained by history and physical examination, blood chemistry, and urinalysis. Exclusion criteria were current use of medications that could confound blood oxygen level-dependent brain response, such as antidopaminergic agents, anticonvulsants, and β-blockers; current psychosis, dementia, intellectual disability, or lifetime history of schizophrenia; clinically significant cardiovascular, hematologic, pulmonary, hepatic, renal, metabolic, gastrointestinal, neurologic, or endocrine abnormalities; pregnancy or breastfeeding; history of clinically significant head trauma; contraindications for XR-NTX, such as medical conditions requiring opioid analgesics such as chronic pain disorder, planned surgery, obesity, elevated liver enzymes >3 times the upper limit of normal, or failure to complete opioid detoxification; contraindications for MRI, such as indwelling magnetically active foreign bodies, or fear of enclosed spaces.
A convenience cohort of fifty-six non-OUD individuals who completed one single MRI session was included as a comparison group [31]. The inclusion and exclusion criteria for the non-OUD group were similar to those for the OUD group, with the exception that all non-OUD individuals were tobacco cigarette smokers, and diagnosis of opioid dependence and eligibility for XR-NTX were not required. The non-OUD group was comparable to the OUD group with regard to demographics (except for race), tobacco smoking severity, comorbid non-opioid substance use disorders (except for stimulant use disorder), and current substance use verified by urine toxicology (see Table 1).
Table 1.
Participant characteristics (count or mean ± SD).
| Variable | OUD | Non-OUD | P-value¹ | |
|---|---|---|---|---|
| pre-treatment | on-treatment | |||
| N | 47 | 56 | – | |
| Sex | 26 male, 21 female | 31 male, 25 female | >0.99 | |
| Age (years) | 29.04 ± 8.72 | 30.09 ± 10.81 | 0.59 | |
| Years of education | 13.77 ± 2.12 | 13.81 ± 2.29 | 0.91 | |
| Race | 44 White, 2 AA, 1 Asian | 23 White, 19 AA, 4 Asian, 10 other | <0.001 | |
| Ethnicity | 3 Hispanic | 7 Hispanic | 0.34 | |
| Years of opioid use | 5.83 ± 5.95 | – | – | |
| Alcohol use disorder2 | 9 | 9 | >0.99 | |
| Stimulant use disorder2 | 13 | 2 | 0.001 | |
| Cannabis use disorder2 | 15 | 14 | 0.66 | |
| Prescription opioid use | 32 | – | – | |
| Heroin use | 33 | – | – | |
| Number of days since last opioid use | 18.23 ± 17.01 | 29.66 ± 17.50 | – | – |
| Number of cigarettes smoked per day3 | 13.23 ± 8.56 | 9.83 ± 8.18 | 12.84 ± 7.34 | 0.81; 0.060 |
| UDS positive for stimulant4 | 5 | 8 | 3 | 0.47; 0.11 |
| UDS positive for cannabis4 | 17 | 20 | 23 | 0.55; >0.99 |
| Opioid craving (0–9)5 | 3.62 ± 2.89 | 2.12 ± 2.50 | – | – |
OUD opioid use disorder, AA African American, UDS urine drug screen.
1P-values were obtained from independent t-tests for numeric variables and Fisher’s exact tests for categorical variables. Separate tests were performed on pre- and on-treatment sessions when applicable.
2Diagnosis of alcohol, stimulant, and cannabis use disorder was missing in 5 non-OUD participants.
3Number of cigarettes per day at on-treatment was missing in 3 OUD participants.
4Urine toxicology data were missing in 2 non-OUD participants.
5Opioid craving at on-treatment was missing in 1 OUD participant.
The study was approved by the University of Pennsylvania Institutional Review Board (No. 814234/819008/819597). Informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Study medication
To ensure completeness of opioid detoxification, XR-NTX was preceded by a challenge with 0.6 mg of naloxone hydrochloride IV. The OUD participants were offered up to three monthly intramuscular injections of XR-NTX (380 mg of naltrexone-HCl gradually released from dissolvable polymer microspheres over a period of one month, manufactured by Alkermes Inc, Cambridge, MA, under the brand name Vivitrol®). As part of the consent procedure, participants were briefed about the expected loss of pharmacological effects of opioids resulting from the XR-NTX treatment, and the dangers of attempting to overcome the opiate receptor blockade with higher than usual opioid doses [40, 41]. Medication was provided in the context of ongoing psychosocial support (two weekly sessions of professional drug counseling and anti-relapse strategies by trained clinical psychologists) and twice-weekly UDS monitoring. Plasma concentrations of naltrexone and 6β-naltrexol (an active metabolite of naltrexone) were measured on the day of the on-treatment session using established liquid chromatography and tandem mass spectrometry technique [19, 42]. Upon study completion, continuation of care was discussed with the patients, and they were given referrals to treatment providers in the community.
Procedure
The OUD individuals completed baseline assessments including demographics, history and physical exam, UDS, and the MINI. Eligible OUD participants were offered a total of three monthly injections of XR-NTX. MRI scans and craving assessments were performed both before and after the first XR-NTX injection. Specifically, immediately before the first XR-NTX injection (i.e., pre-treatment), the OUD participants completed an MRI session and reported craving for opioids. Craving was measured by the question “To what degree are you feeling any craving/desire for opiates” using a 10-point scale (0=none; 9=extremely). An average of 11.36 (SD = 3.09) days after the first XR-NTX injection (i.e., on-treatment), MRI and craving assessments were repeated. Craving scores were missing in one OUD participant at on-treatment. An average of 44.00 (SD = 17.06) days after the third and last XR-NTX injection (i.e., post-treatment), a subsample of 25 OUD participants completed a third session of MRI and craving assessments. Timing of the study sessions was based on a prior pharmacokinetic study showing that plasma concentrations of naltrexone and its primary metabolite 6β-naltrexol reach a plateau at approximately 7–14 days following XR-NTX injection and return to baseline after approximately one month [43]. Details on the post-treatment session are reported in the Supplementary Information (see Figure S1 and Table S2). The non-OUD individuals completed assessments of demographics, UDS, the MINI, and one MRI session.
MRI data acquisition and preprocessing
MRI data were collected using a Siemens Tim Trio 3T system at the University of Pennsylvania. High-resolution T1-weighted whole-brain images were acquired using the magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence with repetition time/echo time = 1510/3.71 ms, field of view = 256 × 192 mm², matrix = 256 × 192, slice thickness/gap = 1/0 mm, 160 slices, effective voxel resolution = 1 × 1 × 1 mm³, and flip angle = 9°.
