Abstract
Background
The COVID-19 pandemic raised concern amongst clinicians that disease-modifying therapies (DMT), particularly anti-CD20 monoclonal antibodies (mAb) and fingolimod, could worsen COVID-19 in people with multiple sclerosis (pwMS). This study aimed to examine DMT prescribing trends pre- and post-pandemic onset.
Methods
A multi-centre longitudinal study with 8,771 participants from MSBase was conducted. Two time periods were defined: pre-pandemic (March 11 2018–March 10 2020) and post-pandemic onset (March 11 2020–11 March 2022). The association between time and prescribing trends was analysed using multivariable mixed-effects logistic regression. DMT initiation refers to first initiation of any DMT, whilst DMT switches indicate changing regimen within 6 months of last use.
Results
Post-pandemic onset, there was a significant increase in DMT initiation/switching to natalizumab and cladribine [(Natalizumab-initiation: OR 1.72, 95% CI 1.39–2.13; switching: OR 1.66, 95% CI 1.40–1.98), (Cladribine-initiation: OR 1.43, 95% CI 1.09–1.87; switching: OR 1.67, 95% CI 1.41–1.98)]. Anti-CD20mAb initiation/switching decreased in the year of the pandemic, but recovered in the second year, such that overall odds increased slightly post-pandemic (initiation: OR 1.26, 95% CI 1.06–1.49; Switching: OR 1.15, 95% CI 1.02–1.29. Initiation/switching of fingolimod, interferon-beta, and alemtuzumab significantly decreased [(Fingolimod-initiation: OR 0.55, 95% CI 0.41–0.73; switching: OR 0.49, 95% CI 0.41–0.58), (Interferon-gamma-initiation: OR 0.48, 95% CI 0.41–0.57; switching: OR 0.78, 95% CI 0.62–0.99), (Alemtuzumab-initiation: OR 0.27, 95% CI 0.15–0.48; switching: OR 0.27, 95% CI 0.17–0.44)].
Conclusions
Post-pandemic onset, clinicians preferentially prescribed natalizumab and cladribine over anti-CD20 mAbs and fingolimod, likely to preserve efficacy but reduce perceived immunosuppressive risks. This could have implications for disease progression in pwMS. Our findings highlight the significance of equitable DMT access globally, and the importance of evidence-based decision-making in global health challenges.
Keywords: Multiple sclerosis, COVID-19, Disease-modifying therapy, Anti-CD20 monoclonal antibodies, Cladribine, Natalizumab
Background
The COVID-19 pandemic caused a multitude of unprecedented challenges in healthcare systems across the globe. Amongst the vulnerable populations affected by the COVID-19 pandemic were people with multiple sclerosis (pwMS). The overall COVID-19 mortality rate amongst patients with either suspected or confirmed MS was estimated to be around 3.0% [1].
In general, pwMS, especially those on disease-modifying therapies (DMT), are more susceptible to infectious diseases and are at a higher risk of infection-related hospitalisations compared to the general population [2]. Specifically for COVID-19, older age, African American ethnicity, and a higher level of disability all significantly increase the risk of experiencing severe infections amongst pwMS [1, 3, 4]. A crucial additional risk factor identified for severe COVID-19 infections in pwMS was the use of certain immunosuppressive DMTs. This posed a significant challenge in MS care for clinicians and led to various consensus agreements and recommendations being published [5–7]. General consensus suggested that lower efficacy DMTs such as interferons and glatiramer acetate were unlikely to increase the risk of severe COVID-19 infection and, potentially, that interferon DMTs may even be protective [8]. However, higher efficacy medications, particularly anti-CD20 monoclonal antibodies (such as ocrelizumab and rituximab) and S1P inhibitors (such as fingolimod), were considered to potentially increase the susceptibility to as well as the severity of COVID-19 for pwMS [9–12].
Current literature suggests that there was a shift in DMT prescribing patterns in pwMS during the COVID-19 pandemic. There was a significant reduction in the initiation of high-efficacy immunosuppressive DMTs, such as anti-CD20 monoclonal antibodies and S1P inhibitors [13–15]. Instead, there was an increased preference for lower efficacy, self-injectable DMTs such as interferon-beta and glatiramer acetate, which were perceived as safer options during the pandemic [13, 16]. Despite the overall reduction in high-efficacy DMT prescriptions, some clinicians continued or initiated these therapies with modifications, such as extended interval dosing, to reduce the risk of severe infections whilst maintaining disease control [17, 18].
These studies, however, were limited by sample size and country-based variation in practice, and the implications of these changes on disease activity in pwMS are yet to be fully elucidated [15]. In this study, we performed a longitudinal multi-centre study across over 25 countries using the MSBase Registry to evaluate prescription patterns of DMTs and to analyse the impact of the COVID-19 pandemic on the care of pwMS.
Methods
Participant selection and patient consent
We conducted a multi-centre, retrospective study using 8,771 participants from the MSBase Registry. All participants provided written informed consent to be a part of the study. Ethics approval for the MSBase registry was granted by the Alfred Health Human Research and Ethics Committee and the local ethics committees of all the participating centres that comprise the MSBase. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study participants
The inclusion criteria for this study were: (1) 18 years of age or over; (2) definite diagnosis of MS according to the McDonald Criteria [21]; and (3) at least one visit recorded in the pre-pandemic or post-pandemic period AND at least one visit after 11 March 2022. Patients with incomplete demographic (sex, age) or clinic (disease duration, the date of starting and/or stopping DMTs, Expanded Disability Status Scale, EDSS, assessments and dates of relapses for the duration of the study) were excluded.
Two time periods were defined as: (1) pre-pandemic (March 11 2018 to March 10 2020) and (2) post-pandemic (March 11 2020, when the COVID-19 pandemic was announced by World Health Organisation, to 11 March 2022) [18].
