Abstract
This work uses response surface methodology (RSM) to study the co-cultivation of symbiotic indigenous wastewater microalgae and bacteria under different conditions (inoculum ratio of bacteria to microalgae, CO2, light intensity, and harvest time) for optimal bioenergy feedstock production. The findings of this study demonstrate that the symbiotic microalgae-bacteria culture not only increases total microalgal biomass and lipid productivity, but also enlarges microalgal cell size and stimulates lipid accumulation. Meanwhile, inoculum ratio of bacteria to microalgae, light intensity, CO2, and harvest time significantly affect biomass and lipid productivity. CO2 concentration and harvest time have significant interactive effect on lipid productivity. The response of microalgal biomass and lipid productivity varies significantly from 2.1 × 105 to 1.9 × 107 cells/mL and 2.8 × 102 to 3.7 × 1012 Total Fluorescent Units/mL respectively. Conditions for optimum biomass and oil accumulation are 100% of inoculation ratio (bacteria/microalgae), 3.6% of CO2 (v/v), 205.8 µmol/m2/s of light intensity, and 10.6 days of harvest time. This work provides a systematic methodology with RSM to explore the benefits of symbiotic microalgae-bacteria culture, and to optimize various cultivation parameters within complex wastewater environments for practical applications of integrated wastewater-microalgae systems for cost-efficient bioenergy production.
Keywords: Co-cultivation of microalgae and bacteria, Bioenergy feedstock, Wastewater, Response surface methodology, Flow cytometry analysis
Subject terms: Environmental sciences, Biochemistry
Introduction
Microalgal biofuel is considered as one of the best energy alternatives attributed to unique and desirable characteristics of microalgae such as rapid growth and capability of growing in poor quality water1. Despite its promise, there remain a number of challenges for large-scale applications of the technology. Key barriers include limited resource supply and high cost for scaled-up microalgae cultivation. The water and nutrients needed for large scale microalgae cultivation make up 35% of the total cultivation cost2. Supplying these resources is not only costly but also under increasing stress of shortage. Therefore, it is warranted to develop affordable and sustainable methods to cultivate microalgae on a large scale, such as incorporating microalgae cultivation with wastewater resources3,4. Wastewater consists of abundant nutrients that can provide free nutrient supply. Additionally, wastewater can supplement significant freshwater demand to alleviate concerns about the intensive water footprint of biofuel feedstock production.
The key challenge of microalgal culture in wastewater is the threat of contamination that results in microalgae crush and low productivity, especially in wastewater open ponds where microalgae is directly exposed to various contaminants5. Therefore, the cultivation of microalgal poly-cultures is attractive for improving crop protection and productivity6,7. Multispecies cultures are more likely to reach ecological equilibrium, which is more resilient to contamination and more productive compared to single species. Among cultivation strategies of poly-cultures, microalgal-bacterial mix-cultures have received particular interest. Microalgae and bacteria always co-exist together in the natural environment, especially in wastewater where bacteria are abundant. Bacteria are critical for the functionality, productivity, and risk profile of microalgal systems8,9. They interact directly or indirectly with microalgae through mechanisms from mutualism to antagonism8.
To date, studies investigating microalgal-bacterial processes primarily focused on wastewater treatment, from nutrients recovery to hazardous contaminants treatment. These studies demonstrated the promise of microalgal-bacterial cultures for wastewater treatment10–12. A few studies reported the potential of microalgal-bacterial cultures for bioenergy or dual-purpose systems (simultaneously producing bioenergy feedstock and treating wastewater)4,13. Zhou et al. investigated the productivity of Chlorella pyrenoidosa with three strains of ammonia-oxidizing bacteria JN1, FN3, and FN513. Miyawaki et al. studied the biomass and lipid production of microalgae using biodigested swine manure, cattle manure and domestic sewage with biogas/air mixture4. However, insufficient information is available to fully understand how other important cultivation parameters (such as ratio of bacteria and microalgae, light, and CO2) affect the performance of mixed microalgal-bacterial cultures. These cultivation parameters have significant impacts on the complexity and dynamic interactions of microalgal-bacterial mixture and, as such, can affect bioenergy productivity. In addition, few studies have examined the impact of these cultivation parameters on the change of microalgal cell size and lipid accumulation, although microalgal cell size and lipid content are critical for downstream processes such as microalgae harvest/concentrating and lipid extraction.
