Abstract
Purpose
Consumption of ultra-processed foods (UPF) has increased despite potential adverse health effects. Recent studies showed an association between UPF consumption and some gastrointestinal disorders. We evaluated the association between UPF consumption and peptic ulcer disease (PUD) in a large Spanish cohort.
Methods
We conducted a prospective analysis of 18,066 participants in the SUN cohort, followed every two years. UPF was assessed at baseline and 10 years after. Cases of PUD were identified among participants reporting a physician-made diagnosis of PUD during follow-ups. Cases were only partially validated against medical records. Cox regression was used to assess the association between baseline UPF consumption and PUD risk. Based on previous findings and biological plausibility, socio-demographic and lifestyle variables, BMI, energy intake, Helicobacter pylori infection, gastrointestinal disorders, aspirin and analgesic use, and alcohol and coffee consumption were included as confounders.We fitted GEE with repeated dietary measurements at baseline and after 10 years of follow-up. Vanderweele’s proposed E value was calculated to assess the sensitivity of observed associations to uncontrolled confounding.
Results
During a median follow-up of 12.2 years, we recorded 322 new PUD cases (1.56 cases/1000 person-years). Participants in the highest baseline tertile of UPF consumption had an increased PUD risk compared to participants in the lowest tertile (HR = 1.52, 95% CI: 1.15, 2.00, Ptrend=0.002). The E-values for the point estimate supported the observed association. The OR using repeated measurements of UPF intake was 1.39 (95% CI: 1.03, 1.87) when comparing extreme tertiles.
Conclusion
The consumption of UPF is associated with an increased PUD risk.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00394-024-03439-2.
Keywords: Ultra-processed food, Peptic ulcer disease, NOVA food classification system, Gastrointestinal disorders
Introduction
Ultra-processed foods (UPF) are industrial formulations including, besides salt, sugar, oils, and fats, food-derived substances and additives, the aim of which is to make these products attractive, extremely palatable and convenient (ready-to-eat and with a long shelf life) [1]. Their consumption has increased dramatically in recent decades, so much so that nowadays they contribute 50% or more of daily calorie intake in some Western countries [2–4]. It has been reported that a diet rich in UPF is nutritionally unbalanced [5], and several prospective cohorts have repeatedly reported an association between UPF consumption and the risk of obesity [6–8], diabetes (both type 2 and gestational diabetes) [9, 10], cardiovascular diseases [11, 12], and other non-communicable diseases [13]. Moreover, recent prospective studies have showed an association between UPF consumption and some functional gastrointestinal disorders or diseases, such as irritable bowel syndrome, functional dyspepsia [14] and Crohn’s disease [15, 16]. However, the association between UPF consumption and peptic ulcer disease (PUD) has not been investigated.
PUD is characterized by an acid peptic lesion of the gastrointestinal mucosa, with depth to the submucosa. Ulcers are generally located in the stomach and proximal duodenum, but can sometimes affect the lower esophagus, distal duodenum or jejunum. The most common symptom of PUD is the burning epigastric pain occurring after meals. Gastrointestinal bleeding is the most common complication, with a mortality rate of 2.5–10%, mainly due to non-hemorrhagic causes such as multiorgan failure, pulmonary complications, and malignancy. Other PUD-related complications are perforation, with a mortality rate of 20%, and gastric outlet obstruction [17]. It has been estimated that 5–10% of individuals in the general population develop PUD during their lifetime. Helicobacter pylori infection and use of nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin are the most common risk factors for PUD. However, the pathophysiology of ulcers not associated with H. pylori or NSAID ingestion is becoming more relevant as the incidence of H. pylori is dropping, particularly in the Western world [17].
Diet has been thought to play a role in the development of PUD, but the evidence is limited and controversial. Certain diet components, such as salt, refined carbohydrates, alcohol, fiber, vitamins and polyphenols have been suspected to be linked to the PUD risk [18–20]. Since UPF are characterized by a low content of protective nutrients, such as fiber, vitamins and polyphenols, and a high density of sugars and salt, a diet rich in these products could contribute to the development of PUD. To elucidate this issue, we conducted an analysis in the SUN cohort to appraise whether UPF consumption was associated with the incident risk of PUD.
Materials and methods
Study design and participants
The SUN project is a Spanish dynamic prospective cohort aimed on studying the relationship between dietary habits, lifestyle and health status. The recruitment began in 1999 and it still on going. Participants are former graduates of University of Navarra, health professionals and other graduates [21]. Participants were invited to participate by means of a letter (e-mail or regular mail) outlining the objective of the project, what their participation entailed, the information required over time, and the arrangements put in place to safeguard their privacy. Along with the invitation letter, participants received a questionnaire (online or paper) designed to collect basic information on sociodemographic and lifestyle variables, eating habits, and medical history. They were given the option to decline participation in the study simply by not submitting the completed questionnaire. Therefore, voluntary completion of the first questionnaire was considered as informed consent as it was approved by the Ethical Committee. Every two years, participants received a new questionnaire investigating the occurrence of new diseases. Ten years after entering the cohort, the questionnaire sent to participants also investigated dietary habits so that they could be updated. This does not imply termination of the study. In fact, participants continue to receive the health status update questionnaire every two years. Participants who were recruited in 1999 have more than 20 years of follow-up within the SUN database used for this study. The project conformed to the guidelines established in the Declaration of Helsinki, and the Human Research Ethical Committee at the University of Navarra approved all the study procedures (091/2008).