Outcomes and definitions
The primary outcome was to analyse the prescribing patterns of high- and low-efficacy DMTs pre- and post-pandemic onset. We classified initiation and switching to DMT as follows: DMT initiation referred to the first prescription of any DMT. DMT switching referred to change in DMT regimen within 6 months of last DMT use.
High-medium efficacy DMTs (called high-efficacy from here on) were defined as ocrelizumab, rituximab, ofatumumab, cladribine, alemtuzumab, natalizumab, and fingolimod. Low-efficacy DMTs were defined as interferon-beta/alpha, glatiramer acetate, teriflunomide and dimethyl fumarate (DMF). To reduce groups for comparison, interferon-beta/alpha and glatiramer acetate were grouped together as “BRACE”, and rituximab, ocrelizumab and ofatumumab were grouped as “Anti-CD20 mAbs”.
Statistical analysis
The demographic information and the baseline characteristics were reported as number and percentage for discrete variables and as mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous variables, as appropriate and according to the data distribution.
Using a pre-post design, we applied generalised linear mixed models with a binomial link function and a random effect for each country to assess associations between DMT initiation or switching (outcomes) as a function of DMT class across pandemic periods (exposures). In the models, the random effect was country of residence, whilst fixed covariates were age, gender, MS phenotype, disease duration, EDSS, and relapse count in the previous 24 months. All statistical tests were two-sided with a statistical significance defined as p ≤ 0.05. Analyses were performed in R version.4.3.0. (R Foundation for Statistical Computing).
Results
Participant demographics
8,771 participants were selected for this study. There were 4,533 initiations and 5899 switches recorded in 5165 unique individuals. Note that this discrepancy in sample numbers arises from instances where some participants may have had an initiation of DMT followed by a subsequent switch, thus contributing to both counts. Table 1 outlines the participant demographics for total participants and participants where initiations and switches were recorded.
Table 1.
Participant demographics
| Characteristic | Total n = 8771 (%) | Initiation n = 4533 (%) | Switching n = 5165 (%) |
|---|---|---|---|
| Gender | |||
| F | 6,222 (71%) | 3,150 (69%) | 3,747 (73%) |
| M | 2,549 (29%) | 1,383 (31%) | 1,418 (27%) |
| Age category (years) | |||
| 0–20 | 326 (3.7%) | 257 (5.7%) | 147 (2.8%) |
| 21–30 | 1,682 (19%) | 1,130 (25%) | 841 (16%) |
| 31–40 | 2,556 (29%) | 1,386 (31%) | 1,455 (28%) |
| 41–50 | 2,318 (26%) | 1,011 (22%) | 1,490 (29%) |
| 51–60 | 1,392 (16%) | 520 (11%) | 944 (18%) |
| > 60 | 497 (5.7%) | 229 (5.1%) | 288 (5.6%) |
| Country | |||
| Australia | 2,512 (29%) | 1,293 (29%) | 1,414 (27%) |
| Turkey | 1,991 (23%) | 927 (20%) | 1,346 (26%) |
| Italy | 706 (8.0%) | 442 (9.8%) | 352 (6.8%) |
| Spain | 590 (6.7%) | 288 (6.4%) | 364 (7.0%) |
| Kuwait | 559 (6.4%) | 353 (7.8%) | 244 (4.7%) |
| Iran | 429 (4.9%) | 163 (3.6%) | 294 (5.7%) |
| Croatia | 309 (3.5%) | 234 (5.2%) | 121 (2.3%) |
| Belgium | 267 (3.0%) | 150 (3.3%) | 161 (3.1%) |
| Tunisia | 103 (1.2%) | 74 (1.6%) | 47 (0.9%) |
| Japan | 94 (1.1%) | 70 (1.5%) | 43 (0.8%) |
| Netherlands | 87 (1.0%) | 37 (0.8%) | 59 (1.1%) |
| Other* | 497 (5.7%) | 274 (6.0%) | 273 (5.3%) |
| MS course | 1,293 (29%) | ||
| Relapsing remitting | 7,610 (87%) | 3,906 (86%) | 4,570 (88%) |
| Secondary progressive | 537 (6.1%) | 113 (2.5%) | 443 (8.6%) |
| Primary progressive | 303 (3.5%) | 253 (5.6%) | 66 (1.3%) |
| Progressive relapsing | 95 (1.1%) | 60 (1.3%) | 42 (0.8%) |
| Radiologically isolated syndrome | 4 (< 0.1%) | 3 (< 0.1%) | 2 (< 0.1%) |
| Clinically isolated syndrome | 222 (2.5%) | 198 (4.4%) | 42 (0.8%) |
| EDSS | 2.4 (1.9%) | 2.0 (1.7%) | 2.6 (2.0%) |
| Relapse count (in previous 24 months) | |||
| 0 | 4,708 (54%) | 2,016 (44%) | 3,005 (58%) |
| 1 | 2,847 (32%) | 1,790 (39%) | 1,478 (29%) |
| 2 | 918 (10%) | 570 (13%) | 498 (9.6%) |
| 3 | 228 (2.6%) | 126 (2.8%) | 137 (2.7%) |
| 4 | 46 (0.5%) | 23 (0.5%) | 28 (0.5%) |
| 5 | 16 (0.2%) | 7 (0.2%) | 12 (0.2%) |
| 6 | 3 (< 0.1%) | 1 (< 0.1%) | 2 (< 0.1%) |
| 7 | 4 (< 0.1%) | - | 4 (< 0.1%) |
| 10 | 1 (< 0.1%) | - | 1 (< 0.1%) |
| Disease duration (years) | 8.2 (8.3%) | 4.4 (6.7%) | 10.6 (8.3%) |
*See appendix for full list of countries
Comparison of high- and low-efficacy DMT prescription pre- and post-pandemic onset
There was an overall decrease in initiating and switching DMTs post-pandemic compared to pre-pandemic (Table 2). There was a significant increase in initiation of low-efficacy DMTs post-pandemic compared to pre-pandemic (54.1 to 59.6%) and a decrease in initiation of high-efficacy DMTs (45.9 to 40.4%). There was a significant increase in switching to low-efficacy DMTs post-pandemic (27.4 to 29%) and a decrease in switching to high-efficacy DMTs post-pandemic (72.6 to 71%).
