Key summary points
Aim
This study aimed to (1) identify which chronic health conditions may cause change in oncologic-decision-making and care in older patients and (2) provide guidance on how to incorporate these in decision-making and care provision of older patients with cancer.
Findings
Thirty-four relevant health conditions were identified and subsequently combined in five profiles, consisting of conditions with similarities regarding involvement of healthcare professionals, consequences for oncologic treatment decisions, or the care trajectory. Furthermore, seven reasons related to decision-making and support or care were identified for why the presence of these profiles would influence oncologic decision-making and/or the subsequent care trajectory.
Message
Assessing a patient’s health condition in light of these profiles and reasons, could aid clinicians in the management of older patients with multimorbidity, including cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41999-023-00919-2.
Keywords: Multimorbidity, Chronic health conditions, Older patients, Decision-making, Geriatric oncology
Abstract
Purpose
A substantial proportion of patients with cancer are older and experience multimorbidity. As the population is ageing, the management of older patients with multimorbidity including cancer will represent a significant challenge to current clinical practice.
Methods
This study aimed to (1) identify which chronic health conditions may cause change in oncologic decision-making and care in older patients and (2) provide guidance on how to incorporate these in decision-making and care provision of older patients with cancer. Based on a scoping literature review, an initial list of prevalent morbidities was developed. A subsequent survey among healthcare providers involved in the care for older patients with cancer assessed which chronic health conditions were relevant and why.
Results
A list of 53 chronic health conditions was developed, of which 34 were considered likely or very likely to influence decision-making or care according to the 39 healthcare professionals who responded. These conditions were further categorized into five patient profiles. From these conditions, five patient profiles were developed, namely, (1) a somatic profile consisting of cardiovascular, metabolic, and pulmonary disease, (2) a functional profile, including conditions that cause disability, dependency or a high caregiver burden, (3) a psychosocial profile, including cognitive impairment, (4) a nutritional profile also including digestive system diseases, and finally, (5) a concurrent cancer profile. All profiles were considered likely to impact decision-making with differences between treatment modalities. The impact on the care trajectory was generally considered less significant, except for patients with care dependency and psychosocial health problems.
Conclusions
Chronic health conditions have various ways of influencing oncologic decision-making and the care trajectory in older adults with cancer. Understanding why specific chronic health conditions may impact the oncologic care trajectory can aid clinicians in the management of older patients with multimorbidity, including cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41999-023-00919-2.
Introduction
As the population is ageing, an increasing proportion of patients with cancer are old. By 2040, 77% of patients with cancer will be older than 65 [1]. Older patients have a high prevalence of multimorbidity [2–5]. In the future, the management of older patients with multimorbidity including cancer will become a core task for cancer specialists [6]. These patients require a different approach and adaptation of current oncologic care.
Over the past decades, geriatric assessment (GA) has become the gold standard to assess older patients with cancer [7]. In a GA, multiple domains of an older patient’s health status, such as the somatic, psychological, functional and social domain, are systematically evaluated. So far, geriatric oncology research has given little attention towards the incorporation of multimorbidity into oncologic care.
In the early days of geriatric oncology, the results of the GA were simplified and used to develop fit-vulnerable-frail algorithms that decided whether patients could receive standard, adapted or no oncologic treatment. However, such simple categorizations cannot replace an overall assessment interpreted within the context of the patients and the disease [8, 9]. Tailoring oncologic care to older oncologic patients can be challenging. Geriatricians, who will all be familiar with GA and multimorbidity, may lack knowledge on how to translate its outcome to an oncologic treatment decision. Whereas, cancer specialists are often not familiar with GA and multimorbidity. Further guidance on how to incorporate chronic health conditions—both as toxicity/complication risk modifiers and as competing causes of death—in oncologic decision-making, and on how adjust care accordingly may aid these clinicians.
Previous research on multimorbidity in oncology has mainly focussed on summarizing comorbidity indexes for older patients, such as cumulative illness rating scale-geriatric (CIRS-G) and Charlson comorbidity index (CCI) [10, 11]. However, these instruments lack context for the individual and do not guide clinicians on how to incorporate chronic health conditions into oncologic care [12]. Other research addressed multimorbidity using latent class analyses to find frequently co-occurring disease-clusters [13, 14], or by clustering illness based on their impact on prognosis or quality of life domains [4, 13–15]. None of these studies were designed to cluster patients based on similar care needs or similar decision-making challenges. Thus, although the relevance of chronic health conditions in oncologic care is widely acknowledged, guidance on how to incorporate into the patient’s care pathway is lacking.
Multiple definitions of multimorbidity exist. In this study, we will define it as the co-occurrence of multiple health conditions without one holding priority [3]. Moreover, relevant geriatric impairments were included as health conditions, because comorbidities mostly cover the somatic dimension, whereas older patients may also have relevant deficits in social, psychological and functional domains [16–18]. Since we are speaking of both (comorbidities and deficits), the term “chronic health conditions” was used throughout the paper.
In this study, we gathered input from literature and geriatric oncology and geriatric experts on what chronic health conditions they consider relevant and how they use information on chronic conditions in oncologic decision-making and the subsequent care trajectory.
Methods
This study was part of the “Streamlined Geriatric and Oncological evaluation based on IC Technology” (GERONTE) study (geronteproject.eu). This project aims to develop and test a new patient-centred, holistic care pathway for older patients with breast, colorectal, lung, or prostate cancer as well as other significant chronic health conditions.
The study protocol was reviewed by the ethics committee (Medical Research Ethics Committees United) and performed in accordance with the declaration of Helsinki.