To address the aforementioned knowledge gaps, this study uses response surface methodology (RSM) to understand the effect of microalgal-bacterial composition and three important cultivation parameters (CO2 concentration, light intensity and harvest time), as well as the synergized impact of these factors for bioenergy feedstock production. RSM can evaluate the interactions of up to 50 input variables, and optimize these inputs for optimal outputs. Furthermore, RSM is a proven approach for experimental design, kinetic study and system optimization14,15. This method has been used to optimize conditions for microalgae cultivation16,17. Our previous work utilized RSM to optimize microalgal biomass/lipid productivity by using mixed cultures of chlorella sp. and wild microalgae selected from wastewater18. The current study is the logical extension from microalgal poly-cultures to microalgal-bacterial cultures. The main objectives of this study include (i) elucidating the interactions of wastewater-borne heterogeneous microalgae and bacteria and the consequential impact on biomass and lipid productivity at a cellular level; (ii) understanding the effect of important cultivation parameters, and their synergized impact on microalgal-bacterial mixed culture; and (iii) establishing a systematic methodology using RSM to optimize microalgal bioenergy feedstock production within complex wastewater conditions by using symbiotic microalgal-bacterial culture. The results of this work provide a holistic understanding of integrated wastewater-microalgae systems for cost-efficient microalgal bioenergy production.
Materials and methods
Wastewater-based symbiotic microalgal-bacterial seed culture
This research used enhanced microalgal-bacterial seed cultures from the Detroit Water Resource Recovery Facility (Detroit WRRF) (Michigan, United States). Secondary wastewater samples were collected in sterile falcon tubes from Detroit WRRF and transferred to the laboratory where samples were kept at a temperature of 4 °C. As shown in SI Fig. S1, the symbiotic microalgal-bacterial culture was chosen via an iterative screening procedure. In brief, microalgal-bacterial seed was raised from collected wastewater samples in sterile Erlenmeyer flasks under light/dark (12/12 h) (300 μmol/m2/s) and shaking (30 rev/min) conditions. Enriched microalgal-bacterial cultures were challenged with wastewater iteratively for five times. Following that, the culture with the highest growth rate was selected. The chosen microalgal-bacterial culture consisted of a combination of microalgae and bacteria naturally existing in wastewater. Microalgae and bacteria were then separated by a multi-step filtration process using filters with a pore size of 5 μm for microalgae and 0.2 μm for bacteria19. Separated seed cultures of microalgae and bacteria were kept in sterilized wastewater media. To preserve microalgal and bacterial diversity, these seed cultures were used without additional species separation.
Cultivation parameter
The following four cultivation parameters were considered in this study: A—inoculum ratio of bacteria to microalgae, B—CO2 supply, C—light intensity, and D—harvest time. To remain the consistence of nutrient composition for a better comparison, a synthetic wastewater—designed to simulate the effluent from a domestic wastewater treatment facility—was used as the growth medium19. The synthetic wastewater consists of glucose (0.4125 g/L), NH4Cl (0.078 g/L), KH2PO4 (0.018 g/L), MgSO4·7H2O (0.013 g/L), CaCl2·2H2O (0.043 g/L), FeSO4·7H2O (0.005 g/L), and Trace Metal Mix A5 with Co solution (MilliporeSigma, MA) (1 mL/L). The media was sterilized by autoclaving at 121 °C for 20 min after adjusting the pH of the media to between 7.0 and 8.0. The inoculum ratio of bacteria to microalgae changed from 25 to 100%, with initial microalgae concentrations remaining constant (2 × 105 cells/mL). BD Accuri™ C6 Plus Flow Cytometer (BD Biosciences, California, USA) was used to quantify the cell concentration of the microalgae and bacteria inoculum, respectively. CO2 was supplied at 0.25 vvm (volume/volume/min) within the concentration range of 1–5% (v/v) by mixing pure CO2 with air at different proportions. The CO2 supply was limited to this range because in our early experiments cultures fed with CO2 concentrations more than 5% had not survived longer than ten days. Light conditions were 12-h light/12-h dark with light intensity with the range of 50–300 µmol/m2/s. Previous studies have reported various light intensity for optimal microalgae growth (80–260 µmol/m2/s) and further increase of light intensity would resulted in a decrease in the growth rate20–22. Also, cultures exposed to light intensity above 300 µmol/m2/s in our preliminary study did not show a good response to growth. Therefore, in our study we chose the light intensity of 50–300 µmol/m2/s for the experimental design. Harvest time ranged between 1 and 15 days as the culture entered the death phase beyond 15 days. Baffled Elden Mayer flasks (500 mL) were used to cultivate microalgal-bacterial cultures under continuous shaking (100 RPM) with a MaxQ™ HP table top shaker (Thermo Scientific™ U.S.A).
Design of experiment
Central composite design with five levels in each factor was used to design the experiment for the four cultivation parameters (inoculum ratio of bacteria/microalgae, CO2, light intensity, and harvest time). The methodology of the experiment design and statistical analysis was based on the method described by Gopalakrishnan18. Briefly, Design Expert® (Version 10) (Stat-Ease Inc., Minneapolis, USA) was used to design the experiment with different levels for each cultivation parameter and to analyze experimental data. Every cultivation parameter was signed with five levels based on the central composite model, resulting in thirty experiments for four factors (Table 1). The correlation between the cultivation parameters and two output responses (biomass and lipid) was determined by using fitted quadratic equations (SI Eqs. S1–2). The detailed experimental design and results of responses were described in Table 1.