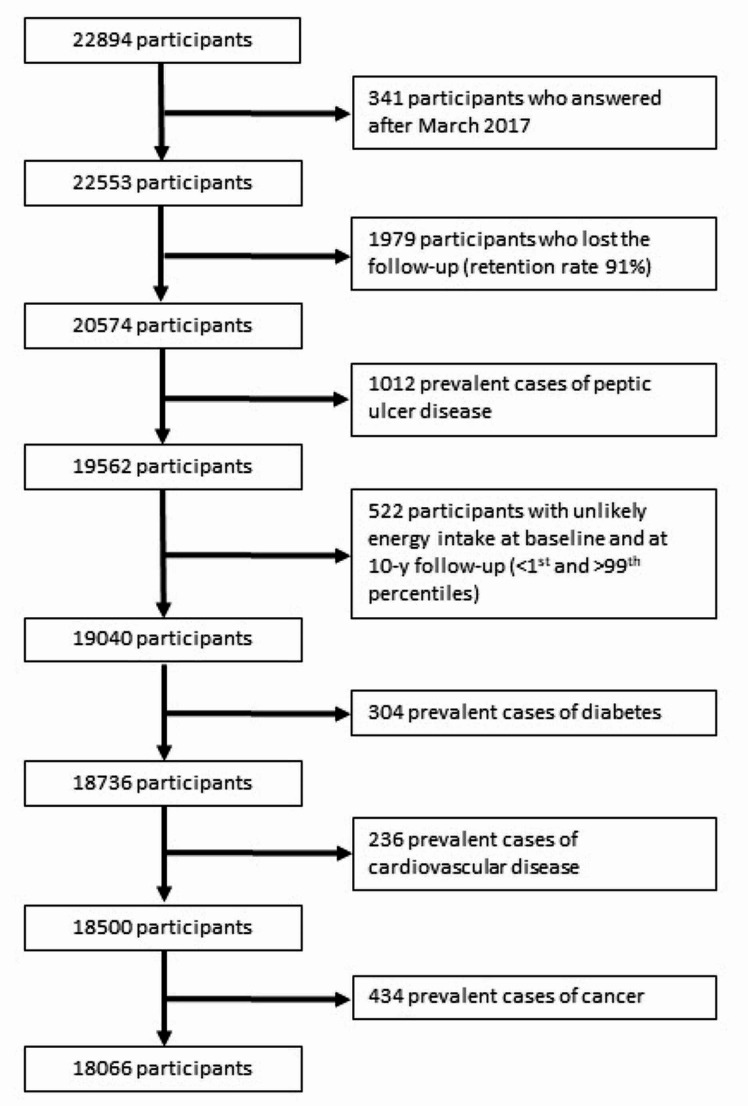
Up to December 2019, 22,894 participants completed the baseline questionnaire. To ensure two years in the cohort, 341 participants who responded the baseline questionnaire after March 2017 were excluded. We further excluded 1979 participants with no follow-up (retention rate 91%), 1012 participants with a history of PUD, 522 participants with unlikely energy intake (< 1st percentile and > 99th percentile), 304 participants with diabetes, 236 prevalent cases of cardiovascular disease, and 434 participants with a prior diagnosis of cancer. The final dataset included 18,066 participants who answered at least 1 follow-up questionnaire (Fig. 1).
Fig. 1.
Flow chart showing the selection process of participants in the SUN project to be included in the present analysis
Exposure Assessment
We assessed dietary habits at baseline and again after 10 years using a validated semi-quantitative food frequency questionnaire consisting of 136-food items [22]. Each food item included a typical portion size. Participants were asked to report the frequency of consumption of each food item by selecting one of 9 frequency options reported in the questionnaire, ranged from never or almost never to more than 6 servings per day. We multiplied the frequency of consumption by the typical portion size to estimate the daily consumption of each food. Foods and beverages were then classified into one of the four categories of the NOVA classification [23]. The UPF group included processed meat, sausages, cookies and pastries, chocolate and candy, breakfast cereals, sweet or salty packaged snacks, margarine, instant soups, pre-prepared pies and pizza dishes, fruit yogurt, carbonated beverages, sweetened milk and fruit drinks, and alcoholic beverages produced by fermentation followed by distillation such as whiskey, gin and rum (a total of 34 items). To estimate the amount of UPF consumed daily, we summed the amount consumed (g/day) of each food item included in the UPF group. We then adjusted the consumption of UPF for the daily energy intake through the residuals method [24]. The use of daily grams of UPF instead of its caloric contribution made it possible to also consider foods that do not provide calories (e.g., calorie-free sweetened beverages). After that, we divided the sample into tertiles according to the total UPF consumption.
Outcome Assessment
Participants reporting a medical diagnosis of PUD in one of the follow-up questionnaires were defined as incident cases of PUD. To validate the self-reported diagnosis of PUD, a subgroup of 139 participants from the SUN cohort was randomly selected (51 reporting PUD and 88 not reporting PUD), and the information reported was compared against the medical history in the clinical records available at the university clinic. A gastroenterologist, blinded to the exposure, handled the comparison. From those who reported a diagnosis of PUD (n = 51), 30 (58.8%; 95% confidence interval [CI], 44.2-72.4% were confirmed through their medical history. From the rest (n = 21), 19 (90.5%, 95% CI: 69.6-98.8%) were diagnosed as gastritis (n = 14), esophagitis (n = 18) and/or epigastric pain (n = 1), and only 2 they did not have any diagnostic related to gastric disease. From the 88 who did not report a diagnosis of PUD, all (100%; 95% CI: 88.4-100%) were confirmed as non-cases of PUD.
Covariates
The baseline questionnaire collected information on sex, age, sociodemographic characteristics, weight and height, smoking, physical activity, and medical history. Self-reported weight and height were previously validated in a subgroup of our cohort [25]. Physical activity was assessed using the Spanish version of the 17-item Harvard Nurses’ Health Study physical activity questionnaire [26]. Leisure time activities were measured in metabolic activity equivalents (METs) per week by assigning habitual energy expenditure to each activity and multiplying by the time spent (in hours per week) on each activity. Total energy and nutrient intake were estimated from food consumption analyzed by the semi-quantitative FFQ using the most up-to-date version of the Food Composition Database for Spain.
Statistical analysis
Continuous variables are reported as median and interquartile range (IQR) because some descriptive variables did not have a Gaussian distribution. Discrete variables are reported as count and percent. A Cox regression model, stratified by smoking and physical activity, was conducted to evaluate the association between tertiles of UPF consumption and PUD risk. The hazard ratio (HR) was calculated using the lowest tertile as the reference category. To control for possible confounders, sex, age (decades), BMI (quartiles), calendar year of recruitment (1999–2001, 2002–2004, 2005–2007, 2008–2010, and from 2011 onwards), health career (yes/no), education (3–4, 5–6, 9 years), marital status (unmarried, married, other, missing), packs of cigarettes (0, 1–12 packs/year, 13–24 packs/year, > 24 packs/year, missing), energy intake (quartiles), known H. pylori infection (yes/no), gastroesophageal reflux (yes/no), hiatal hernia (yes/no), aspirin use (yes/no), NSAIDs use (yes/no), coffee consumption (no, 1–2 cups/day, > 2 cups/day), and alcohol intake from wine and beer (quartiles) were included in the model. Confounders were selected based on biological plausibility and previous causal knowledge on the topic as it is recommended by Hernan et al. [27]. Although we adjusted for a large number of confounding factors, we cannot rule out residual confounding. UPF consumption could also be closely related to other aspects of an unhealthy lifestyle. To assess this aspect, we calculated Vanderweele’s proposed E value [28]. This value represents the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the UPF consumption and the PUD to fully explain away a specific exposure-outcome association, conditional on the measured covariates. The existence of a linear trend between exposure and outcome was assessed by assigning the median value of each tertile and treating the new variable as continuous. To confirm the result, we also ran the Cox regression model by including UPF consumption as a continuous variable (g/day). The linearity of exposure was assessed by the fractional polynomials method. We used repeated measures of dietary intake in order to consider possible changes in the consumption of UPF between baseline and after 10 years of follow-up. We used generalized estimation equations with family binomial and link logit to compute the odds ratio (OR). Only baseline exposure was considered for participants who developed PUD before the 10-year follow-up and those who did not develop PUD but had been in the cohort for less than 10 years. We also represented Nelson-Aalen survival curves, adjusted for potential confounding variables by means of inverse probability weighting methods, to describe the incidence of PUD over time across tertiles of UPF consumption. Sensitivity analyses was also carried out by rerunning the models under different scenarios: (1) changing energy limits, (2) including participants with prevalent diabetes, CVD and cancer, (3) excluding participants taking NSAIDs and aspirin, (4) excluding participants with prevalent gastrointestinal disorders, (5) excluding participants with obesity, (6) excluding participants with an alcohol intake > 25 g/day if woman and > 50 g/day if man, (7) additionally adjusting for sodium intake, (8) additionally adjusting for the adherence to the Mediterranean diet assessed by Trichopoulou score [29]. A p value < 0.05 was considered statistically significant. Statistical analysis was performed by means of STATA program, version 12.0 (StataCorp LP).