MRI images were processed using the volume-based cortical thickness pipeline implemented in Advanced Normalization Tools (ANTs) [34, 44]. Details of the pipeline can be found in Tustison et al. [34]. Specifically, N4 bias field correction was performed on the raw structural images to minimize the impact of low-frequency field inhomogeneity [45]. Bias-corrected images were subjected to brain extraction via optimized template registration and Atropos three-tissue segmentation. Atropos six-tissue segmentation was then performed iteratively with N4 bias field correction using atlases derived from the Open Access Series of Imaging Studies (OASIS) dataset [46]. Voxelwise cortical thickness was estimated within the volumetric domain using the diffeomorphic registration-based cortical thickness (DiReCT) algorithm that measures of the distance between the gray matter-white matter interface and the gray matter-cerebrospinal fluid interface while taking into consideration neuroanatomical constraints [47]. Cortical thickness measure from ANTs has shown high test-retest reliability and external validity [34]. Individual-level cortical thickness maps were created and spatially smoothed with full width at half maximum (FWHM) set to 4 mm using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). For confirmatory purpose, we repeated the main analyses using cortical thickness data that were unsmoothed and smoothed with 12-mm FWHM as well as using voxel-based morphometry data that were estimated by the Computational Anatomy Toolbox (CAT) (see the Supplementary Information).
Data analysis
A whole-brain paired t-test examined the change in regional cortical thickness from pre-treatment to on-treatment among the OUD individuals. Significant changes were determined using the threshold-free cluster enhancement (TFCE) algorithm at cluster-level familywise error-corrected p < 0.05 [48]. Regions showing a significant change were defined as the regions of interest (ROIs). Mean cortical thickness were extracted from the ROIs and subjected to subsequent statistical analyses in R (www.R-project.org). Specifically, we performed two-sample t-test to compare the cortical thickness at the ROIs between the non-OUD and OUD individuals (for both pre-treatment and on-treatment timepoints). However, given that the non-OUD group completed only one MRI session, comparisons between the two groups should be treated as exploratory. We also performed Pearson correlation to test for the association between the change in cortical thickness and the change in opioid craving among the OUD individuals. Exploratory whole-brain analyses were conducted to examine the group difference and the correlation with the change in craving in brain regions beyond the ROIs.
We further examined whether cortical thickness change was correlated with MOR binding potential across brain regions in the OUD cohort. A whole-brain MOR binding potential map was obtained from a previous study in which 204 adults underwent [¹¹C]carfentanil positron emission tomography (PET) [38]. A polynomial regression analysis was performed to test the association between mean cortical thickness change and MOR binding potential across the 360 cortical regions defined by the Human Connectome Project multimodal parcellation (HCP-MMP) atlas of the cerebral cortex [49]. Starting with a basic model that only included the constant term, we added linear, cubic, quadratic, and subsequent higher-order terms to the model one at a time. F-test was used to determine whether the addition of each term significantly improved the fit of the model. The process was continued until the improvement in the model fit was no longer significant.
We performed additional exploratory analyses to examine (1) the association between cortical thickness change and the duration of abstinence (i.e., the number of days since last opioid use), (2) the association between cortical thickness change and data quality, and (3) cortical thickness at post-treatment. We also performed confirmatory analyses on un-smoothed data as well as data that were smoothed using 12-mm FWHM. Details of these analyses are in the Supplementary Information.
Results
Participant characteristics
Participant characteristics of the OUD and the non-OUD groups are summarized in Table 1. The OUD and non-OUD groups significantly differed in race and diagnosis of stimulant use disorder, which were controlled for in subsequent comparisons between the two groups. The OUD individuals showed a significant reduction in opioid craving from pre-treatment to on-treatment (difference = 1.39, 95% confidence interval [CI] = [0.49, 2.30], t(45) = 3.10, p = 0.003). There was also a significant reduction in the number of cigarettes smoked per day (difference = 2.94, 95% CI = [1.34, 4.54], t(43) = 3.70, p < 0.001), a finding that we have reported elsewhere [50]. Positive UDS results for stimulant and cannabis did not differ between pre- and on-treatment sessions (McNemar’s test, χ²(1) = 0.36 & 0.57, p = 0.55 & 0.45).
Cortical thickness change across time in the OUD individuals
Whole-brain comparison between the pre-treatment and on-treatment sessions showed a significant reduction in the cortical thickness of the medial prefrontal cortex (mPFC) and the adjacent anterior cingulate cortex (aCC) among the OUD individuals (whole-brain TFCE-corrected p < 0.05; see Fig. 1a and Table S1). The opposite contrast for increased cortical thickness from pre-treatment to on-treatment did not reveal any significant clusters. Given the spatial proximity of the mPFC and aCC clusters, we combined them into a single ROI in the subsequent analyses. For visualization purposes, we extracted and plotted the cortical thickness of the mPFC/aCC ROI at pre-treatment and on-treatment (see Fig. 1b).
Fig. 1. Cortical thickness change across time.
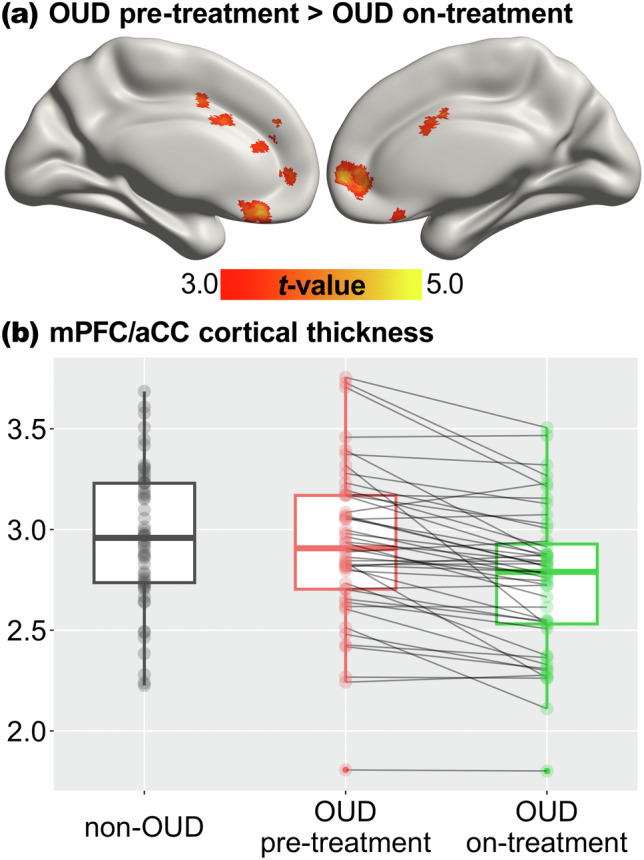
a Significant reduction in cortical thickness from pre-treatment to on-treatment in the OUD group revealed by whole-brain paired t-test, thresholded at corrected p < 0.05. b Cortical thickness of the mPFC/aCC region of interest in the non-OUD group and the OUD group at the pre-treatment and on-treatment sessions. OUD opioid use disorder, mPFC medial prefrontal cortex, aCC anterior cingulate cortex.