Table 1.
Experimental design and the responses obtained using five factorial central composite model.
| Standard | Factors | Responses | ||||
|---|---|---|---|---|---|---|
| IR (%) | Light intensity (µmol/m2/s) | CO2 (%) | Harvest time (days) | Algal biomass (cells/mL) | Lipid productivity (total FL/mL) | |
| 1 | 43.75 | 112.5 | 2 | 4.5 | 2.4 × 105 | 5.5 × 1010 |
| 2 | 81.25 | 112.5 | 2 | 4.5 | 3.2 × 106 | 1.8 × 1011 |
| 3 | 43.75 | 237.5 | 2 | 4.5 | 3.9 × 106 | 1.7 × 1011 |
| 4 | 81.25 | 237.5 | 2 | 4.5 | 5.2 × 106 | 1.2 × 1011 |
| 5 | 43.75 | 112.5 | 4 | 4.5 | 8.5 × 105 | 1.5 × 1010 |
| 6 | 81.25 | 112.5 | 4 | 4.5 | 4.2 × 106 | 3.1 × 1010 |
| 7 | 43.75 | 237.5 | 4 | 4.5 | 4.5 × 106 | 1.1 × 1012 |
| 8 | 81.25 | 237.5 | 4 | 4.5 | 9.2 × 106 | 8.6 × 1010 |
| 9 | 43.75 | 112.5 | 2 | 11.5 | 1.1 × 106 | 1.6 × 1012 |
| 10 | 81.25 | 112.5 | 2 | 11.5 | 4.5 × 106 | 3.3 × 1012 |
| 11 | 43.75 | 237.5 | 2 | 11.5 | 4.1 × 106 | 3.1 × 1012 |
| 12 | 81.25 | 237.5 | 2 | 11.5 | 1.1 × 107 | 3.6 × 1012 |
| 13 | 43.75 | 112.5 | 4 | 11.5 | 4.4 × 106 | 1.8 × 1011 |
| 14 | 81.25 | 112.5 | 4 | 11.5 | 8.3 × 106 | 1.2 × 1011 |
| 15 | 43.75 | 237.5 | 4 | 11.5 | 3.4 × 106 | 3.2 × 1010 |
| 16 | 81.25 | 237.5 | 4 | 11.5 | 1.9 × 107 | 2.5 × 1012 |
| 17 | 25 | 175 | 3 | 8 | 4.3 × 106 | 8.9 × 1011 |
| 18 | 100 | 175 | 3 | 8 | 8.5 × 106 | 3.5 × 1012 |
| 19 | 62.5 | 50 | 3 | 8 | 4.1 × 106 | 3.2 × 1010 |
| 20 | 62.5 | 300 | 3 | 8 | 1.5 × 106 | 5.1 × 1010 |
| 21 | 62.5 | 175 | 1 | 8 | 3.4 × 106 | 1.1 × 1011 |
| 22 | 62.5 | 175 | 5 | 8 | 4.2 × 106 | 1.2 × 1011 |
| 23 | 62.5 | 175 | 3 | 1 | 2.1 × 105 | 3.3 × 1010 |
| 24 | 62.5 | 175 | 3 | 15 | 2.0 × 106 | 2.8 × 102 |
| 25 | 62.5 | 175 | 3 | 8 | 8.3 × 106 | 1.5 × 1012 |
| 26 | 62.5 | 175 | 3 | 8 | 8.0 × 106 | 1.6 × 1012 |
| 27 | 62.5 | 175 | 3 | 8 | 8.2 × 106 | 1.6 × 1012 |
| 28 | 62.5 | 175 | 3 | 8 | 8.0 × 106 | 1.5 × 1012 |
| 29 | 62.5 | 175 | 3 | 8 | 8.2 × 106 | 1.5 × 1012 |
| 30 | 62.5 | 175 | 3 | 8 | 8.5 × 106 | 1.6 × 1012 |
A—Inoculation ratio of bacteria to algae (IR) (25–100%).
B—Light intensity (50–300 µmol/m2/s).
C—Carbon dioxide concentration (1–5%, v/v).
D—Harvest time (1–15 days).