Results
A total of 18,066 participants (62.9% women) were included in the final dataset. The main characteristics of the participants are presented in Table 1. Median age at baseline was 35 years (IQR: 27; 45), and median daily consumption of UPF was 260 g/day (IQR: 187; 357), contributing to 28.9% of total energy intake (IQR: 21.0; 36.0). Table S1 shows the percentage contribution of UPF subgroups to the total UPF consumption.
Table 1.
Baseline characteristics of participants according to UPF consumption (n=18,066)
| Tertiles of energy adjusted ultra-processed food consumption | |||||||
|---|---|---|---|---|---|---|---|
| First (≤212 g/day) (n=6022) |
Second (212–318 g/day) (n=6022) |
Third (>318 g/day) (n=6022) |
|||||
| N | % | N | % | N | % | P valueŦ | |
| Women | 4004 | 66.5 | 3847 | 63.9 | 3506 | 58.2 | <0.001 |
| Married | 3591 | 59.6 | 2849 | 47.3 | 2323 | 38.6 | <0.001 |
| Years of university | <0.001 | ||||||
| 3-4 years | 2191 | 36.4 | 2017 | 33.5 | 1911 | 31.7 | |
| 5-6 years | 3231 | 53.7 | 3411 | 56.6 | 3542 | 58.8 | |
| 9 years | 600 | 10 | 594 | 9.9 | 569 | 9.4 | |
| Health career | 4138 | 68.7 | 3812 | 63.3 | 3646 | 60.5 | <0.001 |
| Smoking status | <0.001 | ||||||
| Current | 1128 | 18.7 | 1324 | 22 | 1545 | 25.7 | |
| Former | 1954 | 32.4 | 1640 | 27.2 | 1351 | 22.4 | |
| Taking aspirin | 220 | 3.7 | 182 | 3 | 208 | 3.5 | 0.147 |
| Taking NSAID | 468 | 7.8 | 498 | 8.3 | 540 | 9 | 0.058 |
| Helicobacter pylori infection | 26 | 0.4 | 40 | 0.7 | 28 | 0.5 | 0.159 |
| Hiatal Hernia | 20 | 0.3 | 20 | 0.3 | 23 | 0.4 | 0.866 |
| Gastroesophageal reflux | 13 | 0.2 | 5 | 0.1 | 5 | 0.1 | 0.062 |
| Median | IQR | Median | IQR | Median | IQR | P value* | |
| Age (years) | 41 | 31; 50 | 34 | 27; 44 | 31 | 26; 39 | <0.001 |
| BMI (kg/m2) | 22.9 | 20.8; 25.3 | 22.8 | 20.7; 25.3 | 22.9 | 20.8; 25.5 | 0.268 |
| Physical activity (METs/week) | 17.6 | 6.7; 32.4 | 15.2 | 5.6; 28.9 | 15.0 | 4.3; 30.0 | <0.001 |
| Energy (kcal/day) | 2549 | 2122; 3071 | 2220 | 1802; 2718 | 2452 | 1996; 3025 | <0.001 |
| Macronutrients intake (% energy) | |||||||
| Carbohydrate | 44.5 | 39.4; 49.4 | 42.8 | 38.3; 47.2 | 43.4 | 38.9; 47.7 | <0.001 |
| Protein | 17.9 | 16.0; 20.1 | 18.1 | 16.2; 20.2 | 17.2 | 15.4; 19.2 | <0.001 |
| Lipid | 35.5 | 30.9; 39.9 | 37.0 | 33.2; 40.9 | 37.5 | 33.6; 41.1 | <0.001 |
| SFA | 11.4 | 9.4; 13.4 | 12.6 | 10.8; 14.5 | 13.1 | 11.3; 15.0 | <0.001 |
| MUFA | 15.3 | 12.9; 18.0 | 15.5 | 13.5; 17.7 | 15.4 | 13.6; 17.5 | 0.030 |
| PUFA | 4.7 | 3.9; 5.6 | 5.0 | 4.2; 6.1 | 5.3 | 4.3; 6.4 | <0.001 |
| Alcohol | 0.8 | 0.2; 2.4 | 0.9 | 0.3; 2.5 | 0.9 | 0.2; 2.5 | <0.001 |
| Alcohol consumption (g/day) | 2.7 | 0.6; 8.8 | 2.8 | 1.0; 8.1 | 3.3 | 1.0; 9.1 | <0.001 |
| Fiber (g/day) | 32.8 | 25.5; 43.3 | 24.6 | 19.1; 31.8 | 23.7 | 17.9; 31.2 | <0.001 |
| Micronutrients intake | |||||||
| Sodium (mg/day) | 2771 | 2097; 3660 | 2763 | 2059; 3766 | 3523 | 2503; 4952 | <0.001 |
| Vit. A (μg/day) | 2213 | 1408; 3253 | 1495 | 1065; 2370 | 1364 | 956; 2214 | <0.001 |
| Vit. C (mg/day) | 324 | 227; 444 | 238 | 169; 332 | 224 | 158; 318 | <0.001 |
| Vit. E (μg/day) | 7.1 | 5.3; 10.1 | 5.9 | 4.4; 8.2 | 6.3 | 4.7; 8.7 | <0.001 |
| Food consumption (servings/day) | |||||||
| Olive oil (g/day) | 25.0 | 10.7; 29.5 | 12.3 | 8.7; 25.8 | 11.8 | 8.0; 25.2 | <0.001 |
| Vegetables | 2.6 | 1.8; 3.6 | 1.9 | 1.3; 2.8 | 1.8 | 1.2; 2.6 | <0.001 |
| Fruit | 2.8 | 1.7; 4.3 | 1.8 | 1.1; 2.8 | 1.6 | 0.9; 2.6 | <0.001 |
| Red meat | 0.5 | 0.3; 0.7 | 0.5 | 0.3; 0.7 | 0.5 | 0.3; 0.7 | 0.002 |
| Processed meat | 1.7 | 1.2; 2.3 | 1.8 | 1.3; 2.3 | 2.0 | 1.5; 2.7 | <0.001 |
| Sugar sweetened beverages | 0.1 | 0.0; 0.1 | 0.1 | 0.1; 0.3 | 0.4 | 0.1; 0.9 | <0.001 |
| High-fat dairy products | 1.4 | 0.6; 2.6 | 1.3 | 0.7; 2.2 | 1.5 | 0.8; 2.4 | <0.001 |
| Coffee consumption | 1 | 0; 3 | 1 | 0; 3 | 1 | 0; 3 | <0.001 |
| Ultraprocessed food (servings/day) | 2.5 | 1.9; 3.2 | 3.7 | 3.2; 4.3 | 4.9 | 4.2; 6.0 | <0.001 |
| Ultraprocessed food (g/day) | 157.0 | 108.8; 186.9 | 259.8 | 235.4; 286.7 | 410.6 | 357.3; 506.5 | <0.001 |