Developing the patient profiles
First, a scoping literature search was performed to identify papers that investigated the most common health problems or examined comorbidity clusters in patients with cancer (results see WebAppendix I). No specific data for older patients were found. From these studies and clinical judgement, a list of prevalent chronic health conditions in older patients was made by geriatricians MH, SR and SOH. Conditions were listed broadly, without strict definitions or subclassifications.
The next step consisted of two rounds of online surveys with an expert panel, consisting of European healthcare professionals involved in oncologic care for older patients. They were approached using purposive sampling. The following respondent data were collected: age, gender, profession, years in clinical practice, and the treatment types and cancer types they were involved in.
In the first online survey round, experts were asked to score each chronic health condition on a four-point Likert scale regarding how likely it was that (a history of) that condition would (1) change their oncologic treatment decision and (2) change the patients’ care and support needs (care trajectory). Respondents were also asked for any relevant chronic conditions that were missing from the initial list. Health conditions were carried forward to the next round if 50% of the participants scored them with likely or very likely for either one of the questions (treatment decision or care trajectory) or if both questions were answered with likely or very likely by at least 30% of the participants.
The aim of the second survey round was to further assess how these chronic health conditions could be incorporated into oncologic care. As it was considered unfeasible to ask this separately for all conditions carried forward, patient profiles were made.
The initial scoping literature review had not yielded any patient profiles that grouped conditions based on their relevance for oncology (Webappendix I). Thus, new patient profiles were designed. Conditions with similar involvement of non-oncologic healthcare professionals, or similar consequences for oncologic treatment decisions or care were grouped together by one author (MH) and subsequently refined, first by co-authors SR, SOH and NS, and afterwards based on feedback from a geriatrician, radiation oncologist, pulmonologist, surgeon and several medical oncologists.
Relevance of patient profiles
Five patient profiles were presented to the expert panel in the second survey round; experts were asked if they agreed with the profiles or if there were relevant conditions missing and why. In addition, experts were asked to rate and describe the relevance of each patient profile for oncologic decision-making and care for the following treatment modalities: surgery, systemic therapy (including chemotherapy/targeted therapy/immunotherapy), radiation therapy, endocrine therapy and other therapy. Scores ranged from 1 (not relevant) to 4 (very relevant), and in an additional open answer field, respondents could present details from their answers.
Answers were coded using inductive coding by MH. The first round of coding consisted of open coding, and in the consecutive rounds focused coding was used. Codes were checked by NS and refined and changed as needed. Only descriptive data were used.