Microalgal biomass and lipid productivity analyses
A modified method based on previous studies14,23 was used to analyze microalgal biomass and lipid productivity using BD Accuri™ C6 Flow Cytometer. This method has three advantages when compared to conventional biomass measurement and lipid extraction. Firstly, it can generate information on microalgal total biomass and lipid productivity while allowing for the examination of cell size and lipid accumulation at a cellular level14. Secondly, the method only measures biomass and lipid from the microalgae, excluding the interference of bacteria. Finally, our results and other studies have demonstrated the reliability of using the flow cytometer for microalgal biomass and lipid analyses. There is a significant correlation between the results from flow cytometry analyses and conventional measurements of microalgal lipid (gravimetric analysis of lipids) and dry biomass (oven drying)23,24 (SI Figs. S2 and S3). Specifically, microalgae biomass was measured as microalgal cells (cells/mL) with the fluorescence of chlorophyll a in algal cells detected by the flow cytometer. BODIPY 505/515, a lipid binding dye, was utilized to analyze microalgal lipids. To a 990 mL microalgal sample, 10 mL of 1.25 mol/L BODIPY dye was applied. Prior to analysis, the mixture was well mixed. For the detection of lipid binding dye signals, a 515 filter in channel 1 (FL1) was employed.18. The 515 filter in channel 1 (FL1) was used for the detection of lipid-binding dye signal14,23 with the result of total fluorescent units per mL sample (Total FL/mL).
Results
Predicted model and statistical analysis
For mixed microalgal-bacterial cultures, multiple factors can affect algal cultivation and productivity. Previous studies have mainly investigated a single factor and its effect instead of two or more factors. To overcome this limitation, the interactions between the inoculum ratio of bacteria to microalgae, CO2 concentration, light intensity, and harvest time, as well as their synergic impact on microalgal biomass and lipid productivity were investigated in this study. This was done by using the central composite statistical model of RSM design. Because the range of microalgal biomass and lipid productivity changed dramatically from 2.1 × 105 to 1.9 × 107 cells/mL and 2.8 × 102 to 3.6 × 1012 Total FL/mL respectively (Table 1), a square root power transformation y′ = √(y + k) was applied (when y + k ≥ 0) for such vigorous change of responses where minimum and maximum output ratios were more than 1018. By using multiple regression analyses of the experimental results, Eqs. (1) and (2) were developed to predict the second order polynomial response of microalgal biomass and lipid productivity.
| 1 |
| 2 |
where A is the inoculum ratio of bacteria to microalgae, B is light intensity, C is CO2 concentration, and D is harvest time.
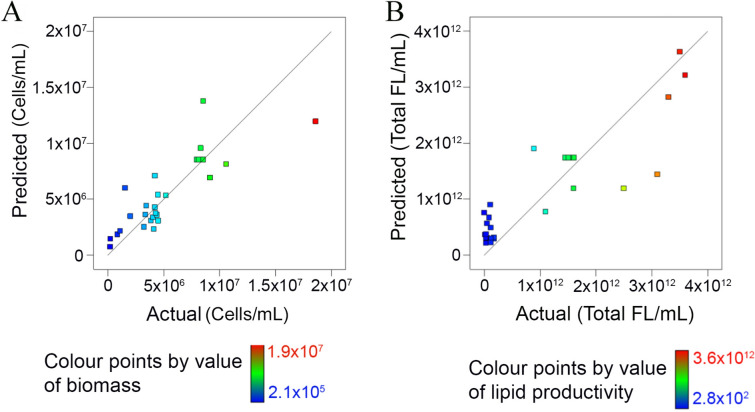
The p values of the predict models were low (0.01 for microalgal biomass and 0.02 for lipid productivity) (SI Table S1a,b), indicating that the fitness of the predicted model was statistically significant. As shown in Fig. 1, the correlation between predicted and actual data for biomass and lipid is significant with a high R2 value (0.75 for cell count and 0.74 for lipid) (SI Table S1a,b). For the impact of cultivation conditions on biomass and lipid productivity, significant parameters were those with p values < 0.05. Inoculum ratio (A) (p = 0.002), harvest time (D) (p = 0.031), quadratic effect of light intensity (B2) (p = 0.047) and harvest time (D2) (p = 0.002) (SI Table S1a) have significant impacts on microalgal biomass. Harvest time (D) (p = 0.016), interactive effect of CO2 concentration with harvest time (CD) (p = 0.023), quadratic effect of light intensity (B2) (p = 0.020), CO2 concentration (C2) (p = 0.045) and harvest time (D2) (p = 0.010) (SI Table S1b) have significant impacts on lipid productivity.
Figure 1.
High closeness of the fitted regression between the actual and predicted biomass (A) and lipid productivity (B).