The observed change in mPFC/aCC thickness was not attributable to duration of abstinence or imaging data quality. Exploratory analysis showed that mPFC/aCC cortical thickness at the post-treatment session was significantly lower than the pre-treatment session but did not differ from the on-treatment session (see Fig. S2). Details of these analyses are reported in the Supplementary Information.
Exploratory comparisons between the OUD and non-OUD individuals
Compared to the non-OUD group (mean ± SD = 2.96 ± 0.34), the OUD group had comparable mPFC/aCC ROI cortical thickness at pre-treatment (2.92 ± 0.39, t(92.8) = 0.55, p = 0.58) but significantly lower thickness at on-treatment (2.75 ± 0.36, t(96.2) = 2.98, p = 0.004) (see Fig. 1b and S2). Linear regression analyses that controlled for race and stimulant use disorder similarly showed differences between non-OUD and OUD in mPFC/aCC thickness (pre-treatment, coefficient=0.10, standard error [SE] = 0.09, t(96) = 1.13, p = 0.26; on-treatment, coefficient = 0.29, SE = 0.09, t(96) = 3.42, p = 0.001). Whole-brain exploration showed widespread, lower frontal, parietal, and temporal cortical thickness in the OUD than the non-OUD group for both pre-treatment and on-treatment (see Fig. S3 and Tables S3 & S4).
Association between changes in cortical thickness and opioid craving
There was a significant positive correlation between the reduction in opioid craving and the reduction in mPFC/aCC thickness from pre-treatment to on-treatment in the OUD group (r = 0.44, p = 0.002) (see Fig. 2b). Whole-brain regression analysis was performed to explore regions beyond the mPFC/aCC ROI and showed that the change in opioid craving was positively associated with the change in cortical thickness of several additional regions including the bilateral insula, bilateral inferior frontal gyrus, left precentral and postcentral gyrus, left temporoparietal junction, and left middle temporal gyrus (corrected p < 0.05; see Fig. 2a and Table S5). There was no significant negative correlation between the change in opioid craving and the change in cortical thickness.
Fig. 2. Association between changes in cortical thickness and opioid craving.
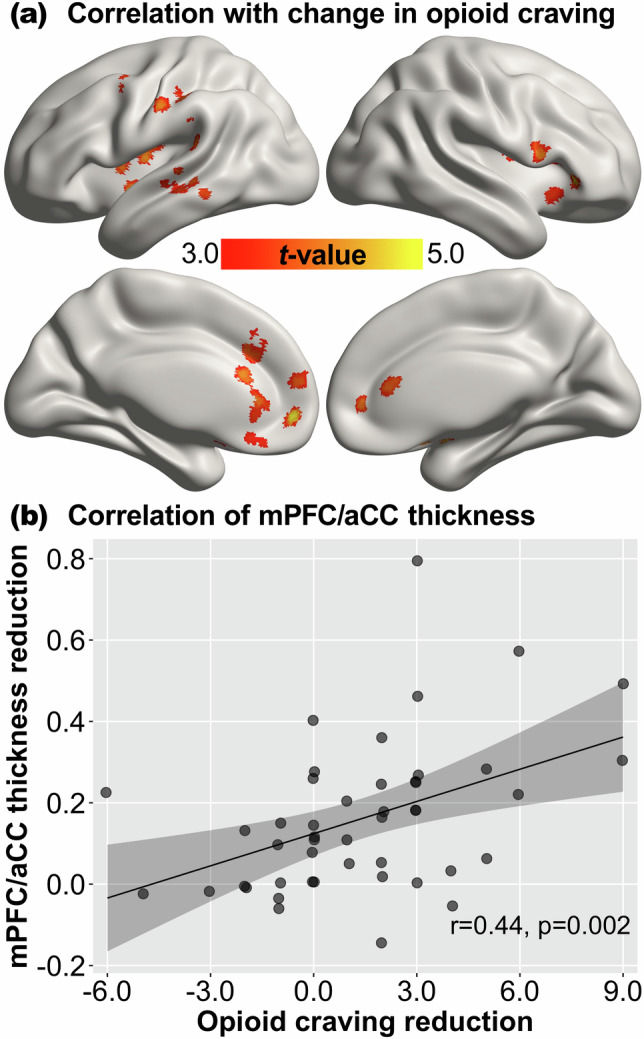
a Significant correlation between the change in opioid craving and the change in cortical thickness from pre-treatment to on-treatment in the OUD group revealed by whole-brain regression, thresholded at corrected p < 0.05. b Association between the change in opioid craving and the change in cortical thickness of the mPFC/aCC region of interest. OUD opioid use disorder, mPFC medial prefrontal cortex, aCC anterior cingulate cortex. Reduction = pre-treatment minus on-treatment. The shaded area represents 95% confidence interval.
Mapping of MOR binding potential to cortical thickness change
We conducted a polynomial regression analysis to examine the relationship between MOR binding potential and cortical thickness reduction in the OUD group. Model comparison using F-tests indicated a cubic model as the optimal model (linear vs. quadratic, F(1,357) = 37.16, p < 0.001; quadratic vs. cubic, F(1,356) = 13.38, p < 0.001; cubic vs. quartic, F(1,355) = 0.11, p = 0.74). The curvilinear relationship was characterized by significant linear, quadratic, and cubic effects of MOR binding potential on cortical thickness reduction (F(1,356) = 93.64, 38.45, & 13.38, ps<0.001, R² = 0.29; see Fig. 3). Examination of the tangent slopes and their 95% CIs suggests a positive association between MOR binding potential and cortical thickness reduction in brain regions with relatively high MOR binding potential (>0.33).
Fig. 3. Mapping of MOR binding potential to cortical thickness change.