Finally, participants were asked which healthcare professionals should be involved in the oncologic care trajectory for each patient profile. They could choose from a list of 15 potential participants and choose if these participants should be involved for all patients, only in specific situations/profiles, or did not need to be involved.
Results
Identifying relevant chronic health conditions
In total, 53 prevalent chronic health conditions for older patients with cancer were elicited from the literature (Fig. 1a). These conditions were used in the two online expert panel survey rounds (April and May 2021). The expert panel was constructed by inviting health care professionals with expertise in geriatrics and/or oncology by email for participation. Out of 87 invited healthcare professionals, 39 agreed to participate, with a range of different specialties and backgrounds (Table 1).
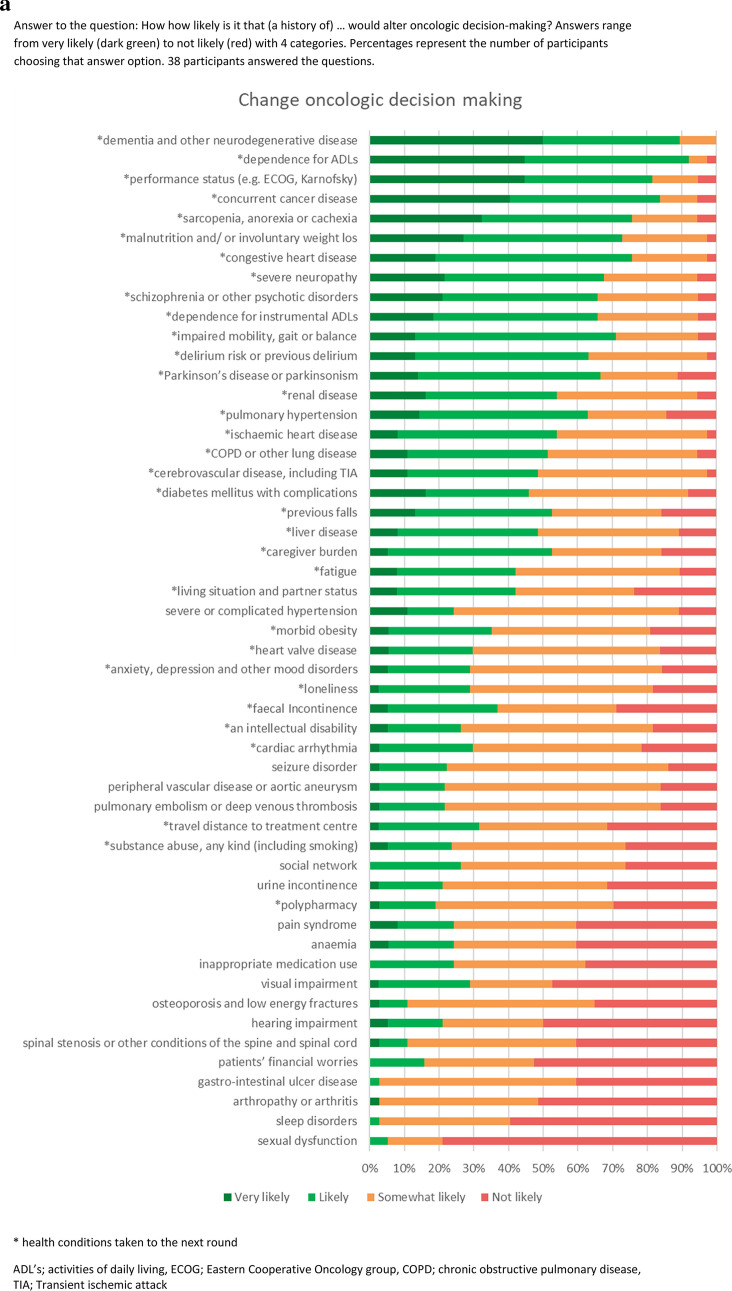
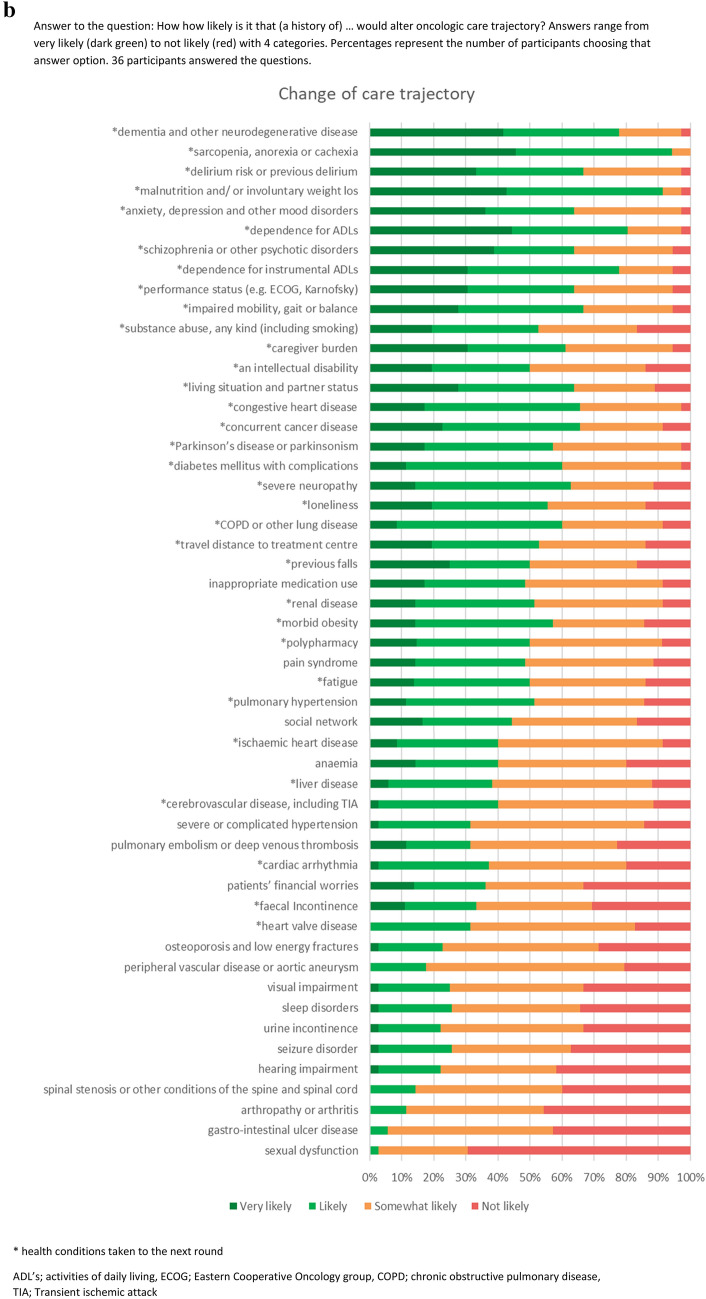
Fig. 1.
(a) Health conditions-likelihood to change oncologic decision-making. Answer to the question: How how likely is it that (a history of) … would alter oncologic decision-making? Answers range from very likely (dark green) to not likely (red) with 4 categories. Percentages represent the number of participants choosing that answer option. 38 participants answered the questions. (b) Health conditions—likelihood to change oncologic care trajectory. Answer to the question: How how likely is it that (a history of) … would alter oncologic care trajectory? Answers range from very likely (dark green) to not likely (red) with 4 categories. Percentages represent the number of participants choosing that answer option. 36 participants answered the questions. *Health conditions taken to the next round. ADL’s activities of daily living, ECOG eastern cooperative oncology group, COPD chronic obstructive pulmonary disease, TIA transient ischemic attack
Table 1.
Demographics of the expert panel (n = 39)
| Healthcare professionals (years) | |
| Mean age | 47 |
| Mean Years in clinical practice | 17.1 |
| Profession (%) | |
| Nurse | 10 |
| Physician | 85 |
| Researcher in Geriatric Oncology (without clinical activities) | 8 |
| Specialty (for physicians) (%) | |
| Medical oncology | 30 |
| Geriatrics | 23 |
| Other specialty … | 23 |
| Surgery | 21 |
| Organ-based specialist | 10 |
| Primary care | 8 |
| Cancer type involved with (physicians and nurses)a (%) | |
| Colorectal cancer | 33 |
| All cancer types | 31 |
| Breast cancer | 23 |
| Prostate cancer | 21 |
| Lung cancer | 18 |
| Which treatments do you provide to patients yourself (physicians and nurses)?a (%) | |
| Chemotherapy | 36 |
| Targeted and/or immune therapy | 36 |
| Hormone therapy | 36 |
| Surgery | 31 |
| None | 23 |
| Other | 18 |
| Radiation therapy | 13 |
aMultiple answers per participant possible
During the first round, 18 health conditions were identified as likely or very likely to change both oncologic treatment decision-making and care trajectory, ten to only change the care trajectory, and one would only change decision-making. Five conditions did not reach >50% likely/very likely; but did reach 30–50% for both decision-making and care trajectory, and were thus carried forward (Fig. 1a, b). Based on these results, 34 health conditions were considered important to incorporate. Details can be found in Appendix 1. With regards to conditions that were considered to be missing from the initial list, only auto-immune disease was mentioned by more than one respondent.