Microalgal biomass
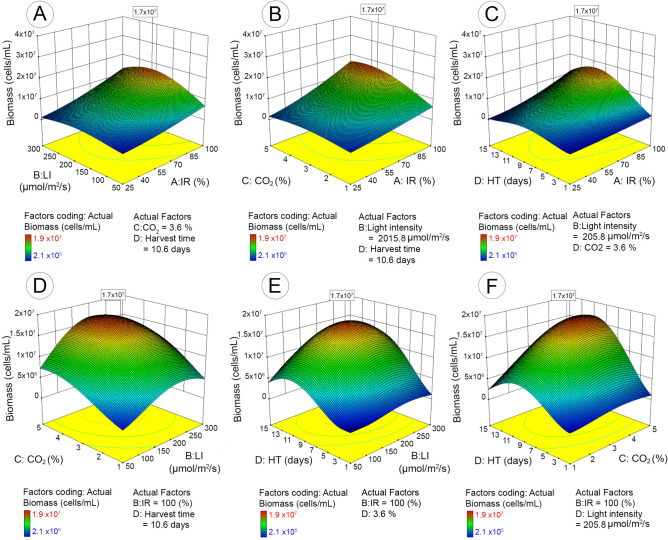
The maximum microalgal biomass (1.7 × 107 cells/mL with the desirability value of 0.939) was obtained with 100% of inoculum ratio (bacteria to microalgae), 4.1% of CO2, 204.8 µmol/m2/s of light intensity, and 10.4 days of harvest time (Table 2). Figure 2A–F illustrates the impact of each cultivation parameter, as well as the interactive impact of different parameters on microalgal biomass yield based on the 3D response surface plots of the RSM predicted model.
Table 2.
Optimal conditions for two outputs (biomass and lipid productivity) individually and together with desirability values.
| Optimal condition | Response | Optimum outcome | Desirabilitya | ||||
|---|---|---|---|---|---|---|---|
| CO2 | Light intensity | Inoculum ratio | Harvest time | ||||
| % | µmol/m2/s | % | Days | ||||
| 4.1 | 204.8 | 100 | 10.4 | Cell count (cells/mL) | 1.7 × 107 | 0.939 | |
| 2.8 | 235.4 | 98.6 | 10.1 | Lipid content (total FL/mL) | 3.6 × 1012 | 1 | |
| 3.6 | 205.8 | 100 | 10.6 | Overall | Cell count (cells/mL) | 1.7 × 107 | 0.985 |
| Lipid content (total FL/mL) | 3.7 × 1012 | ||||||
CO2 Carbon dioxide concentration (1–5%, v/v).
Light intensity (50–300 µmol/m2/s).
Inoculum ratio of bacteria to microalgae (25–100%).
HT Harvest time (1–15 days).
aDesirability: The desirability is a value between 0 and 1. It represents the closeness of a response to its optimal outcome, with a higher value being more optimal.
Figure 2.
3D surface response and contour line of central composite design showing the mutual effect of different parameters on microalgal biomass with maximum response value in boxes. IR (inoculum ratio), inoculation ratio of bacteria to microalgae (25–100%); LI (light intensity), 50–300 µmol/m2/s; HT (harvest time), 1–15 days; CO2, CO2 concentration in mixed CO2/air gas 1–5% (v/v).
The inoculum ratio of bacteria to microalgae had significant impact on microalgal biomass yield. Increasing inoculum ratio from 25 to 100% significantly enhanced microalgal cell proliferation. The maximum biomass was observed when inoculum ratio was maximum (bacteria/microalgae: 100%) (Fig. 2A–C). The impact of inoculum ratio appeared to be more apparent at optimum conditions for the remaining cultivation parameters (CO2, light intensity, and harvest time). The other significant factor was light intensity (Fig. 2A,D,E). Microalgal biomass was enhanced with increase of light intensity up to 206 µmol/m2/s; further increase had negative impact, thus resulting in a slightly declined biomass. In addition, the impact was more significant in exponential and stationary stages than lag- and decline-phases. Unsurprisingly, harvest time was another significant parameter that affected microalgal yield. Microalgal yield boosted from the exponential stage (starting from 2 to 3 days), reached to a maximum biomass yield at 10.4 days, and then declined. In contrast, the response of microalgal biomass marginally varied with the factor of CO2 supply.