Table 2.
Comparison of initiation and switches pre- and post-pandemic with low-efficacy and high-efficacy DMTs
| Initiation | Switching | |||||
|---|---|---|---|---|---|---|
| Overall N = 4,533 |
Pre-pandemic N = 2,443 |
Post-pandemic N = 2,090 |
Overall N = 5,899 |
Pre-pandemic N = 3,306 |
Post-pandemic N = 2,593 |
|
| Low-efficacy DMT | 2,568 (56.6%) | 1,322 (54.1%) | 1,246 (59.6%) | 1,657 (28.1%) | 906 (27.4%) | 751 (29%) |
| High-efficacy DMT | 1,965 (43.4%) | 1,121 (45.9%) | 844 (40.4%) | 4,242 (71.9%) | 2,400 (72.6%) | 1,842 (71.0%) |
Analysis of DMT prescribing patterns pre- and post-pandemic onset
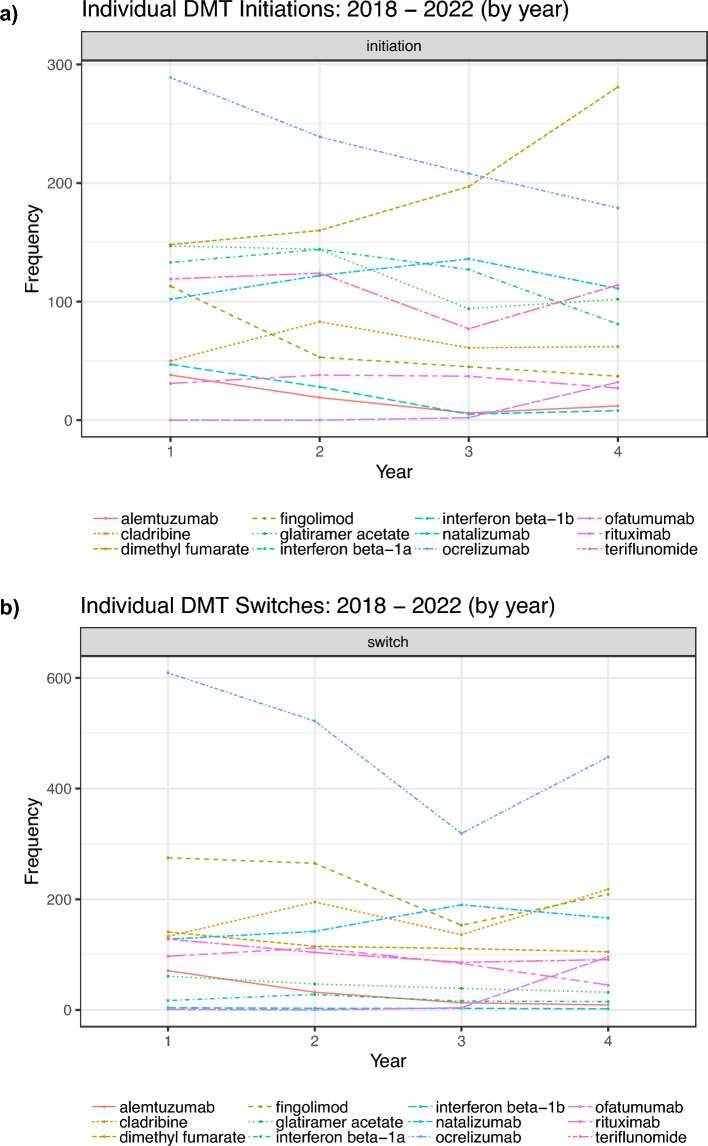
Post-pandemic onset, there was an increase in DMT initiation and switching to natalizumab (OR 1.72, 95% CI 1.39–2.13; OR 1.66, 95% CI 1.40–1.98) and cladribine (OR 1.43, 95% CI 1.09–1.87; OR 1.67, 95% CI 1.41–1.98) (Table 3). The initiation and switching to anti-CD20 monoclonal antibodies (mABs) decreased immediately following the onset of the COVID-19 pandemic in 2020; however, there was a steady increase towards the end of the pandemic, resulting in an overall rise in initiation and switching to anti-CD20 mABs (OR 1.26, 95% CI 1.06–1.49; OR 1.15, 95% CI 1.02–1.29) (Fig. 1). This increase was statistically significant but relatively smaller increase compared to natalizumab or cladribine.
Table 3.
Comparison of DMT initiation and switching from pre- to post-pandemic
| Initiation—OR (95% CI) | Switching—OR (95% CI) | |
|---|---|---|
| Anti-CD20 mAb | ||
| Ocrelizumab, ofatumumab and rituximab | 1.26 (1.06–1.49) | 1.15 (1.02–1.29) |
| Oral immunomodulators | ||
| Dimethyl fumarate | 1.76 (1.49–2.09) | 0.85 (0.69–1.05) |
| Teriflunomide | 0.77 (0.62–0.96) | 0.80 (0.64–0.99) |
| Fingolimod | 0.55 (0.41–0.73) | 0.49 (0.41–0.58) |
| Injectable immunomodulators | ||
| BRACE | 0.78 (0.62–0.99) | 0.78 (0.62–0.99) |
| Integrin antagonist | ||
| Natalizumab | 1.72 (1.39–2.13) | 1.66 (1.40–1.98) |
| Purine analogue | ||
| Cladribine | 1.43 (1.09–1.87) | 1.67 (1.41–1.98) |
| Other | ||
| Alemtuzumab | 0.27 (0.15–0.48) | 0.27 (0.17–0.44) |
BRACE includes interferon-beta and glatiramer acetate, and anti-CD20 mAbs includes rituximab, ocrelizumab and ofatumumab. Data are displayed with Odds ratios and 95% CI
Fig. 1.