Developing patient profiles
Five profiles were created based on the 34 included items carried forward from Round 1, namely:
Profile 1 Cardiovascular, metabolic and pulmonary disease—(somatic)
Profile 2 Disability, dependency and caregiver burden—(functional)
Profile 3 Psychosocial health problems and cognitive impairment—(psychosocial)
Profile 4 Nutritional status and digestive system disease—(nutritional)
Profile 5 Concurrent cancer (treatment)—(concurrent cancer)
These profiles were subsequently presented to the expert panel in the second survey round, in which 37 respondents participated. Thirty participants fully agreed with these profiles (81%). Four did not completely agree and three proposed suggestions for improvement, but since the majority agreed it was decided to keep the profiles as they were (details in Webappendix 2).
Relevance of patient profiles
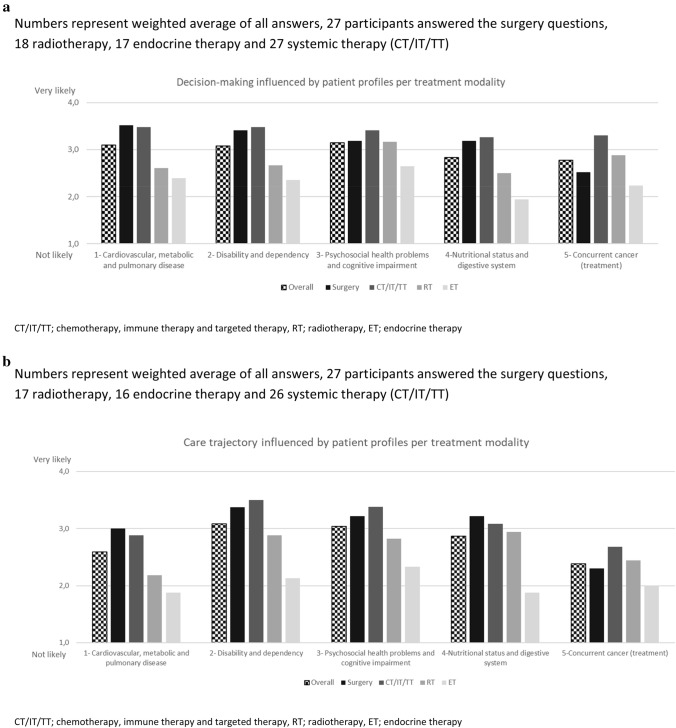
All five profiles had a mean relevance score of 2.8 or higher out of 4 for oncologic decision-making (Fig. 2a). In particular, the somatic, functional and psychosocial profiles were considered most likely to influence oncologic decision-making. The relevance of the patient profiles for the oncologic care trajectory was scored lower than for decision-making. Nonetheless, the functional and psychosocial profiles still received an overall score above 3, and were thus considered likely to impact the care trajectory (Fig. 2b). When assessing specific treatment modalities, decision-making and care for surgery, chemotherapy, immunotherapy and targeted therapy were more likely to be affected by chronic health conditions than radiotherapy or endocrine treatment.
Fig. 2.
(a) Likelihood of the five profiles to change decision-making per treatment modality. Numbers represent weighted average of all answers, 27 participants answered the surgery questions, 18 radiotherapy, 17 endocrine therapy and 27 systemic therapy (CT/IT/TT). (b) Likelihood of the five profiles to change care trajectory per treatment modality. Numbers represent weighted average of all answers, 27 participants answered the surgery questions, 17 radiotherapy, 16 endocrine therapy and 26 systemic therapy (CT/IT/TT). CT/IT/TT chemotherapy, immune therapy and targeted therapy, RT radiotherapy, ET endocrine therapy
Thirty respondents answered open-ended questions regarding why patient profiles would impact treatment decision-making or the care trajectory. Seven overarching reasons were formulated based on inductive coding (Table 2).
Table 2.
Reasons why patient profiles are relevant for oncologic decision-making and oncologic care trajectory
| Reasons | 1. Somatic profile (%) | 2. Functional profile (%) | 3. Psychosocial profile (%) | 4. Nutritional profile (%) | 5. Concurrent cancer profile (%) | |
|---|---|---|---|---|---|---|
| Decision-making | Determine the feasibility of treatment and the risk of complications and toxicity | 65 | 31 | 37 | 52 | 18 |
| Causes Interactions between cancer (treatment) and comorbidity | 24 | 16 | 16 | 13 | 47 | |
| Estimate resilience, impact on functional and cognitive outcomes or on quality of life | 10 | 34 | 15 | 19 | 0 | |
| Predict prognosis, competing causes of death | 6 | 3 | 2 | 2 | 29 | |
| Care trajectory | May lead to pre-treatment optimization and need for post-treatment rehabilitation or additional discharge care | 31 | 31 | 29 | 44 | 6 |
| Tailor support and care during treatment | 26 | 40 | 52 | 19 | 5 | |
| Decision-making and care trajectory | Impact on decision-making capacity and lower health literacy and compliance | 0 | 16 | 55 | 5 | 2 |
The italic values represent the highest values
Percentages represent participants mentioning this reason after asking why a certain profile would alter decision-making or care trajectory. Categories were made after all participants had answered the open-ended questions and were not shown to participants. Bold values represent higher percentages
Irrespective of the patient profile, three types of professionals were considered mandatory for oncologic decision-making in patients with multiple chronic health conditions: cancer specialist(s), the geriatrician and the general practitioner. Over half of respondents felt that organ-specific physicians (56%) and an oncology nurse (54%) should be involved in decision-making as well. For the care trajectory, the same specialties were identified to be important. The involvement of other (para)medical specialists should be tailored to the patient (Webappendix III).