| Ultraprocessed food/energy (%) | 20.3 | 14.9; 25.7 | 29.2 | 23.8; 34.8 | 36.5 | 30.0; 43.2 | <0.001 |
Abbreviations IQR, interquartile range; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids, PUFA, polyunsaturated fatty acids; NSAID, nonsteroidal anti-inflammatory drugs
Ŧ Chi-square test
* Kruskal Wallis test
During a median follow-up of 12.2 years, we recorded 322 new cases of PUD (1.56 cases/1000 person-years). The association between tertiles of UPF consumption and risk of PUD is reported in Table 2. Participants in the highest tertile of UPF consumption had a 52% increased relative risk of PUD compared to participants in the lowest tertile (HR = 1.52, 95%CI: 1.15, 2.00), with a significant dose-response relationship (Ptrend=0.002). A linear association between the UPF consumption and the occurrence of PUD was also confirmed when we included in the Cox regression model the exposure as a continuous variable. We observed a 7% increase in relative risk for every 100 g/day of UPF consumed (HR = 1.07, 95% CI: 1.03, 1.12). Furthermore, when we accounted for changes in UPF consumption (repeated-measures analysis), using the updated data on food consumption after 10 years of follow-up, the association remained statistically significant. Compared with participants in the lowest tertile, those in the highest tertile had a 39% increased risk of PUD (OR = 1.39, 95% CI: 1.03, 1.87; Ptrend = 0.001).
Table 2.
Association between consumption of ultra-processed foods and risk of peptic ulcer
| Tertiles of energy-adjusted UPF consumption | ||||||
|---|---|---|---|---|---|---|
| First (≤212 g/day) |
Second (212–318 g/day) |
Third (>318 g/day) |
P for trend | HR (95% CI) | P value | |
| No. of cases/no. of person-y | 102/69,206 | 94/69,414 | 126/68,218 | |||
| No. of cases/no. of participants | 102/6022 | 94/6022 | 126/6022 | |||
| Crude model | ref. | 1.01 (0.76; 1.35) | 1.47 (1.12; 1.92) | 0.003 | ||
| Multivariable model | ref. | 1.09 (0.81; 1.47) | 1.52 (1.15; 2.00) | 0.002 | ||
| Multivariable model (without BMI) | ref. | 1.10 (0.82; 1.47) | 1.51 (1.15; 2.00) | 0.002 | ||
| Multivariable model (without energy intake) | ref. | 1.05 (0.78; 1.40) | 1.49 (1.13; 1.96) | 0.003 | ||
| Multivariable model (linear, 100 g/day) | 1.07 (1.03; 1.12) | 0.001 | ||||
| Repeated measure multivariable model | ref. | 1.07 (0.79; 1.47) | 1.39 (1.03; 1.87) | 0.001 | ||
Multivariable model: adjusted for sex, age (decades), bmi (quartiles), calendar year of recruitment (1999–2001, 2002–2004, 2005–2007, 2008–2010, and from 2011 onwards), health career (yes/no), education (3-4, 5-6, 9 years), marital status (unmarried, married, other, missing), packs of cigarettes (0, 1-12 packs/year, 13-24 packs/year, >24 packs/year, missing), energy intake (quartiles), known Helicobacter pylori infection (yes/no), gastroesophageal reflux (yes/no), hiatal hernia (yes/no), aspirin (yes/no), analgesics (yes/no), coffee (no, 1-2 cups/day, >2 cups/day), alcohol from wine and beer (quartile), and stratified for smoking (categories) and physical activity (quartiles)
The observed HR of 1.52 in our analysis could hypothetically be explained by the presence of an unmeasured confounder associated with UPF consumption and PUD with a HR of 1.78-fold each, beyond the measured confounders, but a weaker confounder could not. Similarly, the lowest confidence interval could be moved to include the null by an unmeasured confounder associated with both UPF consumption and PUD by a HR of 1.36-fold each, above and beyond the measured confounders, but weaker confounding could not do so.
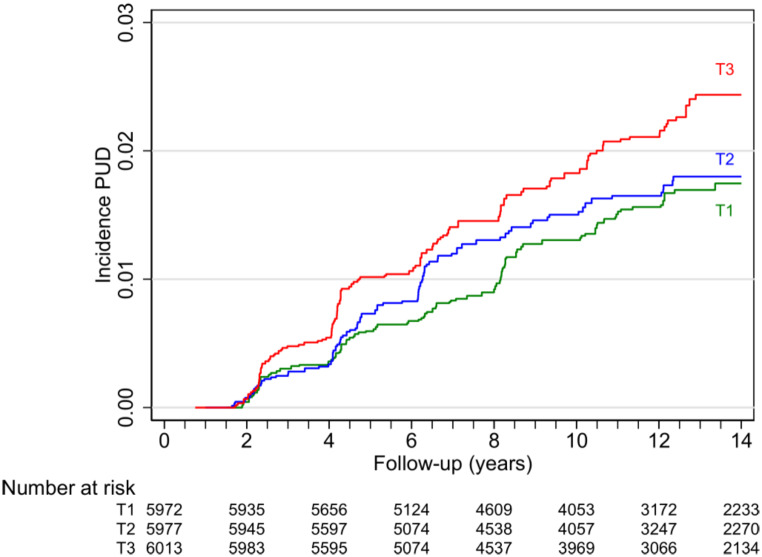
Figure 2 shows the cumulative risk for developing PUD over time across tertiles of UPF consumption. The highest tertile of UPF consumption was associated with higher incidence of PUD.
Fig. 2.
Nelson-Aalen estimate of the incidence of peptic ulcer disease according to tertiles of ultra-processed consumption
We also performed a sensitivity analysis to test the robustness of our results, but we did not observe any substantial change in the magnitude of the association in any of the examined scenarios (Table 3).
Table 3.