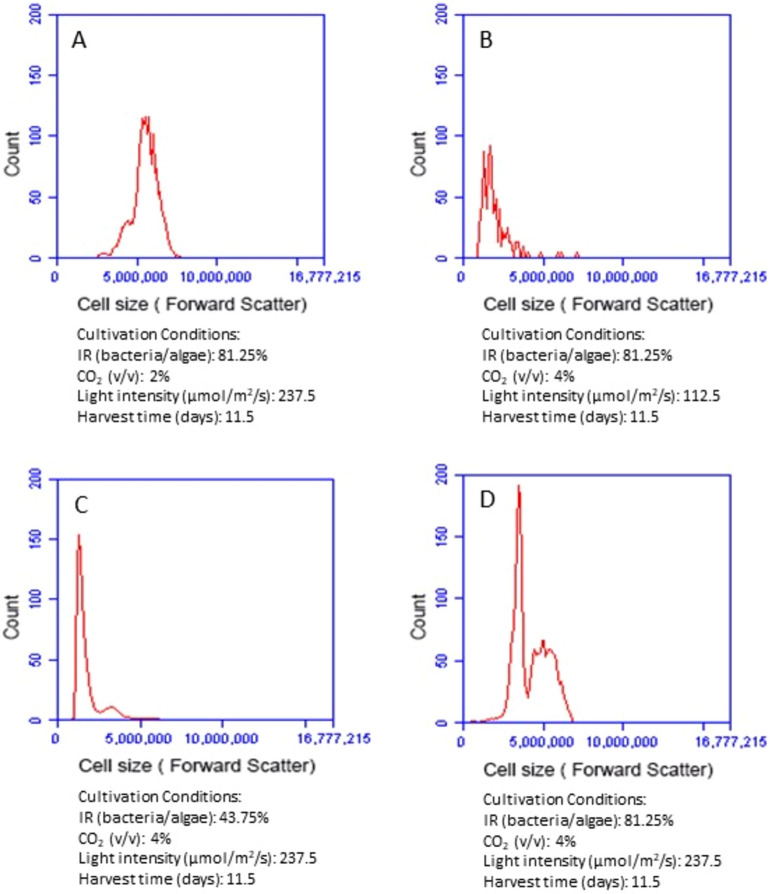
Since microalgal biomass productivity depends on not only microalgal cell number but also cell size, it is necessary to examine changes of microalgal cell sizes under different conditions. Flow cytometry is a powerful tool in analyzing cell sizes at a cellular level. By analyzing forward scatter signal of the flow cytometry histogram, we were able to compare microalgal cell sizes at different cultivation conditions. The larger forward scatter is, the bigger the cell size is. We compared the impact of inoculum ratio, CO2, and light intensity respectively on microalgal cell size by keeping other cultivation conditions at an optimal level. With other conditions held constant, increasing bacteria inoculum clearly enlarged microalgal cell size (Fig. 3C,D). When the ratio of bacteria to microalgae increased from 43.74 to 81.25%, the forward scatter signals of microalgae cells shifted from 1,000,000–4,000,000 to 2,500,000–7,000,000. The comparison of different CO2 conditions (Fig. 3A,D) indicate that enhancing CO2 supply from 2 to 4% did not significantly increase the average size of microalgal cells. Higher CO2 concentration actually promoted a large number growth of small-size microalgae cells (as evidenced with the high peak in the forward scatter of 2,500,000–4,500,000 in Fig. 3D). Light intensity had a significant impact on microalgal cell size (Fig. 3B,D). The forward scatter signals of microalgal cell were 2,500,000–7,000,000 under high light intensity (237.5 µmol/m2/s) but only 1,000,000–3,000,000 under low light intensity (112.5 µmol/m2/s).
Figure 3.
Comparison of forward scatter signals of the flow cytometry histogram showing microalgal cell sizes at different cultivation conditions. IR (inoculum ratio), inoculation ratio of bacteria to microalgae.
Lipid productivity
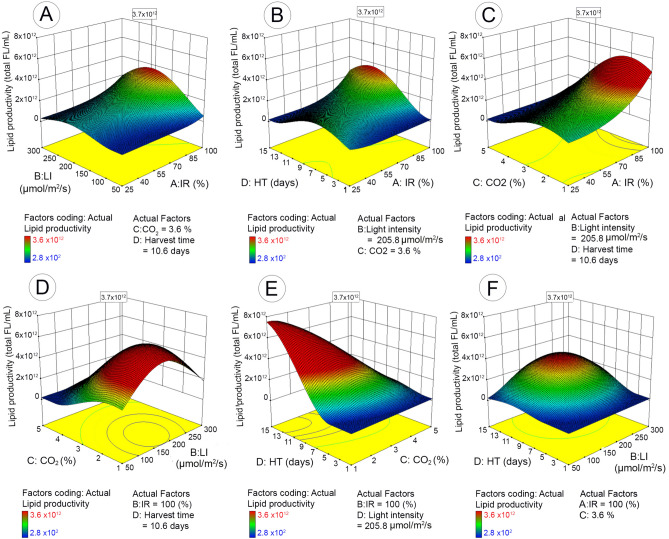
The maximum lipid productivity (3.6 × 1012 Total FL/mL with the desirability value of 1.0) was obtained with 98.6% of inoculum ratio (bacteria to microalgae), 2.8% of CO2 concentration, 235.4 µmol/m2/s of light intensity, and 10.1 days of harvest time (Table 2). Figure 4A–F illustrates the impact of each cultivation parameter, as well as the interactive impact of different parameters, on microalgal lipid productivity yield based on the 3D response surface plots of the RSM predicted model.
Figure 4.
3D surface response and contour line of central composite design showing the mutual effect of different parameters on microalgal lipid productivity with maximum response value in boxes. IR (inoculum ratio), inoculation ratio of bacteria to microalgae (25–100%); LI (light intensity), 50–300 µmol/m2/s; HT (harvest time), 1–15 days; CO2, CO2 concentration in mixed CO2/air gas 1–5% (v/v).