Discussion
This study was designed to provide an overview of chronic health conditions that may alter oncologic decision-making or care, and why. Thirty-four relevant health conditions were identified that were likely to alter oncologic decision-making an care. They were subsequently combined in five profiles, consisting of conditions with similarities regarding involvement of healthcare professionals, consequences for oncologic treatment decisions, or the care trajectory. Furthermore, seven reasons related to decision-making and support or care were identified for why the presence of these profiles would influence oncologic decision-making and/or the subsequent care trajectory. By assessing a patient’s health condition in light of these profiles and reasons, it is possible to develop a tailored, patient-centred treatment plan.
In this study, geriatric syndromes, deficits, and symptoms in other domains than the somatic domain were included as chronic health conditions in addition to more traditional diseases [18]. This was done, because these conditions may also alter the overall benefit–risk ratio of treatment and may thus lead to a different treatment decision. For example, because they are associated with increased treatment risk such as dependency for ADLs and the unavailability of someone to take them to the doctor [19]. Moreover, in case of similar benefits of treatment, a patient who lives far away may chose the treatment that requires the least hospital visits. Traditional diseases are important to take into account, but also other conditions may be determinative in the treatment choice.
The most frequently mentioned reason to alter decision-making was the effect of a chronic health condition on the feasibility of treatment and complication risks. To determine whether impact on feasibility is expected from conditions and whether this may impact treatment decision or care provision, a clinician may discuss these questions with the medical team within the context of the patients, including all treatment options and their effect on various outcomes, such as survival, functional outcomes and quality of life. The answer to this question may shift the balance between risk and benefit and could thus alter the treatment recommendation. Of note, identified deficits should not automatically lead to decline of treatment, as some conditions may benefit from optimization or additional support after which patients are still able to undergo oncologic treatment [20].
Examples of the above-mentioned are pre-treatment optimization, post-treatment rehabilitation, additional discharge care or additional support during treatment. For example, prior to surgery, physical prehabilitation may be an option to improve functional outcomes [7, 20–23]. For psychosocial health problems, the impact on decision-making capacity, self-management capacity, and compliance should be considered [21–23] and caregiver support and increased monitoring may be needed [22, 23]. Previous research can be used to identify supportive interventions to optimize the patients’ overall health to improve feasibility of treatment in vulnerable patients and thus make treatment more accessible for these patients [22, 23]. These interventions should be part of the overall oncologic treatment.
Thus, the developed profiles may help to guide the questions in relation to the chronic health conditions that are identified from the patient’s medical history or geriatric assessment. Nonetheless, these profiles cannot be simplified to easy yes-or-no/one-size-fits-all algorithms, since older patients with multimorbidity including cancer are a heterogeneous group. Moreover, the impact will not merely depend on the presence of a condition, but also on its severity, impact on daily life, and on the combination of chronic conditions in the same patient [24]. Combining conditions into profiles may also cause a risk of concealing interactions in a more broad way, for example, between other diseases and treatments that are not within one profile [25]. Therefore, the profiles should not be used to dictate treatment or care but rather as suggestions of questions that need to be elucidated before making treatment decisions.
This study has some limitations. First, due to the pragmatic method to identify potentially relevant and prevalent chronic health conditions in older patients with cancer, some conditions may have been missed. The expert panel found only one condition to be missing, and the conditions that were ultimately selected by our expert panel are similar to the selections made in studies operationalizing multimorbidity [24, 26]. Second, this study tried to identify relevant chronic health conditions for a heterogeneous group of patients, including four cancer types with all stages and different treatment modalities. Some nuances may have been lost by not considering all the details of the cancer type, stage of disease, or treatment regimens. However, with an ever-evolving scope of treatment options, such detailed guidance may be difficult to provide and could soon become obsolete. Therefore, we chose to provide guidance based on a broad classification that can be used in a wide range of situations and thus will be generalizable and remain useful in the care for the heterogenous older population. Finally, this study was not designed to look at exact percentages or differences between profiles, but to identify how chronic health conditions could impact oncologic decision-making or care. Caution is thus needed when interpreting the reported percentages. Low percentages do not necessarily mean that these reasons are less important, but only mean that the answer was given less frequently. Participants were not given the opportunity to check if reasons mentioned by others were equally or even more important to them.
Previous studies have shown that performing assessments without interventions will have little impact on clinical outcomes [6]. Therefore, this study aimed to assist clinicians in utilizing the information about chronic health conditions from medical history and geriatric assessment in oncologic care. Questions relating to the reasons mentioned in Table 2 are complex and require expertise and collaboration from various disciplines. Ideally, a multidisciplinary team discussion takes place to discuss how chronic health conditions should be incorporated into the oncologic care pathway.