Patterns of frequency of DMT initiation (a) and DMT switching (b) divided by year. Year 1 represents March 2018 to March 2019, Year 2 represents March 2019 to March 2020, Year 3 represents March 2021 to March 2022, and Year 4 represents March 2021 to March 2022
There was a decrease in initiating and switching patients to fingolimod (OR 0.55, 95% CI 0.41–0.73; OR 0.49, 95% CI 0.41–0.58), interferon-beta (OR 0.48, 95% CI 0.41–0.57; OR 0.78, 95% CI 0.62–0.99) and alemtuzumab (OR 0.27, 95% CI 0.15–0.48; OR 0.27, 95% CI 0.17–0.44) post-pandemic onset (Table 3). There was an increase in initiating (OR 1.76, 95% CI 1.49–2.09), but a decrease in switching (OR 0.85, 95% CI 0.69–1.05) patients to DMF.
Discussion
We performed a retrospective multi-site analysis of the prescribing patterns of DMTs in pwMS after the onset of the COVID-19 pandemic. We hypothesised there would be a significant decrease in DMT prescription, particularly anti-CD20 mAbs and fingolimod, given concerns regarding immunosuppression during the COVID-19 pandemic. Our results demonstrate a significant decrease in initiation and switching to fingolimod, alemtuzumab and interferon-beta post-pandemic onset, and a significant increase in initiation and switching patients to natalizumab and cladribine There was also a slight increase to anti-CD20 mAbs (though notably less than other higher-efficacy DMTs). This supports our hypothesis that concerns around more severe COVID-19 outcomes for pwMS on fingolimod and anti-CD20 mAbs influenced prescribing patterns during the pandemic. The increased usage of natalizumab and cladribine post-pandemic onset was likely driven by clinicians attempting to maintain prescribing high-efficacy treatments for patients but avoiding the usage of anti-CD20 mAb therapies due to perceived immunosuppression risks.
Our results show an overall decrease in the initiation and switching of DMTs during the COVID-19 pandemic. We specifically observed an approximate 5% decrease in initiation and 1% decrease in switching patients to high-efficacy DMTs post-pandemic onset, which was likely driven by clinicians choosing to reduce the prescribing of high-efficacy treatments based on concerns of worsening COVID-19 susceptibility and severity in patients. This is consistent with retrospective cohort studies, which have noted an overall decrease in DMT prescription or a change in dosing regimen, specifically in high-efficacy DMTs [13, 16]. This significant shift in underutilisation of higher efficacy DMTs and increased initiation and switching to lower efficacy DMTs has significant implications for relapse probability in pwMS at a population scale and could have a negative impact on overall health outcomes for pwMS [22].
Our results reveal that clinicians increased the prescription of cladribine and natalizumab during the COVID-19 pandemic, likely as they were considered safer, high-efficacy treatments for pwMS. Current evidence indicates that cladribine does not increase susceptibility to COVID-19 infections or exacerbate infection severity. Case studies have shown that pwMS treated with cladribine mount an appropriate immunological response and typically experience mild symptoms following COVID-19 infection [23–25]. This may be due to the immune reconstitution properties of cladribine, wherein it causes selectively transient reductions in CD19+ B and T cells, followed by reconstitution and restoration of the body’s adaptive immunity [26]. Natalizumab is not associated with worse COVID-19 clinical outcomes [27, 28]. Indeed, there is some postulation that natalizumab may be protective against COVID-19 infection by limiting viral entry into cells through the integrin blockade [29, 30].
Anti-CD20 mAb initiation and switching decreased in 2020. Still, it returned to pre-pandemic levels in 2021, such that overall, there was a slight increase in anti-CD20 mAb prescription (though less than other high-efficacy DMTs). These results are consistent with other studies that also observed a decrease in prescribing anti-CD20 mAbs during the COVID-19 pandemic [14]. Multiple cohort studies have indicated a significant relationship between anti-CD20 mAbs and increased severity of COVID-19 infection, thereby indicating the rapid response from clinicians to avoid prescribing, initiating or switching patients to these therapies was appropriate from a COVID-19 disease severity perspective [17, 18, 31, 32].
Concerns with its lymphopenic effects may have driven the decrease in initiating and switching to fingolimod as it is specifically associated with higher rates of Herpes Zoster virus infections compared to other DMTs [33, 34]. Whilst individual case studies have suggested a potential increase in the severity of COVID-19 infections with fingolimod use, larger cohort studies and current evidence indicate that fingolimod is not associated with worse COVID-19 outcomes or increased hospitalisation rates [32, 35–37]. The continued decrease in usage of fingolimod in 2021 implies that clinicians might be progressively opting for other high-efficacy DMTs over fingolimod.
The decrease in initiation and switching patients to alemtuzumab was likely due to published recommendations at the start of the pandemic, which suggested delaying lymphodepleting treatments such as alemtuzumab until the COVID-19 pandemic was more controlled [7]. In addition, prescription may have been influenced by limited access to inpatient healthcare services as various countries navigated the pandemic with various lockdown restrictions.
The increase in DMF initiation may have been based on recommendations from early guidelines that were published at the start of the pandemic, which encouraged the prescription of first-line DMTs such as teriflunomide and dimethyl fumarate [7]. The observed decrease in initiation and switching to interferon treatments post-pandemic onset may have been driven by concerns over immune system modulation and a preference for oral medications during the pandemic due to decreased access to healthcare facilities.