Sensitivity analysis
| Tertiles of energy-adjusted UPF consumption | |||||
|---|---|---|---|---|---|
| No. of cases/no. of person-y | T1 | T2 | T3 | P for trend | |
| Overall results | 322/206,837 | 1 (ref.) | 1.09 (0.81; 1.47) | 1.52 (1.15; 2.00) | 0.002 |
| Willett’s energy limits (<800 kcal/d or >4000 kcal/d in men and <500 kcal/d or >3500 kcal/d in women) | 298/192,450 | 1 (ref.) | 0.99 (0.73; 1.35) | 1.44 (1.08; 1.91) | 0.008 |
| Including participants with prevalent diabetes, CVD or cancer | 341/217,437 | 1 (ref.) | 1.06 (0.80; 1.41) | 1.47 (1.17; 1.92) | 0.003 |
| Excluding participants taking aspirin and analgesics | 271/183,571 | 1 (ref.) | 1.09 (0.79; 1.50) | 1.57 (1.17; 2.13) | 0.003 |
| Excluding participants with prevalent gastrointestinal disorders | 316/204,812 | 1 (ref.) | 1.07 (0.80; 1.45) | 1.48 (1.12; 1.95) | 0.004 |
| Excluding participants with obesity | 303/198,598 | 1 (ref.) | 1.12 (0.82; 1.52) | 1.61 (1.21; 2.15) | 0.001 |
| Excluding participants with alcohol consumption >25 g/d for women or >50 g/d for men | 314/203,550 | 1 (ref.) | 1.12 (0.83; 1.50) | 1.51 (1.14; 2.00) | 0.003 |
| Additionally adjusted for sodium intake | 322/206,837 | 1 (ref.) | 1.10 (0.82; 1.48) | 1.55 (1.16; 2.06) | 0.002 |
| Additionally adjusted for Mediterranean diet | 322/206,837 | 1 (ref.) | 1.08 (0.80; 1.46) | 1.50 (1.13; 1.99) | 0.003 |
Multivariable model: adjusted for sex, age (decades), bmi (quartiles), calendar year of recruitment (1999–2001, 2002–2004, 2005–2007, 2008–2010, and from 2011 onwards), health career (yes/no), education (3-4, 5-6, 9 years), marital status (unmarried, married, other, missing), packs of cigarettes (0, 1-12 packs/year, 13-24 packs/year, >24 packs/year, missing), energy intake (quartiles), Helicobacter infection (yes/no), gastroesophageal reflux (yes/no), hiatal hernia (yes/no), aspirin (yes/no), analgesics (yes/no), coffee (no, 1-2 cups/day, >2 cups/day), alcohol from wine and beer (quartile), and stratified for smoking (categories) and physical activity (quartiles)
Discussion
In this prospective cohort study, we found that a higher consumption of UPF was associated with the risk of incident PUD. This result remained consistent in the sensitivity analysis even when excluding participants taking NSAIDs and aspirin and with gastrointestinal disorders, including a known H. pylori infection. However, it should be kept in mind that H. pylori infection is often asymptomatic. Therefore, it is highly likely that many participants did not know they were infected. The further adjustment for the adherence to the Mediterranean diet, dietary pattern rich in unsaturated fatty acids, fiber, vitamins and minerals and antioxidants, and low in salt, did not affect the result. Thus, our findings support the hypothesis that the consumption of UPF could be an environmental factor that increases the risk of PUD. Recent systematic reviews and meta-analyses reported an increased risk of functional gastrointestinal disorders or diseases, including dyspepsia, irritable bowel syndrome [13], and Crohn’s disease [30], associated with higher consumption of UPF. Furthermore, a recent case-control study showed the consumption of UPF associated with a higher risk of stomach adenocarcinoma [31]. However, to the best of our knowledge, this is the first study investigating the association between UPF consumption and PUD.
Several mechanisms could explain the relationship between UPF and PUD. Recent meta-analysis reported that a higher UPF consumption contributed to increase dietary intake of salt and refined carbohydrates, and to reduce the intakes of fiber, vitamins, and antioxidants [32]. Our data confirmed these findings, also showing higher alcohol intake among those who consumed higher amounts of UPF, presumably due to higher consumption of distilled spirits and liquor (alcoholic beverages that fall under the definition of UPF). Epidemiological studies have reported these components in the diet to be positive or negative associated with PUD risk. In addition, results from animal studies have corroborated these findings, providing evidence on possible mechanisms. In particular, some diet components may undermine the integrity of the mucosal barrier, resulting in inflammation and damage, and subsequently, erosion of the gastric mucosa. The mucosal damage could enhance H. pylori colonization and the presence of certain nutrients in the gastric lumen may influence the expression of H. pylori virulence factors associated with the development of PUD and other gastroduodenal diseases [33]. In vivo studies shown salt to alter the viscosity and composition of the protective mucosal barrier [34, 35], potentially exposing the mucosa to the toxic effects of acid and intragastric enzymes, resulting in mucosal damage and inflammation [36]. Moreover, salt has been also shown to facilitate H. pylori colonization both in human and animals [37–39] and increase gene expression of virulence factors which resulted in more virulent bacterial strains [40]. Prospective and geographical studies have confirmed the role of salt, documenting an increased risk of gastric ulcer associated with higher salt intake [20, 41]. Other prospective studies reported the intakes of refined carbohydrates and fiber, especially soluble fiber from fruits, vegetables, and legumes, associated with higher and lower risk of duodenal ulcer, respectively [18, 19]. These findings were corroborated by additional studies showing an increased risk of H. pylori infection associated with carbohydrate/sugar intake and diet glycemic index [42, 43]. Moreover, in patients with duodenal ulcer, it has been observed that the liquid phase of a meal is emptied more rapidly into the duodenum, compared with controls [35]. A rapid rate of gastric emptying in the presence of gastric hypersecretion may play an important role in the pathogenesis of duodenal ulcer. Dietary fiber might delay the gastric emptying explaining its apparent benefit. The relationship between alcohol intake and PUD risk is uncertain. However, alcohol is known to dose-dependently damage the gastric mucosa through numerous mechanisms, including alterations in epithelial transport, disruption of the intercellular junction and mucosal barrier, which allow hydrogen ions to penetrate the mucosa [44]. Moreover, ethanol activates an inflammatory reaction that also participates in gastric mucosal damage [45]. Histological studies indicate that after ethanol administration in concentrations comparable to distillates and spirits (20% and 40% ethanol), the mucosal layer and mucin content of the lining epithelial cells decreased significantly. In addition, the presence of acids aggravated the injury and induced bleeding. The restoration of mucosal damage was completed in 24 h [46]. Alcoholic beverages such as wine and beer may be less harmful to the gastroduodenal mucosa both for lower ethanol content and for the presence of polyphenols, which have shown anti-ulcer effects such as reducing acid secretion, inhibiting pepsin level and activity, and increasing gastric mucus and bicarbonate secretion, as well as enhancing cytoprotective, antioxidative, anti-inflammatory, and antibacterial mucosal defenses against peptic ulcer [47]. Finally, it has been suggested that some vitamins, whose intakes were lower in participants consuming larger amounts of UPF, may protect against PUD through several mechanisms. In animal models, vitamin A increased gastric and duodenal mucus production. Moreover, dietary supplementation of vitamin A reduced the incidence of stress- and aspirin-induced ulcers in rats [48]. Vitamin E, particularly tocopherol and tocotrienol, conferred its protection against ulcerogenic factors/agents mainly through its antioxidant and anti-inflammatory mechanisms [49]. In addition, vitamin C attenuated the oxidative damage induced by NSAIDs and H. pylori to the gastric mucosa [50, 51]. Note, however, that so far only vitamin A intake has been found to be associated with a lower risk of duodenal ulcer in humans [18].