In conclusion, chronic health conditions have various ways of influencing oncologic decision-making and may impact oncologic care differently throughout the care trajectory. Knowing which conditions may impact the oncologic care trajectory at what stages by asking the right questions may improve the management of older patients with cancer. Nevertheless, treatment recommendations and subsequent management of this complex population require a patient-centred and multidisciplinary approach.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix 1: Likelihood of health conditions to change treatment decision-making or care trajectory
Percentages represent the proportion of participants stating that the health condition would likely or very likely change the treatment decision or the care trajectory. Dark colour represents the 34 conditions that were forwarded to the next round.
| Treatment decision (%) | Care trajectory (%) | |
|---|---|---|
| … dependence for ADLs | 92 | 81 |
| … dementia and other neurodegenerative disease | 89 | 78 |
| … concurrent cancer disease | 84 | 66 |
| … performance status (e.g. ECOG, Karnofsky) | 82 | 64 |
| … congestive heart disease | 76 | 66 |
| … sarcopenia, anorexia or cachexia | 76 | 94 |
| … malnutrition and/or involuntary weight loss | 73 | 91 |
| … impaired mobility, gait or balance | 71 | 67 |
| … severe neuropathy | 68 | 63 |
| … Parkinson’s disease or parkinsonism | 67 | 57 |
| … schizophrenia or other psychotic disorders | 66 | 64 |
| … dependence for instrumental ADLs | 66 | 78 |
| … delirium risk or previous delirium | 63 | 67 |
| … pulmonary hypertension | 63 | 51 |
| … ischaemic heart disease | 54 | 40 |
| … renal disease | 54 | 51 |
| … previous falls | 53 | 50 |
| … caregiver burden | 53 | 61 |
| … COPD or other lung disease | 51 | 60 |
| … cerebrovascular disease, including TIA | 49 | 40 |
| … liver disease | 49 | 38 |
| … diabetes mellitus with complications | 46 | 60 |
| … fatigue | 42 | 50 |
| … living situation and partner status | 42 | 64 |
| … faecal Incontinence | 37 | 33 |
| … morbid obesity | 35 | 57 |
| … travel distance to treatment centre | 32 | 53 |
| … cardiac arrhythmia | 30 | 37 |
| … heart valve disease | 30 | 31 |
| … anxiety, depression and other mood disorders | 29 | 64 |
| … visual impairment | 29 | 25 |
| … loneliness | 29 | 56 |
| … an intellectual disability | 26 | 50 |
| … social network | 26 | 44 |
| … severe or complicated hypertension | 24 | 31 |
| … pain syndrome | 24 | 49 |
| … anaemia | 24 | 40 |
| … inappropriate medication use | 24 | 49 |
| … substance abuse, any kind (including smoking) | 24 | 53 |
| … seizure disorder | 22 | 26 |
| … pulmonary embolism or deep venous thrombosis | 22 | 31 |
| … peripheral vascular disease or aortic aneurysm | 22 | 18 |
| … hearing impairment | 21 | 22 |
| … urine incontinence | 21 | 22 |
| … polypharmacy | 19 | 50 |
| … patients’ financial worries | 16 | 36 |
| … spinal stenosis or other conditions of the spine and spinal cord | 11 | 14 |
| … osteoporosis and low energy fractures | 11 | 23 |
| … sexual dysfunction | 5 | 3 |
| … gastro-intestinal ulcer disease | 3 | 6 |
| … arthropathy or arthritis | 3 | 11 |
| … sleep disorders | 3 | 26 |
Appendix 2: Reasons why patient profiles are relevant for decision-making and care trajectory per patient profile
Percentages represent participants mentioning this reason. Darker colours represent higher percentages. Details on the 5 profiles are shown in Webappendix II.
| Determine the feasibility of treatment and the risk of complications and toxicity (%) | Predict prognosis, competing causes of death (%) | Causes Interactions between cancer (treatment) and comorbidity (%) | Estimate resilience, impact on functional and cognitive outcomes or on quality of life of life (%) | Impact on decision-making capacity and lower health literacy and compliance (%) | May lead to pre-treatment optimization and need for post-treatment rehabilitation or additional discharge care (%) | Tailor support and care during treatment (%) | |
|---|---|---|---|---|---|---|---|
| PROFILE 1 Cardiovascular, metabolic and pulmonary disease—(somatic) | |||||||
| Surgery | 91 | 14 | 0 | 23 | 0 | 55 | 45 |
| Chemotherapy | 71 | 0 | 39 | 4 | 0 | 17 | 22 |
| Radiotherapy | 50 | 9 | 27 | 0 | 0 | 18 | 0 |
| Endocrine therapy | 33 | 0 | 33 | 0 | 0 | 11 | 11 |
| PROFILE 2 Disability, dependency and caregiver burden—(functional) | |||||||
| Surgery | 27 | 9 | 0 | 64 | 0 | 50 | 41 |
| Chemotherapy | 47 | 0 | 39 | 32 | 0 | 17 | 32 |
| Radiotherapy | 42 | 0 | 17 | 0 | 25 | 17 | 50 |
| Endocrine therapy | 0 | 0 | 25 | 0 | 50 | 0 | 38 |
| PROFILE 3 Psychosocial health problems and cognitive impairment—(psychosocial) | |||||||
| Surgery | 59 | 5 | 0 | 36 | 36 | 32 | 64 |
| Chemotherapy | 28 | 0 | 20 | 0 | 70 | 25 | 50 |
| Radiotherapy | 42 | 0 | 21 | 7 | 57 | 21 | 21 |
| Endocrine therapy | 0 | 0 | 27 | 0 | 36 | 27 | 45 |
| PROFILE 4 Nutritional status and digestive system disease—(nutritional) | |||||||
| Chemotherapy | 76 | 0 | 18 | 0 | 6 | 47 | 18 |
| Radiotherapy | 42 | 0 | 23 | 8 | 0 | 23 | 31 |
| Endocrine therapy | 13 | 0 | 22 | 0 | 22 | 11 | 22 |
| PROFILE 5 Concurrent cancer (treatment)—(concurrent cancer) | |||||||
| Surgery | 19 | 62 | 52 | 0 | 0 | 10 | 5 |
| Chemotherapy | 50 | 0 | 0 | 0 | 0 | 0 | 50 |
| Radiotherapy | 33 | 13 | 50 | 0 | 0 | 13 | 0 |
| Endocrine therapy | 33 | 33 | 0 | 0 | 0 | 0 | 0 |
Appendix 3: Reasons why patient profiles are relevant for decision-making and care trajectory per treatment modality
Percentages represent participants mentioning this reason. Darker colours are higher percentages.