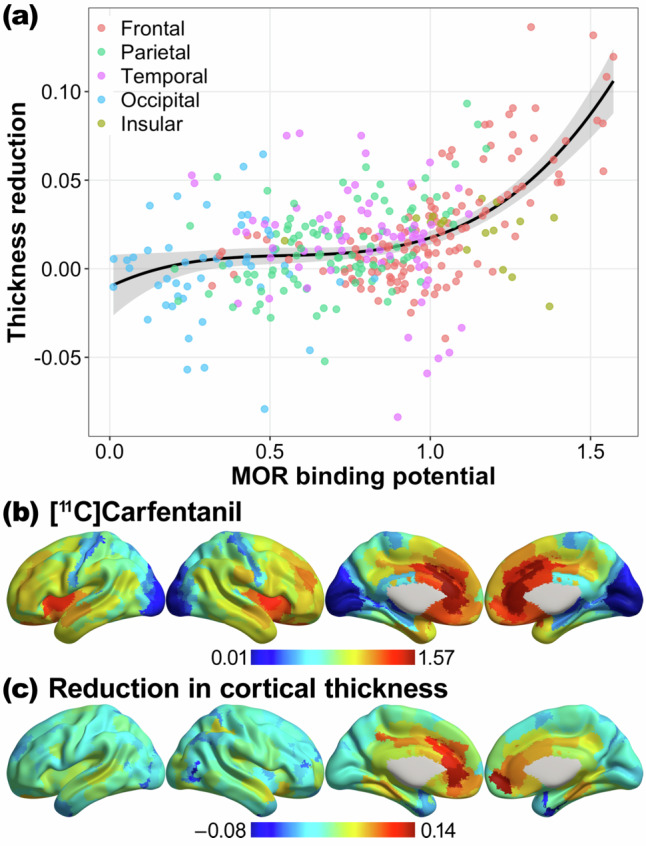
a Curvilinear association between MOR binding potential and the change in cortical thickness (pre-treatment minus on-treatment) across cortical regions in the OUD group. b MOR receptor binding potential map obtained from a large sample (N = 204) [¹¹C]carfentanil positron emission tomographic imaging study (Kantonen et al. [38], NeuroImage). c Unthresholded map of cortical thickness change (pre-treatment minus on-treatment) in the OUD group. OUD opioid use disorder, MOR μ-opioid receptor.
Discussion
We found reduced cortical thickness in the mPFC and the adjacent aCC among the OUD patients during the first two weeks of MOR antagonist treatment with XR-NTX. An exploratory comparison of the OUD and non-OUD individuals showed that the cortical thickness in mPFC/aCC was comparable between the two groups before XR-NTX but was lower in the former during and after XR-NTX. The reduction in mPFC/aCC cortical thickness after treatment correlated with patients’ reductions in craving for opioids, but not with the duration of opioid abstinence. A multimodal analysis with [¹¹C]carfentanil PET data suggests that the relationship between MOR binding potential and cortical thickness reduction followed a curvilinear pattern, with larger thickness reduction observed in brain regions with higher MOR binding potential.
MRI-measured cortical thickness reflects the distance between the cerebral cortex’s pial surface and the white matter-gray matter boundary [34, 47, 51]. The cerebral cortex comprises of neuronal cell bodies, glia cells, and neuropil – a network of axons, dendrites, and synapses that facilitate neural communication [52]. Cortical thickness correlates negatively with neuronal density [53] and positively with dendritic arborization [54, 55] and density of glia cells [56]. Gene expression studies have also associated variations in cortical thickness with the cellular composition of the cortex, including CA1 pyramidal cells, astrocytes, and microglia [57–59]. Therefore, a thicker cortex may reflect not only the number of neurons but also a more intricate neuropil organization attributable to dendritic arborization and support by glial cells that enhance intracortical connectivity [52].
Preclinical evidence suggests a role for opioids in regulating neurodevelopment. Perinatal blockade of opioid receptors with naltrexone and naloxone was found to increase the number of dendritic spines (i.e., protrusions that receive synaptic input) [60, 61], whereas morphine decreased dendritic spine density [61] and dendritic length [62]. Interestingly, in adult rats, repeated morphine exposure upregulated the expression of genes involved in intra- and inter-cellular signaling and synaptic morphology, which may have implications for neuroadaptation and neuroplasticity processes in the development of and recovery from OUD [63].
Studies reporting reduced cortical thickness following a clinically effective pharmacotherapy are a minority within the literature pertaining to the treatment of mental disorders [64]. In most cases, reduction in cortical thickness after early adulthood is regarded as a manifestation or consequence of aging [65] and conditions such as neurodegeneration [66], traumatic brain injury [67], and mental disorders [68–71] (but see [72]). Reductions in prefrontal cortical thickness, specifically, can be associated with cognitive decline such as impaired executive functions [73]. The observed decrease in mPFC/aCC cortical thickness in the current study appears to contradict the absence of clinical evidence in the literature that indicates any cognitive impairment during XR-NTX treatment of OUD [2, 74, 75]. On the contrary, preclinical studies show that naltrexone promotes early neurodevelopment [60] and attenuates age-related decline in attentional set-shifting [76]. In human subjects, naltrexone has been shown to increase cognitive flexibility [77] and adaptive cognitive control [78]. The mPFC and aCC are implicated in a range of cognitive functions (e.g., incentive salience processing [8], impulsivity control [79], decision making [80]), which either remain unchanged or show partial recovery as a result of abstinence [25, 27, 81–84], psychotherapy [85–87], and pharmacotherapy [20, 88–90] in individuals with substance use disorders. The discrepancy between previous findings and the observed mPFC/aCC thickness reduction raises intriguing questions about the mechanisms underlying XR-NTX’s effect on brain structure and potential clinical implications of our findings.
One possible interpretation is that MOR blockade by XR-NTX alters the neurotransmission within and thus morphology of MOR-rich brain structures. A previous study showed that the dopamine D2 antagonist haloperidol (a first-generation antipsychotic medication) reduced the volume of the striatum, a region rich in D2 receptors, within 2 hours of administration [91]. Similarly, loss of brain gray matter has been associated with the use of other first-generation antipsychotics such as chlorpromazine and trifluoperazine which are high-affinity D2 antagonists [92, 93]. To be best of our knowledge, no prior research has examined the effect of opioid antagonism on brain structure in humans. Our finding of reduced cortical thickness in MOR-rich regions during XR-NTX treatment appears to corroborate the D2 literature and suggests a link from neurotransmission and/or receptor occupancy to brain morphology [94–96]. Nevertheless, the molecular and cellular mechanisms underlying such a link remain unclear.
The reduced mPFC/aCC cortical thickness may also reflect neuroplasticity that relates to changes in OUD patients’ responses to external stimuli. The mPFC and the aCC play a key role in processing rewards [97–100]. Individuals with substance use disorders show heightened mPFC/aCC neural response to drug cues [8, 100] that correlates with elevated drug craving [9, 101, 102]. XR-NTX is highly effective in blunting opioid craving [2–6] and reducing the neural response to opioid cues [19, 20]. The reduction in mPFC/aCC cortical thickness may be attributable to the brain’s adaption (e.g., elimination of dysfunctional synapses) to environmental drug cues whose incentive value diminished as a result of XR-NTX treatment. Alternatively, it could reflect changes in the environment (e.g., less exposure to drug cues) that reinforced the craving-reducing effect of XR-NTX.