We are well aware that our study is not free of limitations. First, the ulcer diagnosis was self-reported and validation study showed partial validity. Therefore, we have to acknowledge the existence of some misclassification, taking in mind that some of self-reported diagnoses of PUD are gastritis or esophagitis. Nevertheless, the plausible biological mechanisms explained before can be applied as well. Second, we had no information on ulcer location and therefore could not investigate the impact of UPF on gastric and duodenal ulcer risk separately. Third, we had no information on the use of proton pump inhibitors that may have influenced the risk of PUD. However, we controlled the analysis for gastrointestinal disorders, like hiatal hernia and gastroesophageal reflux, that generally require the use of proton pump inhibitors. Fourth, like any FFQ, the one used in this study has the limitation of investigating the consumption of only the foods listed. We are aware that consumption was not specifically required for all commercially available UPF. This may have led to an underestimation of exposure. Nevertheless, this is a validated FFQ investigating a large number of food items (n = 136). Fifth, SUN cohort involves mainly graduate participants, limiting the generalizability of our results. Sixth, since this was a cohort of Spanish graduates, we can assume that almost all participants were Caucasian, and therefore these results cannot be transferred to other ethnicities without prior confirmation. Finally, as in any observational study, we cannot rule out residual confounding. However, adjusted for a wide range of potential confounders using different statistical methods, and the results were consistent. In addition, the E-values for the point estimate supported the observed association. The point estimate could be theoretically explained only by an unmeasured confounder with a hazard ratio of at least 1.78-fold for PUD and for ultra-processed food consumption.
Our study has several strengths. First, the study addresses a topic not previously covered. Second, the study prospective nature, as the assessment of participants’ dietary habits was carried out before the onset of the disease, which reflects the optimal temporal relation between exposure and disease occurrence. Third, the repeated measurements of exposure variable allowed to take into consideration dietary changes occurred overtime. Fourth, we controlled our analysis for NSAID and aspirin use and for the presence of gastroduodenal disorders, including known H. pylori infection, risk factors for PUD. However, as mentioned earlier, it is likely that many participants did not know they were infected due to lack of symptoms. Therefore, it is not possible to extrapolate detailed information about the underlying mechanisms. Fifth, the high cohort response rate. Sixth, the high educational level of participants, which may have facilitated better understanding of the food frequency questionnaire. Seventh, the food frequency questionnaire used to assess dietary habits has been repeatedly validated [52, 53].
In conclusion, our data suggest that the consumption of UPF may be associated with the risk of PUD and other gastric disorders. Given the importance of the topic - individuals with PUD have an increased risk of developing gastric cancer [54] - further studies confirming our findings are strongly requested. Nevertheless, UPF consumption should still be discouraged because of the known negative associations with health status.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the SUN participants for their continued participation and collaboration with the project.
Author contributions
Conceptualization, A.L. and M.B.-R.; methodology, A.L.; validation, C.DLF-A., M.V., and M.B.-R.; formal analysis, A.L.; writing—original draft preparation, A.L.; writing—review and editing, A.L., C.DLF-A., M.V., C.S.-O., M.Á.M.-G., F.M., and M.B.-R.; supervision, M.B.-R.; funding acquisition, M.Á.M.-G. and M.B.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This research was funded by the Spanish Government—Instituto de Salud Carlos III with the co-funding from the European Union, grants: PI20/00564, and PI23/01332; and the Centro de Investigacion Biomedica en Fisiopatología de la Obesidad y Nutrición [CIBERobn, group: CB12/03/30017].
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Declarations
Ethical approval
The project conformed to the guidelines established in the Declaration of Helsinki, and the Human Research Ethical Committee at the University of Navarra approved all the study procedures (091/2008). Voluntary completion of the first questionnaire was considered to imply informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Gibney MJ (2019) Ultra-processed foods: definitions and Policy issues. Curr Dev Nutr 3(2):nzy077. 10.1093/cdn/nzy077 10.1093/cdn/nzy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauber F, Steele EM, Louzada MLC, Millett C, Monteiro CA, Levy RB (2020) Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008–2016). PLoS ONE 15(5):e0232676–e0232676. 10.1371/journal.pone.0232676 10.1371/journal.pone.0232676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA (2016) Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open 6(3):e009892. 10.1136/bmjopen-2015-009892 10.1136/bmjopen-2015-009892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moubarac JC, Batal M, Louzada ML, Martinez Steele E, Monteiro CA (2017) Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 108:512–520. 10.1016/j.appet.2016.11.006 10.1016/j.appet.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 5.Rauber F, da Costa Louzada ML, Steele EM, Millett C, Monteiro CA, Levy RB (2018) Ultra-processed Food Consumption and chronic non-communicable diseases-related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 10(5). 10.3390/nu10050587 [DOI] [PMC free article] [PubMed]
- 6.Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, Bes-Rastrollo M (2016) Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 104(5):1433–1440. 10.3945/ajcn.116.135004 10.3945/ajcn.116.135004 [DOI] [PubMed] [Google Scholar]