| Determine the feasibility of treatment and the risk of complications and toxicity (%) | Predict prognosis, competing causes of death (%) | Causes Interactions between cancer (treatment) and comorbidity (%) | Estimate resilience, impact on functional and cognitive outcomes or on quality of life of life (%) | Impact on decision-making capacity and lower health literacy and compliance (%) | May lead to pre-treatment optimization and need for post-treatment rehabilitation or additional discharge care (%) | Tailor support and care during treatment (%) | |
|---|---|---|---|---|---|---|---|
| Surgery | |||||||
| Profile 1 | 91 | 14 | 0 | 23 | 0 | 55 | 45 |
| Profile 2 | 27 | 9 | 0 | 64 | 0 | 50 | 41 |
| Profile 3 | 59 | 5 | 0 | 36 | 36 | 32 | 64 |
| Profile 4 | 59 | 5 | 0 | 50 | 0 | 68 | 14 |
| Profile 5 | 19 | 62 | 52 | 0 | 0 | 10 | 5 |
| Chemo | |||||||
| Profile 1 | 71 | 0 | 39 | 4 | 0 | 17 | 22 |
| Profile 2 | 47 | 0 | 27 | 32 | 14 | 27 | 32 |
| Profile 3 | 28 | 0 | 20 | 0 | 70 | 25 | 50 |
| Profile 4 | 76 | 0 | 18 | 0 | 6 | 47 | 18 |
| Profile 5 | 19 | 29 | 93 | 0 | 7 | 7 | 14 |
| RT | |||||||
| Profile 1 | 50 | 9 | 27 | 0 | 0 | 18 | 0 |
| Profile 2 | 42 | 0 | 17 | 0 | 25 | 17 | 50 |
| Profile 3 | 42 | 0 | 21 | 7 | 57 | 21 | 21 |
| Profile 4 | 42 | 0 | 23 | 8 | 0 | 23 | 31 |
| Profile 5 | 33 | 13 | 50 | 0 | 0 | 13 | 0 |
| ET | |||||||
| Profile 1 | 33 | 0 | 33 | 0 | 0 | 11 | 11 |
| Profile 2 | 0 | 0 | 25 | 0 | 50 | 0 | 38 |
| Profile 3 | 0 | 0 | 27 | 0 | 36 | 27 | 45 |
| Profile 4 | 13 | 0 | 22 | 0 | 22 | 11 | 22 |
| Profile 5 | 0 | 0 | 20 | 0 | 0 | 0 | 0 |
Profile 1 Cardiovascular, metabolic and pulmonary disease—(Somatic)
Profile 2 Disability, dependency and caregiver burden—(functional)
Profile 3 Psychosocial health problems and cognitive impairment—(psychosocial)
Profile 4 Nutritional status and digestive system disease—(nutritional)
Profile 5 Concurrent cancer (treatment)—(concurrent cancer)
Author contribution
Conception and design: MH. Data collection: NS. Analysis and interpretation of data: NS, MH, SR, SH. Manuscript writing: NS, MH. Approval of final article: All authors.
Funding
This research was funded by GERONTE. The GERONTE project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 945218.
Declarations
Conflict of interests
Pierre Soubeyran: Board member with TEVA, Sandoz, BMS and EISAI. All other authors: No competing interests to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All participants provided informed consent prior to their participation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. CEBP 25(7):1029–1036. 10.1158/1055-9965.EPI-16-0133 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams GR, Mackenzie A, Magnuson A, Olin R, Chapman A, Mohile S, Allore H, Somerfield MR, Targia V, Extermann M et al (2016) Comorbidity in older adults with cancer. J Geriatr Oncol 7(4):249–257. 10.1016/j.jgo.2015.12.002 10.1016/j.jgo.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skou ST, Mair FS, Fortin M, Guthrie B, Nunes BP, Miranda JJ, Boyd CM, Pati S, Mtenga S, Smith S, Multimorbidity M (2022) Nat Rev Dis Primers 8(1). 10.1038/s41572-022-00376-4 [DOI] [PMC free article] [PubMed]
- 4.Sarfati D, Gurney J, Lim BT, Bagheri N, Simpson A, Koea J, Dennett E (2016) Identifying important comorbidity among cancer populations using administrative data: prevalence and impact on survival. Asia Pac J Clin Oncol 12(1):e47–e56. 10.1111/ajco.12130 10.1111/ajco.12130 [DOI] [PubMed] [Google Scholar]
- 5.Nicholson K, Makovski TT, Griffith LE, Raina P, Stranges S, van den Akker M (2019) Multimorbidity and comorbidity revisited: refining the concepts for international health research. J Clin Epidemiol 105:142–146. 10.1016/j.jclinepi.2018.09.008 10.1016/j.jclinepi.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Corbett T, Cummings A, Calman L, Farrington N, Fenerty V, Foster C, Richardson A, Wiseman T, Bridges J (2020) Self-management in older people living with cancer and multi-morbidity: a systematic review and synthesis of qualitative studies. Psycho-oncology 29(10):1452–1463. 10.1002/pon.5453 10.1002/pon.5453 [DOI] [PubMed] [Google Scholar]
- 7.Hamaker M, Lund C, te Molder M, Soubeyran P, Wildiers H, van Huis L, Rostoft S (2022) Geriatric assessment in the management of older patients with cancer—a systematic review (update). J Geriatr Oncol. 10.1016/j.jgo.2022.04.008 10.1016/j.jgo.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 8.Balducci L, Extermann M (2000) Management of cancer in the older person: a practical approach. Oncologist 5(3):224–237. 10.1634/theoncologist.5-3-224 10.1634/theoncologist.5-3-224 [DOI] [PubMed] [Google Scholar]