The observation that post-treatment mPFC/aCC cortical thickness was comparable to on-treatment and lower than pre-treatment suggests an acute effect of XR-NTX that occurred during the first two weeks of treatment. Prior research showed that craving for opioids immediately decreased following the first injection of XR-NTX and remained stable throughout subsequent injections. Given the similar temporal profiles, structural changes of the mPFC/aCC may mediate XR-NTX-induced attenuation of opioid craving. Such speculation was corroborated by the observed positive correlation between the reduction in mPFC/aCC cortical thickness and the reduction in opioid craving.
In an exploratory analysis, we compared the single session of cortical thickness data of the non-OUD group to each of the pre-treatment and on-treatment sessions of the OUD group. We found that, relative to the non-OUD individuals, the OUD patients had comparable pre-treatment mPFC/aCC cortical thickness but significantly lower on-treatment and post-treatment thickness. Hence, the observed thickness reduction in the OUD group may not signify a restorative or normalization process but rather a treatment-related effect that is independent of OUD diagnosis itself. The results parallel those reported in patients with psychosis, where antipsychotics modulate cortical thickness in brain regions unaffected by the illness at baseline, suggesting treatment-induced neuroanatomical alterations beyond the primary pathology [93]. The exact significance of these alterations, whether they are essential for the therapeutic effects or are mere side effects, remains unclear. Our findings thus underscore the complex and multifaceted effects of XR-NTX, whose impact extends beyond mere reversal of impairments caused by OUD.
Our study has several limitations. First, the absence of untreated, placebo-controlled, or agonist-treated OUD cohorts for comparison limited our ability to determine whether the observed reduction in mPFC/aCC cortical thickness was specific to XR-NTX. Implementing a placebo control condition for XR-NTX would pose challenges, as patients almost invariably test opioid blockade in the early stages of treatment, which could increase the risk of relapse and overdose under placebo [103]. Additionally, we did not find an association between the duration of abstinence and cortical thickness, suggesting that abstinence alone could not fully account for the results. Secondly, the non-OUD cohort only completed a single session of MRI, which prevented us from isolating the main effect of time (i.e., pre vs. on). Potential confounding variables, such as signals from non-cortical tissues, would be better addressed with repeated MRI scans for both cohorts. Moreover, the non-OUD cohort was a convenient sample and was not well matched with the OUD cohort on variables including race and comorbid stimulant use disorder (although controlling for these variables produced similar results). Therefore, caution is required in the interpretation of the wide-spread cortical thickness differences between the two cohorts, which were somewhat inconsistent with the results of a prior study [29]. Thirdly, the study did not examine the underlying cellular and molecular processes. Prior research has highlighted the contribution of genetic factors to regional cortical thickness [57–59]. The brain opioidergic system has also been implicated in shaping brain morphology during both early development and adulthood [60–63]. It would be interesting to examine the role of genetic factors and opioidergic neurotransmissions in brain structural changes during pharmacotherapy of OUD. Fourthly, a lack of neuropsychological assessments prevented us from examining the relationship between cortical thickness reduction and potential cognitive functions. A significant reduction in cortical thickness over a short period (e.g., ≈2 weeks) is uncommon and can be of neurological concern [65–71]. Although cognitive deterioration has not been reported in previous trials involving XR-NTX treatment of OUD [2, 74, 75], the observed acute reduction in mPFC/aCC thickness during the early phase of the treatment underscores the need for more in-depth investigation to determine if it constitutes a side effect. Specifically, future research should combine neuroimaging and neuropsychological assessments to investigate XR-NTX’s effects on cognitive functions associated with the mPFC/aCC such as decision making [104], inhibitory control [79], and social cognition [105]. Lastly, the three-month duration of XR-NTX treatment was relatively short compared to the extended period required for recovery from OUD. Although a single on-treatment time point after the first injection minimized the impact of patient dropout, it limited our ability to fully characterize the time course of brain structural changes during the three-month treatment. In addition, our data were collected before the regional drug market became dominated by fentanyl, a synthetic opioid with much higher potency and cytotoxicity than heroin. Effective treatment of OUD amid the fentanyl epidemic will likely require longer (>6 months) treatment duration [106, 107]. It would be valuable to evaluate brain structure at additional time points and their correlation with long-term outcomes such as relapse and overdose.
In conclusion, we demonstrated that XR-NTX treatment is associated with reduced cortical thickness among OUD patients in the mPFC and the adjacent aCC, regions known for their high MOR binding potential. Importantly, cortical thickness reductions covaried with the clinical effectiveness of XR-NTX, as indexed by the reduction in opioid craving. These findings present a novel neurobiological phenomenon in XR-NTX treatment for OUD. Understanding the impact of XR-NTX on cortical morphology and its relationship with clinical outcomes contributes to our knowledge of the underlying mechanisms of the treatment and may guide future interventions aimed at improving the clinical management of OUD.
Supplementary information
Author contributions
JL and DDL designed the study. ZS, JL, and DDL collected the data. ZS, WC, and KGL analyzed the data. ZS, XL, DRT, KGL, JAD, DDL, & CEW interpreted the results. ZS, XL, DRT, DDL, & CEW wrote the manuscript. All authors contributed to the revision of the manuscript for critical intellectual content and approved the final version.
Funding
This work was supported by the Commonwealth of Pennsylvania CURE grant SAP#4100055577 (Anna Rose Childress, University of Pennsylvania), the NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (#30780) (ZS), and the following National Institutes of Health grants: K01DA051709 (ZS), R00AA026892 (CEW), T32DA028874 (XL), and R01DA036028 (DDL). The funding sources had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Data availability
The cortical thickness change map is available at NeuroVault (http://neurovault.org/collections/15137). The [¹¹C]carfentanil PET map is available at NeuroVault (http://neurovault.org/collections/5706) [38]. The HCP-MMP atlas is available at NeuroVault (https://neurovault.org/collections/1549) [49]. Clinical data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Daniel D. Langleben, Corinde E. Wiers.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03061-0.