Figure 4A–C shows the effect of inoculum ratio (bacteria to microalgae) on lipid productivity with change in the remaining parameters, respectively (CO2, light intensity, and harvest time). When light intensity or harvest time were low (Fig. 4A,B), the lipid productivity was very low and no change was observed when inoculum ratio (bacteria to microalgae) was increased from minimum to maximum. When light intensity or harvest time enhanced gradually to optimal conditions, the increase of inoculum ratio started to show impact on lipid productivity and the maximum yield was achieved when inoculum ratio was 100%. This indicates that when other conditions are suitable for microalgal growth, the enhancement of bacteria to microalgae ratio can increase the lipid productivity. Interestingly, the lipid productivity with respect to the interaction of carbon dioxide and inoculum ratio (Fig. 4C) showed that increasing inoculum ratio could only enhance lipid yield when CO2 concentration was less than 3%. When CO2 concentration was above 3%, no significant impact was observed with the change of inoculum ratio. Figures 4A,D,F show the impact of light intensity along with the change of the other three factors. Generally, the lipid productivity increased along with the enhanced light intensity until the optimal condition was reached (235.4 µmol/m2/s) and then declined with further increase of light intensity. The impact of light intensity on lipid productivity tended to be more significant at high inoculum ratio (> 80%) (Fig. 4A), low CO2 concentration (< 3%) (Fig. 4D) and exponential/stationary growth stages (Fig. 4F). Similar to the biomass yield, harvest time had significant impact on lipid productivity that reached to maximum productivity at the stationary stage (10.4 days) and then declined. Interestingly, harvest time and CO2 concentration had significant interactive impact on the lipid productivity (p = 0.023) (Fig. 4E). At low CO2 concentration (< 3%), the lipid accumulation increased with extended harvest time until optimal time, but decreased with the same extended harvest time when CO2 continued to increase above 3%.
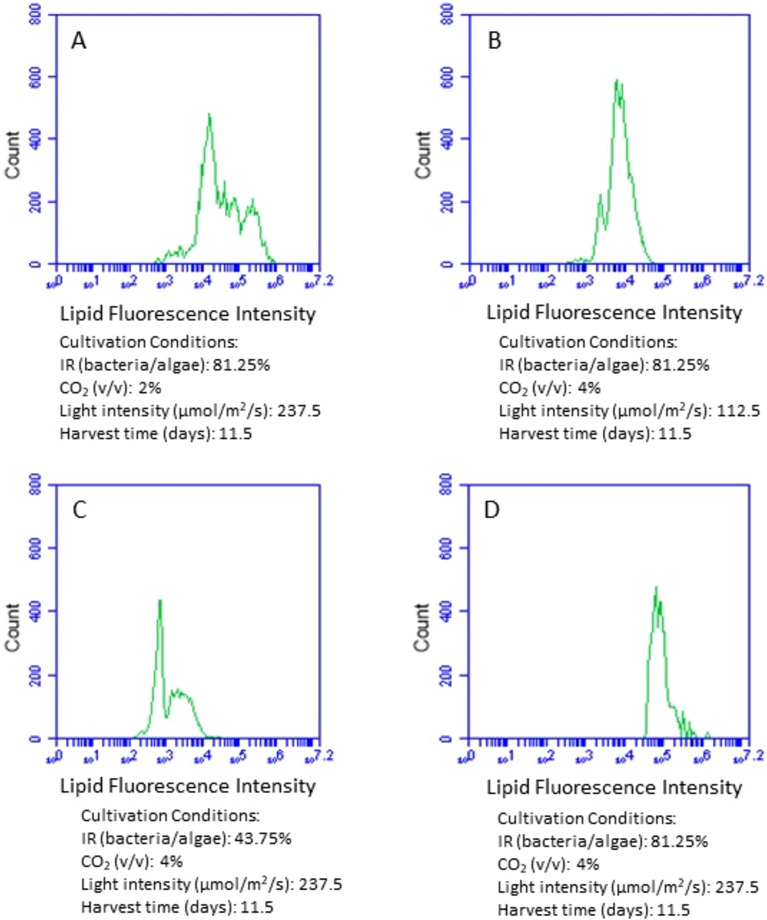
The lipid content in microalgal cells at different cultivation conditions was also examined by analyzing the lipid fluorescence intensity of the flow cytometry histogram. The greater the lipid fluorescence intensity, the higher lipid content was. Similar to the analysis of microalgal cell size, we compared the impact of inoculum ratio, CO2, and light intensity respectively on microalgal lipid content by keeping the other three cultivation conditions the same. With other conditions held constant, increasing the bacteria inoculum clearly stimulated lipid accumulation in microalgal cells (Fig. 5C,D). When the ratio of bacteria to microalgae increased from 43.74 to 81.25%, the average lipid fluorescence intensity of lipid enhanced 14 times and shifted from 9.4 × 103 (43.74% inoculum ratio of bacteria to microalgae) to 1.3 × 105 (81.25% inoculum ratio of bacteria to microalgae). Light intensity also significantly enhanced microalgal lipid content (Fig. 5B,D). The average lipid fluorescence intensity increased 10 times when the light intensity enhanced, being 1.4 × 104 at 112.5 µmol/m2/s and 1.3 × 105 at 237.5 µmol/m2/s. Compared to inoculation ratio and light intensity, CO2 supply had much less impact on the lipid content (Fig. 5A,D), with an average lipid fluorescence intensity of 3.3 × 105 at 2% of CO2 supply and 1.3 × 105 at 4% of CO2 supply.