The limitations of our study include the fact that we did not record the reasons for clinicians’ choices of DMT initiation or switching. Patients’ co-morbidities and COVID-19 vaccine status were not recorded, which could have influenced DMT choice. Furthermore, concerns regarding DMTs affecting vaccine efficacy may also have influenced DMT therapy initiation choices [38, 39]. The temporal dynamics of the pandemic which varied substantially between countries, characterised by various waves and changing public health responses, alongside the evolution in the availability and types of COVID-19 vaccines, may have also influenced clinicians’ choices for DMT initiation and switching. In addiiton, individual access to DMTs, which varied greatly between countries and supply chain issues, may have influenced prescribing preferences.
Considered collectively, it is evident that during the COVID-19 pandemic, there was a nuanced balance between mitigating severe COVID-19 infections and ensuring continued use of high-efficacy DMTs to minimise MS disease activity. The approach to prescribing DMTs for pwMS demonstrated a significant evolution from initial, recommendation-driven practices to more robust, data-driven strategies. The initial hesitancy to prescribe certain high-efficacy DMTs, such as anti-CD20 monoclonal antibodies and fingolimod, was influenced by concerns regarding their impact on COVID-19 severity and vaccine efficacy. Over time, however, prescribing patterns adapted in response to accumulating clinical evidence, highlighting the resilience and adaptability of clinicians in managing treatment of pwMS under global health challenges.
Moreover, this shift highlights a crucial need for international equity in access to DMTs. Our findings suggest that the ability to select the most appropriate therapy based on up-to-date evidence was at times limited by availability and accessibility, affecting treatment choices globally. As such, ensuring equitable access to a range of DMTs is essential, not only for managing MS more effectively but also for preparing healthcare systems to respond more effectively to future global health emergencies. Our research highlights the necessity of evidence-based decision-making and collaborative efforts amongst researchers, clinicians, and healthcare systems to optimise care and protect the health outcomes of pwMS amid ongoing global health challenges.
Abbreviations
- MS
Multiple sclerosis
- pwMS
People with multiple sclerosis
- DMT
Disease-modifying therapy
- mAb
Monoclonal antibodies
- DMF
Dimethyl fumarate
- RAT
Rapid-antigen testing
- PCR
Polymerase chain reaction
- EDSS
Expanded Disability Status Scale
Appendix 1
See Table 4.
Table 4.
Participant demographics
| Characteristic | Total n = 8771 (%) | Initiation n = 4533 (%) | Switching n = 5165 (%) |
|---|---|---|---|
| Gender | |||
| F | 6,222 (71%) | 3,150 (69%) | 3,747 (73%) |
| M | 2,549 (29%) | 1,383 (31%) | 1,418 (27%) |
| Age category (years) | |||
| 0–20 | 326 (3.7%) | 257 (5.7%) | 147 (2.8%) |
| 21–30 | 1,682 (19%) | 1,130 (25%) | 841 (16%) |
| 31–40 | 2,556 (29%) | 1,386 (31%) | 1,455 (28%) |
| 41–50 | 2,318 (26%) | 1,011 (22%) | 1,490 (29%) |
| 51–60 | 1,392 (16%) | 520 (11%) | 944 (18%) |
| > 60 | 497 (5.7%) | 229 (5.1%) | 288 (5.6%) |
| Country | |||
| AU | 2,512 (29%) | 1,293 (29%) | 1,414 (27%) |
| AE | 30 (0.3%) | 15 (0.3%) | 18 (0.3%) |
| BE | 267 (3.0%) | 150 (3.3%) | 161 (3.1%) |
| CA | 627 (7.1%) | 228 (5.0%) | 447 (8.7%) |
| CO | 68 (0.8%) | 63 (1.4%) | 16 (0.3%) |
| EE | 70 (0.8%) | 34 (0.8%) | 45 (0.9%) |
| ES | 590 (6.7%) | 288 (6.4%) | 364 (7.0%) |
| GB | 38 (0.4%) | 13 (0.3%) | 29 (0.6%) |
| GR | 4 (< 0.1%) | 1 (< 0.1%) | 3 (< 0.1%) |
| HR | 309 (3.5%) | 234 (5.2%) | 121 (2.3%) |
| HU | 2 (< 0.1%) | 2 (< 0.1%) | 1 (< 0.1%) |
| IR | 429 (4.9%) | 163 (3.6%) | 294 (5.7%) |
| IT | 706 (8.0%) | 442 (9.8%) | 352 (6.8%) |
| JP | 94 (1.1%) | 70 (1.5%) | 43 (0.8%) |
| KW | 559 (6.4%) | 353 (7.8%) | 244 (4.7%) |
| LB | 45 (0.5%) | 20 (0.4%) | 31 (0.6%) |
| NL | 87 (1.0%) | 37 (0.8%) | 59 (1.1%) |
| NZ | 28 (0.3%) | 17 (0.4%) | 14 (0.3%) |
| OM | 53 (0.6%) | 23 (0.5%) | 37 (0.7%) |
| Other | 107 (1.2%) | 72 (1.6%) | 40 (0.8%) |
| PL | 1 (< 0.1%) | 1 (< 0.1%) | 0 (0%) |
| PT | 50 (0.6%) | 12 (0.3%) | 39 (0.8%) |
| TN | 103 (1.2%) | 74 (1.6%) | 47 (0.9%) |
| TR | 1,991 (23%) | 927 (20%) | 1,346 (26%) |
| US | 1 (< 0.1%) | 1 (< 0.1%) | 0 (0%) |
| MS course | 1,293 (29%) | ||
| Relapsing remitting | 7,610 (87%) | 3,906 (86%) | 4,570 (88%) |
| Secondary progressive | 537 (6.1%) | 113 (2.5%) | 443 (8.6%) |
| Primary progressive | 303 (3.5%) | 253 (5.6%) | 66 (1.3%) |
| Progressive relapsing | 95 (1.1%) | 60 (1.3%) | 42 (0.8%) |
| Radiologically isolated syndrome | 4 (< 0.1%) | 3 (< 0.1%) | 2 (< 0.1%) |
| Clinically isolated syndrome | 222 (2.5%) | 198 (4.4%) | 42 (0.8%) |
| EDSS | 2.37 (1.88) | 1.99 (1.67) | 2.64 (1.96) |
| Relapse count (in previous 24 months) | |||
| 0 | 4,708 (54%) | 2,016 (44%) | 3,005 (58%) |
| 1 | 2,847 (32%) | 1,790 (39%) | 1,478 (29%) |
| 2 | 918 (10%) | 570 (13%) | 498 (9.6%) |
| 3 | 228 (2.6%) | 126 (2.8%) | 137 (2.7%) |
| 4 | 46 (0.5%) | 23 (0.5%) | 28 (0.5%) |