References
- 1.Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020;87:82–8. 10.1016/j.biopsych.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 2.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–13. 10.1016/S0140-6736(11)60358-9 [DOI] [PubMed] [Google Scholar]
- 3.Tanum L, Solli KK, Latif ZE, Benth JŠ, Opheim A, Sharma-Haase K, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197–205. 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309–18. 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, et al. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016;374:1232–42. 10.1056/NEJMoa1505409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan MariaA, Bisaga Adam, Pavlicova Martina, Carpenter KennethM, Choi CJean, Mishlen Kaitlyn, et al. A randomized trial comparing extended-release injectable suspension and oral naltrexone, both combined with behavioral therapy, for the treatment of opioid use disorder. Am J Psychiatry. 2019;176:129–37. 10.1176/appi.ajp.2018.17070732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology. 2019;44:259–73. 10.1038/s41386-018-0232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard AA, Hauson AO, Lackey NS, Zhang E, Khayat S, Carson B, et al. Functional neuroanatomy of craving in heroin use disorder: voxel-based meta-analysis of functional magnetic resonance imaging (fMRI) drug cue reactivity studies. Am J Drug Alcohol Abuse. 2023;49:418–30. 10.1080/00952990.2023.2172423 [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012;1469:63–72. 10.1016/j.brainres.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2015;20:968–78. 10.1111/adb.12182 [DOI] [PubMed] [Google Scholar]
- 11.Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control patients: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–62. 10.1037/1064-1297.5.3.256 [DOI] [PubMed] [Google Scholar]
- 12.Lee TMC, Pau CWH. Impulse control differences between abstinent heroin users and matched controls. Brain Inj. 2002;16:885–89. 10.1080/02699050210128915 [DOI] [PubMed] [Google Scholar]
- 13.Zeng H, Lee TMC, Waters JH, So K-F, Sham PC, Schottenfeld RS, et al. Impulsivity, cognitive function, and their relationship in heroin-dependent individuals. J Clin Exp Neuropsychol. 2013;35:897–905. 10.1080/13803395.2013.828022 [DOI] [PubMed] [Google Scholar]
- 14.Fu L-p, Bi G-h, Zou Z-t, Wang Y, Ye E-m, Ma L, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438:322–26. 10.1016/j.neulet.2008.04.033 [DOI] [PubMed] [Google Scholar]
- 15.Yang B, Yang S, Zhao L, Yin L, Liu X, An S. Event-related potentials in a Go/Nogo task of abnormal response inhibition in heroin addicts. Sci China Ser C: Life Sci. 2009;52:780–88. 10.1007/s11427-009-0106-4 [DOI] [PubMed] [Google Scholar]
- 16.Ceceli AO, King SG, McClain N, Alia-Klein N, Goldstein RZ. The neural signature of impaired inhibitory control in individuals with heroin use disorder. J Neurosci. 2023;43:173–82. 10.1523/JNEUROSCI.1237-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Wang Y, Zhang Y, Li W, Zhu J, Zheng Y, et al. Assessing cue-induced brain response as a function of abstinence duration in heroin-dependent individuals: an event-related fMRI study. PLoS One. 2013;8:e62911. 10.1371/journal.pone.0062911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, et al. Acute effect of methadone maintenance dose on brain fmri response to heroin-related cues. Am J Psychiatry. 2008;165:390–4. 10.1176/appi.ajp.2007.07010070 [DOI] [PubMed] [Google Scholar]
- 19.Langleben DD, Ruparel K, Elman I, Loughead JW, Busch EL, Cornish J, et al. Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol. 2014;19:262–71. 10.1111/j.1369-1600.2012.00462.x [DOI] [PubMed] [Google Scholar]
- 20.Shi Z, Wang A-L, Jagannathan K, Fairchild VP, O’Brien CP, Childress AR, et al. Effects of extended-release naltrexone on the brain response to drug-related stimuli in patients with opioid use disorder. J Psychiatry Neurosci. 2018;43:254–61. 10.1503/jpn.170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Xu Q, Li S, Zhao X, Ma L, Zheng Y, et al. The effects of methadone maintenance treatment on heroin addicts with response inhibition function impairments: evidence from event-related potentials. J Food Drug Anal. 2015;23:260–66. 10.1016/j.jfda.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriegler J, Wegener S, Richter F, Scherbaum N, Brand M, Wegmann E. Decision making of individuals with heroin addiction receiving opioid maintenance treatment compared to early abstinent users. Drug Alcohol Depend. 2019;205:107593. 10.1016/j.drugalcdep.2019.107593 [DOI] [PubMed] [Google Scholar]
- 23.Yang T, Zhao X, Zhang F, Li X, Zhu H, Zhang M, et al. Craving-induced effects of different drug cues on persons abstaining from heroin. Addict Res Theory. 2019;27:235–41. 10.1080/16066359.2018.1496425 [DOI] [Google Scholar]
- 24.Tolomeo S, Gray S, Matthews K, Steele JD, Baldacchino A. Multifaceted impairments in impulsivity and brain structural abnormalities in opioid dependence and abstinence. Psychol Med. 2016;46:2841–53. 10.1017/S0033291716001513 [DOI] [PubMed] [Google Scholar]
- 25.Biernacki K, McLennan SN, Terrett G, Labuschagne I, Rendell PG. Decision-making ability in current and past users of opiates: a meta-analysis. Neurosci Biobehav Rev. 2016;71:342–51. 10.1016/j.neubiorev.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Li B, Zhou X, Liao Y, Tang J, Liu T, et al. Changes in brain gray matter in abstinent heroin addicts. Drug Alcohol Depend. 2012;126:304–08. 10.1016/j.drugalcdep.2012.05.030 [DOI] [PubMed] [Google Scholar]
- 27.Ieong HF-H, Yuan Z. Resting-state neuroimaging and neuropsychological findings in opioid use disorder during abstinence: a review. Front Hum Neurosci. 2017;11:169. 10.3389/fnhum.2017.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, et al. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460:72–7. 10.1016/j.neulet.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 29.Li M, Tian J, Zhang R, Qiu Y, Wen X, Ma X, et al. Abnormal cortical thickness in heroin-dependent individuals. Neuroimage. 2014;88:295–307. 10.1016/j.neuroimage.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 30.Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, et al. Gray matter abnormalities in opioid-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2017;43:505–17. 10.1080/00952990.2016.1245312 [DOI] [PubMed] [Google Scholar]
- 31.Shi Z, Li X, Byanyima JI, O’Brien CP, Childress AR, Lynch KG, et al. Effects of current smoking severity on brain gray matter volume in opioid use disorder–a voxel-based morphometry study. Am J Drug Alcohol Abuse. 2023;49:180–89. 10.1080/00952990.2023.2169616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–80. 10.1016/j.neuroimage.2009.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–79. 10.1016/j.neuroimage.2014.05.044 [DOI] [PubMed] [Google Scholar]
- 35.Yang W, Zhang M, Tang F, Du Y, Fan L, Luo J, et al. Recovery of superior frontal gyrus cortical thickness and resting-state functional connectivity in abstinent heroin users after 8 months of follow-up. Hum Brain Mapp. 2022;43:3164–75. 10.1002/hbm.25841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt A, Vogel M, Baumgartner S, Wiesbeck GA, Lang U, Borgwardt S, et al. Brain volume changes after long-term injectable opioid treatment: a longitudinal voxel-based morphometry study. Addict Biol. 2021;26:e12970. 10.1111/adb.12970 [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Wang S, Liu Y, Wang F, Li Q, Li Z, et al. The influence of methadone on cerebral gray matter and functional connectivity. Annals of Palliative Medicine. 2021;10:9497–507. 10.21037/apm-21-2012 [DOI] [PubMed] [Google Scholar]