- 7.Rauber F, Chang K, Vamos EP, da Costa Louzada ML, Monteiro CA, Millett C, Levy RB (2021) Â Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr 60(4):2169–2180. 10.1007/s00394-020-02367-1 10.1007/s00394-020-02367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mambrini SP, Menichetti F, Ravella S, Pellizzari M, De Amicis R, Foppiani A, Battezzati A, Bertoli S, Leone A (2023) Ultra-processed Food Consumption and Incidence of Obesity and cardiometabolic risk factors in adults: a systematic review of prospective studies. Nutrients 15(11). 10.3390/nu15112583 [DOI] [PMC free article] [PubMed]
- 9.Llavero-Valero M, Escalada-San Martín J, Martínez-González MA, Basterra-Gortari FJ, de la Fuente-Arrillaga C, Bes-Rastrollo M (2021) Ultra-processed foods and type-2 diabetes risk in the SUN project: a prospective cohort study. Clin Nutr 40(5):2817–2824. 10.1016/j.clnu.2021.03.039 10.1016/j.clnu.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 10.Leone A, Martínez-González M, Craig W, Fresán U, Gómez-Donoso C, Bes-Rastrollo M (2021) Pre-gestational consumption of Ultra-processed Foods and Risk of Gestational Diabetes in a Mediterranean Cohort. SUN Project Nutrients 13(7). 10.3390/nu13072202 [DOI] [PMC free article] [PubMed]
- 11.Srour B, Fezeu LK, Kesse-Guyot E, Alles B, Mejean C, Andrianasolo RM, Chazelas E, Deschasaux M, Hercberg S, Galan P, Monteiro CA, Julia C, Touvier M (2019) Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante). BMJ 365:l1451. 10.1136/bmj.l1451 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N (2021) Ultra-processed Foods and Incident Cardiovascular Disease in the Framingham offspring study. J Am Coll Cardiol 77(12):1520–1531. 10.1016/j.jacc.2021.01.047 10.1016/j.jacc.2021.01.047 [DOI] [PubMed] [Google Scholar]
- 13.Lane MM, Davis JA, Beattie S, Gómez-Donoso C, Loughman A, O’Neil A, Jacka F, Berk M, Page R, Marx W, Rocks T (2021) Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev 22(3):e13146. 10.1111/obr.13146 10.1111/obr.13146 [DOI] [PubMed] [Google Scholar]
- 14.Schnabel L, Buscail C, Sabate JM, Bouchoucha M, Kesse-Guyot E, Allès B, Touvier M, Monteiro CA, Hercberg S, Benamouzig R, Julia C (2018) Association between Ultra-processed Food Consumption and Functional Gastrointestinal disorders: results from the French NutriNet-Santé cohort. Am J Gastroenterol 113(8):1217–1228. 10.1038/s41395-018-0137-1 10.1038/s41395-018-0137-1 [DOI] [PubMed] [Google Scholar]
- 15.Lo CH, Khandpur N, Rossato SL, Lochhead P, Lopes EW, Burke KE, Richter JM, Song M, Ardisson Korat AV, Sun Q, Fung TT, Khalili H, Chan AT, Ananthakrishnan AN (2022) Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: a prospective cohort study. Clin Gastroenterol Hepatol 20(6):e1323–e1337. 10.1016/j.cgh.2021.08.031 10.1016/j.cgh.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narula N, Wong ECL, Dehghan M, Mente A, Rangarajan S, Lanas F, Lopez-Jaramillo P, Rohatgi P, Lakshmi PVM, Varma RP, Orlandini A, Avezum A, Wielgosz A, Poirier P, Almadi MA, Altuntas Y, Ng KK, Chifamba J, Yeates K, Puoane T, Khatib R, Yusuf R, Boström KB, Zatonska K, Iqbal R, Weida L, Yibing Z, Sidong L, Dans A, Yusufali A, Mohammadifard N, Marshall JK, Moayyedi P, Reinisch W, Yusuf S (2021) Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ 374:n1554. 10.1136/bmj.n1554 10.1136/bmj.n1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loscalzo J, Fauci AS, Kasper DL, Hauser S, Longo DL, Jameson JL (2022) Harrison’s principles of internal medicine. Principles of internal medicine, Twenty First edition. edn. New York
- 18.Aldoori WH, Giovannucci EL, Stampfer MJ, Rimm EB, Wing AL, Willett WC (1997) Prospective study of diet and the risk of duodenal ulcer in men. Am J Epidemiol 145(1):42–50. 10.1093/oxfordjournals.aje.a009030 10.1093/oxfordjournals.aje.a009030 [DOI] [PubMed] [Google Scholar]
- 19.Katschinski BD, Logan RF, Edmond M, Langman MJ (1990) Duodenal ulcer and refined carbohydrate intake: a case-control study assessing dietary fibre and refined sugar intake. Gut 31(9):993–996. 10.1136/gut.31.9.993 10.1136/gut.31.9.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato I, Nomura AM, Stemmermann GN, Chyou PH (1992) A prospective study of gastric and duodenal ulcer and its relation to smoking, alcohol, and diet. Am J Epidemiol 135(5):521–530. 10.1093/oxfordjournals.aje.a116319 10.1093/oxfordjournals.aje.a116319 [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Gonzalez MA (2006) The SUN cohort study (Seguimiento University of Navarra). Public Health Nutr 9(1a):127–131 10.1079/PHN2005935 [DOI] [PubMed] [Google Scholar]
- 22.Martin-Moreno JM, Boyle P, Gorgojo L, Maisonneuve P, Fernandez-Rodriguez JC, Salvini S, Willett WC (1993) Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 22(3):512–519. 10.1093/ije/22.3.512 10.1093/ije/22.3.512 [DOI] [PubMed] [Google Scholar]
- 23.Monteiro CA, Cannon G, Levy R, Moubarac J-C, Jaime P, Martins AP, Canella D, Louzada M, Parra D (2016) NOVA. The star shines bright. World Nutr 7(1–3):28–38 [Google Scholar]
- 24.Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65 (4 Suppl):1220S-1228S; discussion 1229S-1231S. 10.1093/ajcn/65.4.1220S [DOI] [PubMed]
- 25.Bes-Rastrollo M, Pérez Valdivieso JR, Sánchez-Villegas A, Alonso A, Martínez-González MA (2005) Validación Del peso e índice de masa corporal auto-declarados de Los participantes de una cohorte de graduados universitarios. Rev Esp Obes 3(6):183–189 [Google Scholar]
- 26.Martinez-Gonzalez MA, Lopez-Fontana C, Varo JJ, Sanchez-Villegas A, Martinez JA (2005) Validation of the Spanish version of the physical activity questionnaire used in the nurses’ Health Study and the Health professionals’ follow-up study. Public Health Nutr 8(7):920–927 10.1079/PHN2005745 [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA (2002) Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155(2):176–184. 10.1093/aje/155.2.176 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Ding P (2017) Sensitivity analysis in Observational Research: introducing the E-Value. Ann Intern Med 167(4):268–274. 10.7326/m16-2607 10.7326/m16-2607 [DOI] [PubMed] [Google Scholar]
- 29.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D (2003) Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348(26):2599–2608. 10.1056/NEJMoa025039 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 30.Narula N, Chang NH, Mohammad D, Wong ECL, Ananthakrishnan AN, Chan SSM, Carbonnel F, Meyer A (2023) Food Processing and Risk of Inflammatory Bowel Disease: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2023.01.012 10.1016/j.cgh.2023.01.012 [DOI] [PubMed] [Google Scholar]