| 5 | 16 (0.2%) | 7 (0.2%) | 12 (0.2%) |
| 6 | 3 (< 0.1%) | 1 (< 0.1%) | 2 (< 0.1%) |
| 7 | 4 (< 0.1%) | – | 4 (< 0.1%) |
| 10 | 1 (< 0.1%) | – | 1 (< 0.1%) |
| Disease duration (years) | 8.20 (8.31) | 4.44 (6.69) | 10.56 (8.34) |
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflicts of interest
Yi Chao Foong: reports a relationship with Biogen that includes: travel reimbursement. Tim Spelman: received compensation for serving on scientific advisory board for Biogen and speaker honoraria from Novartis. Marzena Fabis-Pedrini: received travel compensation from Merck. Matteo Foschi: received travel and meeting attendance support from Novartis, Biogen, Roche, Sanofi-Genzyme, and Merck. Mario Habek: participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi-Genzyme, Merck, Novartis, Pliva/Teva, Roche, Zentiva, Astra-Zeneca, TG Pharmaceuticals, and CNSystems. Tomas Kalincik: served on scientific advisory boards for the MS International Federation and the World Health Organization, BMS, Roche, Janssen, Sanofi-Genzyme, Novartis, Merck, and Biogen. He served on the steering committee for the Brain Atrophy Initiative by Sanofi-Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi-Genzyme, Teva, BioCSL, and Merck, and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene, and Merck. Izanne Roos: has served on scientific advisory boards and received conference travel support and/or speaker honoraria from Roche, Novartis, Merck, and Biogen. Izanne Roos is supported by MS Australia and the Trish Multiple Sclerosis Research Foundation. Anneke van der Walt: served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck, and Roche. She has received speaker’s honoraria and travel support from Novartis, Roche, and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia. Helmut Butzkueven: received institutional (Monash University) funding from Biogen, F. Hoffmann-La Roche Ltd, Merck, Alexion, CSL, and Novartis; has carried out contracted research for Novartis, Merck, F. Hoffmann-La Roche Ltd, and Biogen; has taken part in speakers’ bureaus for Biogen, Genzyme, UCB, Novartis, F. Hoffmann-La Roche Ltd, and Merck; has received personal compensation from the Oxford Health Policy Forum for the Brain Health Steering Committee. Jeannette Lechner-Scott: received travel compensation from Novartis, Biogen, Roche, and Merck. Her institution receives honoraria for talks and advisory board commitments as well as research grants from Biogen, Merck, Roche, TEVA, and Novartis. Nevin John: is a PI on commercial MS studies sponsored by Novartis, Roche, Biogen, and Sanofi. He has received speaker’s honoraria from Merck and conference travel and registration reimbursement from Novartis. “Emanuele D'amico”: has received speaker honoraria and consultant fees from Biogen-Idec, Novartis, Merck, Janssen, Bristol-Myers, Bayer, Sanofi-Genzyme, and Roche. Riadh Gouider: has received research grant and/or advisory board honoraria from Biogen, Hikma, Merck, Roche, and Sanofi. He has no conflict of interest related to this study. Saloua Mrabet: has received a MENACTRIMS clinical fellowship grant (2020). Katrin Gross-Paju: received honoraria as a consultant on scientific advisory boards for Biogen, Roche, and Novartis; has received travel grants from Biogen, Roche, and Novartis; and has participated in clinical trials by Biogen, Merck, Sanofi, Roche, and Novartis. Simón Cárdenas-Robledo: has received travel expenses for scientific meetings from Biopas, Roche, Merck, and Genzyme; compensation for consulting services or participation on advisory boards from Merck, Biogen-Idec, Sanofi, and Novartis; lecture fees from Biopas, Novartis, Merck, Sanofi, Janssen, and Biogen-Idec; and research support from Biogen-Idec and Novartis. He is a subject editor on Multiple Sclerosis for Acta Neurológica Colombiana and a member of the editorial board of Frontiers of Neurology. Abdorreza Naser Moghadasi: has served on scientific advisory boards and received conference travel support and/or speaker honoraria from Roche, Novartis, Genzyme, Merck, and Biogen. Maria Jose Sa: received consulting fees, speaker honoraria, and/or travel expenses for scientific meetings from Alexion, Bayer Healthcare, Biogen, Bristol Myers Squibb, Celgene, Janssen, Merck-Serono, Novartis, Roche, Sanofi, and Teva. Orla Gray: received honoraria as a consultant on scientific advisory boards for Genzyme, Biogen, Merck, Roche, and Novartis; has received travel grants from Biogen, Merck, Roche, and Novartis; has participated in clinical trials by Biogen and Merck. Her institution has received research grant support from Biogen. Jiwon Oh: has received research funding from the MS Society of Canada, National MS Society, Brain Canada, Biogen, Roche, and EMD Serono (an affiliate of Merck KGaA); and personal compensation for consulting or speaking from Alexion, Biogen, Celgene (BMS), EMD Serono (an affiliate of Merck KGaA), Novartis, Roche, and Sanofi-Genzyme. Sudarshini Ramanathan: has received research funding from the National Health and Medical Research Council (NHMRC, Australia), the Petre Foundation, the Brain Foundation, the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on