- 38.Kantonen T, Karjalainen T, Isojärvi J, Nuutila P, Tuisku J, Rinne J, et al. Interindividual variability and lateralization of μ-opioid receptors in the human brain. Neuroimage. 2020;217:116922. 10.1016/j.neuroimage.2020.116922 [DOI] [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatr. 1998;59:22–33. [PubMed] [Google Scholar]
- 40.Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. J Pharmacol Exp Ther. 2011;336:488–95. 10.1124/jpet.110.173823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan X, Chen T, Gudin J, Couch JP, Chiravuri S. Acute opioid withdrawal precipitated by ingestion of crushed embeda (morphine extended release with sequestered naltrexone): case report and the focused review of the literature. J Opioid Manag. 2010;6:300–03. 10.5055/jom.2010.0028 [DOI] [PubMed] [Google Scholar]
- 42.Slawson MH, Chen M, Moody D, Comer SD, Nuwayser ES, Fang WB, et al. Quantitative analysis of naltrexone and 6β-naltrexol in human, rat, and rabbit plasma by liquid chromatography-electrospray ionization tandem mass spectrometry with application to the pharmacokinetics of Depotrex® in rabbits. J Anal Toxicol. 2007;31:453–61. 10.1093/jat/31.8.453 [DOI] [PubMed] [Google Scholar]
- 43.Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcoholism: Clinical and Experimental Research. 2006;30:480–90. 10.1111/j.1530-0277.2006.00052.x [DOI] [PubMed] [Google Scholar]
- 44.Tustison NJ, Cook PA, Holbrook AJ, Johnson HJ, Muschelli J, Devenyi GA et al. The ANTsX ecosystem for quantitative biological and medical imaging. Sci Rep. 2021;11:9068. 10.1038/s41598-021-87564-6. [DOI] [PMC free article] [PubMed]
- 45.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–20. 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. 10.1007/s12021-011-9109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. Neuroimage. 2009;45:867–79. 10.1016/j.neuroimage.2008.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- 49.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–78. 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang A-L, Shi Z, Elman I, Langleben DD. Reduced cigarette smoking during injectable extended-release naltrexone treatment for opioid use disorder. Am J Drug Alcohol Abuse. 2020;46:472–77. 10.1080/00952990.2020.1741001 [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–55. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagstyl K, Lerch JP. Cortical thickness. In: Spalletta G, Piras F, Gili T, editors. Brain Morphometry. New York, NY: Springer; 2018. p. 35–49.
- 53.la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, et al. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage. 2011;56:951–60. 10.1016/j.neuroimage.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 54.Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol. 2011;95:275–300. 10.1016/j.pneurobio.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 55.Russell TA, Blizinsky KD, Cobia DJ, Cahill ME, Xie Z, Sweet RA, et al. A sequence variant in human KALRN impairs protein function and coincides with reduced cortical thickness. Nature Communications. 2014;5:4858. 10.1038/ncomms5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlo CN, Stevens CF. Structural uniformity of neocortex, revisited. Proc Natl Acad Sci USA 2013;110:1488–93. 10.1073/pnas.1221398110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vidal-Pineiro D, Parker N, Shin J, French L, Grydeland H, Jackowski AP, et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep. 2020;10:21803. 10.1038/s41598-020-78471-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin J, French L, Xu T, Leonard G, Perron M, Pike GB, et al. Cell-specific gene-expression profiles and cortical thickness in the human brain. Cereb Cortex. 2017;28:3267–77. 10.1093/cercor/bhx197 [DOI] [PubMed] [Google Scholar]
- 59.Writing Committee for the Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, Bipolar Disorder, Major Depressive Disorder, Obsessive-Compulsive Disorder, and Schizophrenia ENIGMA Working Groups. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry. 2021;78:47–63. [DOI] [PMC free article] [PubMed]
- 60.Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J Comp Neurol. 1989;281:13–22. 10.1002/cne.902810103 [DOI] [PubMed] [Google Scholar]
- 61.Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA 2005;102:1725–30. 10.1073/pnas.0406797102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricalde AA, Hammer RP. Perinatal opiate treatment delays growth of cortical dendrites. Neurosci Lett. 1990;115:137–43. 10.1016/0304-3940(90)90444-E [DOI] [PubMed] [Google Scholar]
- 63.Liu SX, Gades MS, Swain Y, Ramakrishnan A, Harris AC, Tran PV, et al. Repeated morphine exposure activates synaptogenesis and other neuroplasticity-related gene networks in the dorsomedial prefrontal cortex of male and female rats. Drug Alcohol Depend. 2021;221:108598. 10.1016/j.drugalcdep.2021.108598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bansal R, Hellerstein DJ, Peterson BS. Evidence for neuroplastic compensation in the cerebral cortex of persons with depressive illness. Mol Psychiatry. 2018;23:375–83. 10.1038/mp.2017.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52:1215–23. 10.1016/j.neuroimage.2010.04.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apostolova LG, Thompson PM. Brain mapping as a tool to study neurodegeneration. Neurotherapeutics. 2007;4:387–400. 10.1016/j.nurt.2007.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Govindarajan KA, Narayana PA, Hasan KM, Wilde EA, Levin HS, Hunter JV, et al. Cortical thickness in mild traumatic brain injury. J Neurotrauma. 2016;33:1809–17. 10.1089/neu.2015.4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–09. 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Z, Zhao Y, Wen K, Li Q, Pan N, Fu S, et al. Cortical thickness abnormalities in patients with bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2022;300:209–18. 10.1016/j.jad.2021.12.080 [DOI] [PubMed] [Google Scholar]
- 70.Fouche J-P, du Plessis S, Hattingh C, Roos A, Lochner C, Soriano-Mas C, et al. Cortical thickness in obsessive–compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry. 2017;210:67–74. 10.1192/bjp.bp.115.164020 [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Zhang Q, Shah C, Li Q, Sweeney JA, Li F, et al. Cortical thickness abnormalities at different stages of the illness course in schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:560–70. 10.1001/jamapsychiatry.2022.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Zhang Y, Zhao Y, Li Z, Kemp GJ, Wu M, et al. Cortical thickness abnormalities in patients with post-traumatic stress disorder: a vertex-based meta-analysis. Neurosci Biobehav Rev. 2022;134:104519. 10.1016/j.neubiorev.2021.104519 [DOI] [PubMed] [Google Scholar]
- 73.Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42:180–92. 10.1016/j.neubiorev.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krupitsky E, Nunes EV, Ling W, Gastfriend DR, Memisoglu A, Silverman BL. Injectable extended‐release naltrexone (XR‐NTX) for opioid dependence: long‐term safety and effectiveness. Addiction. 2013;108:1628–37. 10.1111/add.12208 [DOI] [PubMed] [Google Scholar]