- 31.Peres SV, Silva DRM, Coimbra FJF, Fagundes MA, Auzier JJN, Pelosof AG, Araujo MS, Assumpção PP, Curado MP (2022) Consumption of processed and ultra-processed foods by patients with stomach adenocarcinoma: a multicentric case-control study in the Amazon and southeast regions of Brazil. Cancer Causes Control 33(6):889–898. 10.1007/s10552-022-01567-w 10.1007/s10552-022-01567-w [DOI] [PubMed] [Google Scholar]
- 32.Martini D, Godos J, Bonaccio M, Vitaglione P, Grosso G (2021) Ultra-processed Foods and Nutritional Dietary Profile: a Meta-analysis of nationally Representative Samples. Nutrients 13(10). 10.3390/nu13103390 [DOI] [PMC free article] [PubMed]
- 33.Chang WL, Yeh YC, Sheu BS (2018) The impacts of H. Pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci 25(1):68. 10.1186/s12929-018-0466-9 10.1186/s12929-018-0466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, Katsuyama T, Asaka M, Tatematsu M (2006) High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer 119(7):1558–1566. 10.1002/ijc.21810 10.1002/ijc.21810 [DOI] [PubMed] [Google Scholar]
- 35.Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, Shirai T (1975) Effects in rats of sodium chloride on experimental gastric cancers induced by N-methyl-N-nitro-N-nitrosoguanidine or 4-nitroquinoline-1-oxide. J Natl Cancer Inst 55(1):101–106. 10.1093/jnci/55.1.101 10.1093/jnci/55.1.101 [DOI] [PubMed] [Google Scholar]
- 36.Takahashi M, Hasegawa R (1985) Enhancing effects of dietary salt on both initiation and promotion stages of rat gastric carcinogenesis. Princess Takamatsu Symp 16:169–182 [PubMed] [Google Scholar]
- 37.Tsugane S, Tei Y, Takahashi T, Watanabe S, Sugano K (1994) Salty food intake and risk of Helicobacter pylori infection. Jpn J Cancer Res 85(5):474–478. 10.1111/j.1349-7006.1994.tb02382.x 10.1111/j.1349-7006.1994.tb02382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beevers DG, Lip GY, Blann AD (2004) Salt intake and Helicobacter pylori infection. J Hypertens 22(8):1475–1477. 10.1097/01.hjh.0000133736.77866.77 10.1097/01.hjh.0000133736.77866.77 [DOI] [PubMed] [Google Scholar]
- 39.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC (1999) High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res 59(19):4823–4828 [PubMed] [Google Scholar]
- 40.Gancz H, Jones KR, Merrell DS (2008) Sodium chloride affects Helicobacter pylori growth and gene expression. J Bacteriol 190(11):4100–4105. 10.1128/jb.01728-07 10.1128/jb.01728-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stemmermann G, Haenszel W, Locke F (1977) Epidemiologic pathology of gastric ulcer and gastric carcinoma among Japanese in Hawaii. J Natl Cancer Inst 58(1):13–20. 10.1093/jnci/58.1.13 10.1093/jnci/58.1.13 [DOI] [PubMed] [Google Scholar]
- 42.Sohouli MH, Haghshenas N, Pouladi F, Sayyari A, Olang B, Găman MA, Kord-Varkaneh H, Fatahi S (2021) Association between glycemic index and Helicobacter pylori infection risk among adults: a case-control study. Nutrition 83:111069. 10.1016/j.nut.2020.111069 10.1016/j.nut.2020.111069 [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Meng G, Zhang Q, Liu L, Wu H, Shi H, Bao X, Su Q, Gu Y, Fang L, Yu F, Yang H, Yu B, Sun S, Wang X, Zhou M, Jia Q, Zhao H, Song K, Niu K (2016) Dietary patterns are Associated with Helicobacter Pylori infection in Chinese adults: a cross-sectional study. Sci Rep 6:32334. 10.1038/srep32334 10.1038/srep32334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bor S, Bor-Caymaz C, Tobey NA, Abdulnour-Nakhoul S, Orlando RC (1999) Esophageal exposure to ethanol increases risk of acid damage in rabbit esophagus. Dig Dis Sci 44(2):290–300. 10.1023/a:1026646215879 10.1023/a:1026646215879 [DOI] [PubMed] [Google Scholar]
- 45.Gasbarrini A, D’Aversa F, Di Rienzo T, Franceschi F (2014) Nutrients affecting gastric barrier. Dig Dis 32(3):243–248. 10.1159/000357856 10.1159/000357856 [DOI] [PubMed] [Google Scholar]
- 46.Dinoso VP, Chey WY, Siplet H, Lorber SH (1970) Effects of ethanol on the gastric mucosa of the Heidenhain pouch of dogs. Am J Dig Dis 15(9):809–817. 10.1007/bf02236042 10.1007/bf02236042 [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Lian Y, Li Q, Sun L, Chen R, Lai X, Lai Z, Yuan E, Sun S (2020) Preventative and therapeutic potential of flavonoids in peptic ulcers. Molecules 25(20). 10.3390/molecules25204626 [DOI] [PMC free article] [PubMed]
- 48.Mahmood T, Tenenbaum S, Niu XT, Levenson SM, Seifter E, Demetriou AA (1986) Prevention of duodenal ulcer formation in the rat by dietary vitamin a supplementation. JPEN J Parenter Enter Nutr 10(1):74–77. 10.1177/014860718601000174 10.1177/014860718601000174 [DOI] [PubMed] [Google Scholar]
- 49.Kamisah Y, Qodriyah HM, Chua KH, Nur Azlina MF (2014) Vitamin E: a potential therapy for gastric mucosal injury. Pharm Biol 52(12):1591–1597. 10.3109/13880209.2014.902082 10.3109/13880209.2014.902082 [DOI] [PubMed] [Google Scholar]
- 50.Koc M, Imik H, Odabasoglu F (2008) Gastroprotective and anti-oxidative properties of ascorbic acid on indomethacin-induced gastric injuries in rats. Biol Trace Elem Res 126(1–3):222–236. 10.1007/s12011-008-8205-9 10.1007/s12011-008-8205-9 [DOI] [PubMed] [Google Scholar]
- 51.Shi LQ, Zheng RL (2006) DNA damage and oxidative stress induced by Helicobacter pylori in gastric epithelial cells: protection by vitamin C and sodium selenite. Pharmazie 61(7):631–637 [PubMed] [Google Scholar]
- 52.de la Fuente-Arrillaga C, Ruiz ZV, Bes-Rastrollo M, Sampson L, Martinez-Gonzalez MA (2010) Reproducibility of an FFQ validated in Spain. Public Health Nutr 13(9):1364–1372. 10.1017/s1368980009993065 10.1017/s1368980009993065 [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Ballart JD, Pinol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez-Bauer M, Martinez-Gonzalez MA, Salas-Salvado J, Martin-Moreno JM (2010) Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 103(12):1808–1816. 10.1017/s0007114509993837 10.1017/s0007114509993837 [DOI] [PubMed] [Google Scholar]
- 54.Paragomi P, Dabo B, Pelucchi C, Bonzi R, Bako AT, Sanusi NM, Nguyen QH, Zhang ZF, Palli D, Ferraroni M, Vu KT, Yu GP, Turati F, Zaridze D, Maximovitch D, Hu J, Mu L, Boccia S, Pastorino R, Tsugane S, Hidaka A, Kurtz RC, Lagiou A, Lagiou P, Camargo MC, Curado MP, Lunet N, Vioque J, Boffetta P, Negri E, La Vecchia C, Luu HN (2022) The Association between Peptic Ulcer Disease and gastric Cancer: results from the stomach Cancer Pooling (StoP) Project Consortium. Cancers (Basel) 14(19). 10.3390/cancers14194905 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.