the International Steering Committee for a clinical trial led by UCB (NCT05063162). She is on the advisory board for educational activities led by Limbic Neurology. She has been an invited speaker for educational/research sessions coordinated by Biogen, Alexion, Novartis, Excemed, and Limbic Neurology. She is on the medical advisory board (non-remunerated positions) of The MOG Project and the Sumaira Foundation. Ayse Altintas: received speaker honoraria from Novartis and Alexion. Todd A. Hardy: received speaker honoraria/conference travel support or served on advisory boards for Bayer Schering, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Bristol Myers Squibb, and Teva. Raed Alroughani: received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche, and Sanofi-Genzyme. Allan G Kermode: received speaker honoraria and scientific advisory board fees from Bayer, BioCSL, Biogen, Genzyme, Innate Immunotherapeutics, Merck, Novartis, Sanofi, Sanofi-Aventis, and Teva. Andrea Surcinelli: received travel and meeting attendance support from Novartis, Biogen, Roche, Merck, Bristol, Sanofi-Genzyme, Almirall, and Piam. Guy Laureys: received travel and/or consultancy compensation from Sanofi-Genzyme, Roche, Teva, Merck, Novartis, Celgene, and Biogen. Sara Eichau: has received speaker honoraria and consultant fees from Biogen-Idec, Novartis, Merck, Janssen, Bristol-Myers, Bayer, Sanofi-Genzyme, Roche, and Teva. Pierre Duquette: served on editorial boards and has been supported to attend meetings by EMD, Biogen, Novartis, Genzyme, and TEVA Neuroscience. He holds grants from the CIHR and the MS Society of Canada and has received funding for investigator-initiated trials from Biogen, Novartis, and Genzyme. Suzanne Hodgkinson: has received consulting fees and speaker honoraria from Biogen, Novartis, Roche, and Merck, and has received grants for her institution from Biogen, Merck, Novartis, and Roche. Cristina Ramo-Tello: has received consulting fees, speaker honoraria, support for attending meetings and/or travel, participation on advisory boards, and research grants for her institution from Biogen, Novartis, Sanofi, Bristol, Roche, Almirall, Janssen, Sandoz, and Merck. Davide Maimone: received speaker honoraria for the advisory board and travel grants from Alexion, Almirall, Bayer, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Pamela McCombe: received speakers fees and travel grants from Novartis, Biogen, T’évalua, and Sanofi. Daniele Spitaleri: received honoraria as a consultant on scientific advisory boards by Bayer-Schering, Novartis, and Sanofi-Aventis, and compensation for travel from Novartis, Biogen, Sanofi-Aventis, Teva, and Merck. Jose Luis Sanchez-Menoyo: accepted travel compensation from Novartis, Merck, and Biogen; speaking honoraria from Biogen, Novartis, Sanofi, Merck, Almirall, Bayer, and Teva; and has participated in clinical trials by Biogen, Merck, and Roche. Seyed Mohammad Baghbanian: has served on scientific advisory boards and has received conference travel support and/or speaker honoraria from several pharmaceutical companies, including Roche, Novartis, Merck, Cinnagen, Nanoalvand, and Biogen. Rana Karabudak: received consulting fees, payment, or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events; support for attending meetings and/or travel; and participation on a data safety monitoring board or advisory board from Gen Turkey. Abdullah Al-Asmi: received personal compensation for serving as a scientific advisor or speaker/moderator for Novartis, Biogen, Roche, Sanofi-Genzyme, and Merck. Gregor Brecl Jakob: participated as a clinical investigator and/or received consultation and/or speaker fees from Amgen, Astra-Zeneca, Biogen, Janssen, Lek, Merck, Novartis, Pliva/Teva, Roche, Sanofi-Genzyme, and Swixx. Samia J. Khoury: received compensation for scientific advisory board activity from Merck and Roche, and received compensation for serving on the IDMC for Biogen. Vincent van Pesch: received travel grants from Merck Healthcare KGaA (Darmstadt, Germany), Biogen, Sanofi, Bristol Meyer Squibb, Almirall, and Roche. His institution has received research grants and consultancy fees from Roche, Biogen, Sanofi, Merck Healthcare KGaA (Darmstadt, Germany), Bristol Meyer Squibb, Janssen, Almirall, Novartis Pharma, and Alexion. Helmut Butzkueven: received institutional (Monash University) funding from Biogen, F. Hoffmann-La Roche Ltd, Merck, Alexion, CSL, and Novartis; has carried out contracted research for Novartis, Merck, F. Hoffmann-La Roche Ltd and Biogen; has taken part in speakers’ bureaus for Biogen, Genzyme, UCB, Novartis, F. Hoffmann-La Roche Ltd and Merck; has received personal compensation from Oxford Health Policy Forum for the Brain Health Steering Committee. Anneke van der Walt: served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck and Roche She has received speaker’s honoraria and travel support from Novartis, Roche, and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia. The remaining authors have nothing to declare.
Footnotes
Anoushka P. Lal and Yi Chao Foong contributed equally to this work.