- 75.Solli KK, Latif ZeH, Opheim A, Krajci P, Sharma‐Haase K, Benth JŠ, et al. Effectiveness, safety and feasibility of extended‐release naltrexone for opioid dependence: a 9‐month follow‐up to a 3‐month randomized trial. Addiction. 2018;113:1840–49. 10.1111/add.14278 [DOI] [PubMed] [Google Scholar]
- 76.Rodefer JS, Nguyen TN. Naltrexone reverses age-induced cognitive deficits in rats. Neurobiol Aging. 2008;29:309–13. 10.1016/j.neurobiolaging.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 77.Grant JE, Odlaug BL, Schreiber LR, Kim SW. The opiate antagonist, naltrexone, in the treatment of trichotillomania: results of a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34:134–38. 10.1097/JCP.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 78.van Steenbergen H, Weissman DH, Stein DJ, Malcolm-Smith S, van Honk J. More pain, more gain: blocking the opioid system boosts adaptive cognitive control. Psychoneuroendocrinology. 2017;80:99–103. 10.1016/j.psyneuen.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 79.Maartje L, Marise WJM, Dick JV, Robert H, Lieuwe de H, Ingmar HAF. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci. 2014;39:149–69. 10.1503/jpn.130052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rushworth MatthewFS, Noonan MaryAnnP, Boorman ErieD, Walton MarkE, Behrens TimothyE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–69. 10.1016/j.neuron.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 81.Schulte MHJ, Cousijn J, den Uyl TE, Goudriaan AE, van den Brink W, Veltman DJ, et al. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev. 2014;34:531–50. 10.1016/j.cpr.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 82.Guerra D, Solé A, Camí J, Tobeña A. Neuropsychological performance in opiate addicts after rapid detoxification. Drug Alcohol Depend. 1987;20:261–70. 10.1016/0376-8716(87)90036-6 [DOI] [PubMed] [Google Scholar]
- 83.Kaur G, Sidana A, Singh S, Gupta A. Effects of abstinence from opioid on neuropsychological performance in men with opioid use disorder: a longitudinal study. J Addict Med. 2023;17:557–62. 10.1097/ADM.0000000000001177 [DOI] [PubMed] [Google Scholar]
- 84.Wollman SC, Hauson AO, Hall MG, Connors EJ, Allen KE, Stern MJ, et al. Neuropsychological functioning in opioid use disorder: a research synthesis and meta-analysis. Am J Drug Alcohol Abuse. 2019;45:11–25. 10.1080/00952990.2018.1517262 [DOI] [PubMed] [Google Scholar]
- 85.Garland EL. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Ann N Y Acad Sci. 2016;1373:25–37. 10.1111/nyas.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verdejo-Garcia A. Cognitive training for substance use disorders: neuroscientific mechanisms. Neurosci Biobehav Rev. 2016;68:270–81. 10.1016/j.neubiorev.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 87.Valls-Serrano C, Caracuel A, Verdejo-Garcia A. Goal Management Training and Mindfulness Meditation improve executive functions and transfer to ecological tasks of daily life in polysubstance users enrolled in therapeutic community treatment. Drug Alcohol Depend. 2016;165:9–14. 10.1016/j.drugalcdep.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 88.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. 10.1111/j.1360-0443.2009.02791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scott TM, Rivera Mindt M, Cunningham CO, Arias F, Coulehan K, Mangalonzo A, et al. Neuropsychological function is improved among opioid dependent adults who adhere to opiate agonist treatment with buprenorphine-naloxone: a preliminary study. Subst Abuse Treat Prev Policy. 2017;12:48. 10.1186/s13011-017-0133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kosten T, Aharonovich E, Nangia N, Zavod A, Akerman SC, Lopez-Bresnahan M, et al. Cognitive performance of patients with opioid use disorder transitioned to extended-release injectable naltrexone from buprenorphine: post hoc analysis of exploratory results of a phase 3 randomized controlled trial. Addict Behav. 2020;111:106538. 10.1016/j.addbeh.2020.106538 [DOI] [PubMed] [Google Scholar]
- 91.Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, et al. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat Neurosci. 2010;13:920–22. 10.1038/nn.2572 [DOI] [PubMed] [Google Scholar]
- 92.Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–91. 10.1016/j.neubiorev.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ansell BRE, Dwyer DB, Wood SJ, Bora E, Brewer WJ, Proffitt TM, et al. Divergent effects of first-generation and second-generation antipsychotics on cortical thickness in first-episode psychosis. Psychol Med. 2015;45:515–27. 10.1017/S0033291714001652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res Rev. 1988;13:179–212. 10.1016/0165-0173(88)90020-3 [DOI] [PubMed] [Google Scholar]
- 95.MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–80. 10.1016/j.tins.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 96.Hansen JY, Shafiei G, Markello RD, Smart K, Cox SML, Nørgaard M, et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat Neurosci. 2022;25:1569–81. 10.1038/s41593-022-01186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–34. 10.1097/00001756-200011270-00046 [DOI] [PubMed] [Google Scholar]
- 98.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–72. 10.1016/S1053-8119(02)00057-5 [DOI] [PubMed] [Google Scholar]
- 99.McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–87. 10.1016/j.neuron.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 100.Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–26. 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- 101.Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN. Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology. 2016;41:628–37. 10.1038/npp.2015.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koban L, Wager TD, Kober H. A neuromarker for drug and food craving distinguishes drug users from non-users. Nat Neurosci. 2023;26:316–25. 10.1038/s41593-022-01228-w [DOI] [PubMed] [Google Scholar]
- 103.Sullivan MA, Bisaga A, Mariani JJ, Glass A, Levin FR, Comer SD, et al. Naltrexone treatment for opioid dependence: does its effectiveness depend on testing the blockade? Drug Alcohol Depend. 2013;133:80–5. 10.1016/j.drugalcdep.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hampton AN, O’Doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. Proc Natl Acad Sci USA 2007;104:1377–82. 10.1073/pnas.0606297104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30:829–58. 10.1002/hbm.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Volkow ND. The epidemic of fentanyl misuse and overdoses: challenges and strategies. World Psychiatry. 2021;20:195–96. 10.1002/wps.20846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ching JH, Owens DK, Trafton JA, Goldhaber-Fiebert JD, Salomon JA. Impact of treatment duration on mortality among Veterans with opioid use disorder in the United States Veterans Health Administration. Addiction. 2021;116:3494–503. 10.1111/add.15574 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cortical thickness change map is available at NeuroVault (http://neurovault.org/collections/15137). The [¹¹C]carfentanil PET map is available at NeuroVault (http://neurovault.org/collections/5706) [38]. The HCP-MMP atlas is available at NeuroVault (https://neurovault.org/collections/1549) [49]. Clinical data that support the findings of this study are available from the corresponding author upon reasonable request.