- 9.Hamaker ME, Jonker JM (2012) Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 13:437–481 10.1016/S1470-2045(12)70259-0 [DOI] [PubMed] [Google Scholar]
- 10.Extermann M, Lee H (2000) Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol 2000(35):181–200 10.1016/S1040-8428(00)00090-1 [DOI] [PubMed] [Google Scholar]
- 11.Sarfati D (2012) Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol 65(9):924–933. 10.1016/j.jclinepi.2012.02.017 10.1016/j.jclinepi.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 12.Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV (2017) New horizons in multimorbidity in older adults. Age Ageing 46(6):882–888. 10.1093/ageing/afx150 10.1093/ageing/afx150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn EE, Gould MK, Munoz-Plaza CE, Lee JS, Parry C, Shen E (2018) Understanding comorbidity profiles and their effect on treatment and survival in patients with colorectal cancer. J Natl Compr Canc Netw 16(1):23–34. 10.6004/jnccn.2017.7026 10.6004/jnccn.2017.7026 [DOI] [PubMed] [Google Scholar]
- 14.Gould MK, Munoz-Plaza CE, Hahn EE, Lee JS, Parry C, Shen E (2017) Comorbidity profiles and their effect on treatment selection and survival among patients with lung cancer. Ann Am Thorac Soc 14(10):1571–1580. 10.1513/AnnalsATS.201701-030OC 10.1513/AnnalsATS.201701-030OC [DOI] [PubMed] [Google Scholar]
- 15.Rijken M, Van Der Heide I (2019) Identifying subgroups of persons with multimorbidity based on their needs for care and support. BMC Fam Pract 20(1). 10.1186/s12875-019-1069-6. [DOI] [PMC free article] [PubMed]
- 16.Soto-Perez-De-Celis E, Li D, Yuan Y, Lau M, Hurria A (2018) Geriatric oncology 2, functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 19:e305–e316 10.1016/S1470-2045(18)30348-6 [DOI] [PubMed] [Google Scholar]
- 17.Wieland D, Boyd CM, Ritchie CS, Tipton EF, Studenski SA (2005) From bedside to bench: summary from the American geriatrics society/national institute on aging research conference on comorbidity and multiple morbidity in older adults* aging clinical and experimental research. Aging Clin Exp Res 20(3):181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd CM, Fortin M (2010) Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Rev 32(2):451–474 10.1007/BF03391611 [DOI] [Google Scholar]
- 19.Klepin HD, Sun C-L, Smith DD, Elias R, Trevino KM, Leak Bryant A, Li D, Nelson C, Tew WP, Mohile SG et al (2021) Predictors of unplanned hospitalizations among older adults receiving cancer chemotherapy. JCO Oncol Pract 17(6):e740–e752 10.1200/OP.20.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuMontier C, Loh KP, Soto-Perez-de-Celis E, Dale W (2021) Decision making in older adults with cancer. J Clin Oncol 39(19):2164–2174. 10.1200/jco.21.00165 10.1200/jco.21.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohile SG, Mohamed MR, Xu H, Culakova E, Loh KP, Magnuson A, Flannery MA, Obrecht S, Gilmore N, Ramsdale E et al (2021) Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. The Lancet 398(10314):1894–1904. 10.1016/S0140-6736(21)01789-X 10.1016/S0140-6736(21)01789-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Sun CL, Kim H, Soto-Perez-De-Celis E, Chung V, Koczywas M, Fakih M, Chao J, Cabrera Chien L, Charles K, et al (2021) Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol 7(11). 10.1001/jamaoncol.2021.4158 [DOI] [PMC free article] [PubMed]
- 23.Rostoft S, O’Hanlon S, Seghers PAL (Nelleke), Hamaker M (2022) D1.3: Protocol For intrinsic capacity evalution. Zenodo. 10.5281/zenodo.6782759
- 24.Ho ISS, Azcoaga-Lorenzo A, Akbari A, Davies J, Khunti K, Kadam UT, Lyons RA, McCowan C, Mercer SW, Nirantharakumar K et al (2022) Measuring multimorbidity in research: delphi consensus study. BMJ Med 1(1):e000247. 10.1136/bmjmed-2022-000247 10.1136/bmjmed-2022-000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M (2009) Defining comorbidity: implications for understanding health and health services. Ann Fam Med 7(4):357–363. 10.1370/afm.983 10.1370/afm.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho ISS, Azcoaga-Lorenzo A, Akbari A, Black C, Davies J, Hodgins P, Khunti K, Kadam U, Lyons RA, McCowan C et al (2021) Examining variation in the measurement of multimorbidity in research: a systematic review of 566 studies. Lancet Public Health 6(8):e587–e597. 10.1016/S2468-2667(21)00107-9 10.1016/S2468-2667(21)00107-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.