References
- 1.Barzegar M et al (2021) COVID-19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm 8:4 10.1212/NXI.0000000000001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung MW et al (2022) Mapping the risk of infections in patients with multiple sclerosis: a multi-database study in the United Kingdom Clinical Practice Research Datalink GOLD and Aurum. Mult Scler 28(11):1808–1818 10.1177/13524585221094218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry F et al (2020) COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci 418:117147 10.1016/j.jns.2020.117147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louapre C et al (2020) Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 77(9):1079–1088 10.1001/jamaneurol.2020.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee W et al (2020) Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology 94(22):949–952 10.1212/WNL.0000000000009507 [DOI] [PubMed] [Google Scholar]
- 6.Korsukewitz C, Reddel SW, Bar-Or A, Wiendl H (2020) Neurological immunotherapy in the era of COVID-19—looking for consensus in the literature. Nat Rev Neurol 16(9):493–505 10.1038/s41582-020-0385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannoni G et al (2020) The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord 39:102073 10.1016/j.msard.2020.102073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger JR, Brandstadter R, Bar-Or A (2020) COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm 7:4 10.1212/NXI.0000000000000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safavi F, Nourbakhsh B, Azimi AR (2020) B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord 43:102195 10.1016/j.msard.2020.102195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalla Costa G et al (2020) Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurol Sci 41(7):1647–1650 10.1007/s10072-020-04519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Januel E et al (2023) Association between anti-CD20 therapies and COVID-19 severity among patients with relapsing-remitting and progressive multiple sclerosis. JAMA Netw Open 6(6):e2319766–e2319766 10.1001/jamanetworkopen.2023.19766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiavetti I et al (2022) Severe outcomes of COVID-19 among patients with multiple sclerosis under anti-CD-20 therapies: a systematic review and meta-analysis. Multiple Scler Relat Disord 57:103358 10.1016/j.msard.2021.103358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bsteh G et al (2022) Has the pandemic changed treatment strategy in multiple sclerosis? Mult Scler Relat Disord 63:103912 10.1016/j.msard.2022.103912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaheer R et al (2023) Impact of COVID-19 on prescribing patterns and treatment selection of disease modifying therapies in multiple sclerosis. Mult Scler Relat Disord 71:104575 10.1016/j.msard.2023.104575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krett JD, Salter A, Newsome SD (2024) Era of COVID-19 in multiple sclerosis care. Neurol Clin 42(1):319–340 10.1016/j.ncl.2023.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas W et al (2022) Impact of the COVID-19 pandemic on the prescription of disease-modifying therapy for multiple sclerosis in England: a nationwide study. J Neurol Neurosurg Psychiatry 93(11):1229 10.1136/jnnp-2021-328340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sormani MP et al (2021) DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Transl Neurol 8(8):1738–1744 10.1002/acn3.51408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spelman T et al (2021) Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler J 28(7):1051–1059 10.1177/13524585211026272 [DOI] [PubMed] [Google Scholar]
- 19.Zanghì A et al (2022) Is it time for ocrelizumab extended interval dosing in relapsing remitting MS? Evidence from an Italian multicenter experience during the COVID-19 pandemic. Neurotherapeutics 19(5):1535–1545 10.1007/s13311-022-01289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisecco A et al (2024) Covid-19 outbreak in Italy: an opportunity to evaluate extended interval dosing of ocrelizumab in MS patients (P2–6010). Neurology 102(17):6251 10.1212/WNL.0000000000206347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson AJ et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 22.Foong YC et al (2024) Comparing ocrelizumab to interferon/glatiramer acetate in people with multiple sclerosis over age 60. J Neurol Neurosurg Psychiatry 2:4 [DOI] [PubMed] [Google Scholar]
- 23.Carlini F et al (2023) Cladribine tablets mode of action, learning from the pandemic: a narrative review. Neurol Therapy 12(5):1477–1490 10.1007/s40120-023-00520-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preziosa P et al (2021) COVID-19 in cladribine-treated relapsing-remitting multiple sclerosis patients: a monocentric experience. J Neurol 268(8):2697–2699 10.1007/s00415-020-10309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Angelis M et al (2020) Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult Scler Relat Disord 45:102452 10.1016/j.msard.2020.102452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comi G et al (2019) Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord 29:168–174 10.1016/j.msard.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 27.Chisari CG et al (2021) Natalizumab administration in multiple sclerosis patients during active SARS-CoV-2 infection: a case series. BMC Neurol 21(1):462 10.1186/s12883-021-02421-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landi D et al (2022) Safety of Natalizumab infusion in multiple sclerosis patients during active SARS-CoV-2 infection. Mult Scler Relat Disord 57:103345 10.1016/j.msard.2021.103345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguirre C et al (2020) Covid-19 in a patient with multiple sclerosis treated with natalizumab: May the blockade of integrins have a protective role? Mult Scler Relat Disord 44:102250 10.1016/j.msard.2020.102250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigrist CJ, Bridge A, Le Mercier P (2020) A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res 177:104759 10.1016/j.antiviral.2020.104759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hada M, Mosholder AD, Leishear K, Perez-Vilar S (2022) Systematic review of risk of SARS-CoV-2 infection and severity of COVID-19 with therapies approved to treat multiple sclerosis. Neurol Sci 43(3):1557–1567 10.1007/s10072-021-05846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson-Yap S et al (2021) Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 97(19):e1870–e1885 10.1212/WNL.0000000000012753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvin AM et al (2015) Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol 72(1):31–39 10.1001/jamaneurol.2014.3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luna G et al (2020) Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 77(2):184–191 10.1001/jamaneurol.2019.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barzegar M et al (2020) COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm 7:4 10.1212/NXI.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan R et al (2021) COVID-19 infection in fingolimod- or siponimod-treated patients: case series. Neurol Neuroimmunol Neuroinflamm 9:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teymouri S et al (2023) The effect of Fingolimod on patients with moderate to severe COVID-19. Pharmacol Res Perspect 11(1):e01039 10.1002/prp2.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker D et al (2020) COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol 202(2):149–161 10.1111/cei.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciotti JR, Valtcheva MV, Cross AH (2020) Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord 45:102439 10.1016/j.msard.2020.102439 [DOI] [PMC free article] [PubMed] [Google Scholar]