Figure 5.
Comparison of fluorescence intensity of the flow cytometry histogram showing lipid accumulation in microalgal cells at different cultivation conditions. IR (inoculum ratio), inoculation ratio of bacteria to microalgae.
Simultaneous optimization of microalgal biomass and lipid productivity
The optimum conditions of both biomass and lipid yield were 100% of inoculum ratio (bacteria/microalgae), 3.6% of CO2, 205.8 µmol/m2/s of light intensity, and 10.6 days of harvest time. The maximum outputs of biomass and lipid production were 1.7 × 107 cells/mL and 3.7 × 1012 Total FL/mL, corresponding to 1.10 mg/mL of dry biomass and 0.21 mg/mL of total lipid respectively (SI Fig. S3—algal biomass and lipid productivity analyses). While comparing the optimum conditions required for biomass and lipid productivity individually and simultaneously, there was no significant difference with harvest time and inoculum ratio (bacteria/microalgae). The optimal harvest time stayed very close between 10 and 11 days. Similarly, the maximum inoculum ratio (100%) produced the best output in all cases. For light intensity, the maximum biomass was obtained at 204 µmol/m2/s while maximum lipid productivity was achieved at 235 µmol/m2/s. The optimum condition required for both microalgal biomass and lipid productivity was close to the optimum condition for biomass yield. With regards to CO2 supply, there was a difference between optimal concentrations required for biomass (4.1% CO2) and lipid (2.8% CO2). To achieve maximum yields of both the biomass and lipid, CO2 concentration was in the middle of two individual optimum conditions.
Discussion
Although research on co-cultivation of microalgae and bacteria for wastewater treatment and bioenergy production have been conducted, this study is unique in assessing the response caused by multi cultivation factors (inoculum ratio of bacteria/microalgae, CO2, light intensity, and harvest time) and their cross-factor interactions. Bacteria are critical for the functionality, productivity, and risk profile of microalgal systems8. They may interact directly or indirectly with microalgae through mechanisms ranging from mutualism to antagonism. In a natural environment, microalgae grow in the same habitat where bacteria are present. Achieving symbiosis of microalgae and bacteria is particularly critical in wastewater-based cultivation where abundant bacteria exist. Our results demonstrate that co-cultivation of symbiotic microalgae-bacteria mixtures (selected by multiple screening processes, SIS1) could significantly enhance microalgal biomass and lipid productivity. This is consistent with some of the other studies in microalgae-bacteria co-cultivation. For example, Zhou et al. found that one of ammonia-oxidizing bacteria (Kluyvera sp.) screened from wastewater could enhance the biomass in dry weight and lipid content of Chlorella pyrenoidosa by 14.8% and 13.6%, respectively13. In our work, the inoculum ratio of bacteria to microalgae could be as high as 1:1, with a higher ratio leading to better microalgal growth. In this study, the highest inoculum ratio of bacteria to microalgae was 100% (1:1). A further increase in bacterial inoculum ratio could undermine microalgal growth due to competition for other nutrients. Therefore, future studies are necessary to investigate the maximum allowable bacteria ratio. Also, the impact of other cultivation parameters (such as N and P supply) on microalgae-bacteria co-cultivation needs to be examined. It should be noted that this work used the flow cytometer to measure microalgal biomass and lipid yield based on microalgal cell count (microalgal chlorophyll a) and lipid fluorescence. As a consequence, the findings were associated with microalgae, which accurately reflected the effects of various factors on microalgal biomass and lipid production.
One mechanism that contributes to the symbiosis of microalgae and bacteria is the nutrient exchange between microalgae and bacteria, such as CO2–O2 exchange. CO2 produced by bacteria and O2 produced by microalgae are reciprocally utilized by and beneficial for both microalgae and bacteria. Our results further confirm this mechanism. As shown in Fig. 4C, when CO2 concentration was low, the increment of bacterial inoculum ratio resulted in more significant enhancement in lipid production compared to high CO2 concentration (> 3%). One possible explanation for this could be that, at low CO2 concentration, bacteria provided CO2 for microalgal growth and reduced the dependence on external CO2 supply. When CO2 concentration was high, the level of CO2 supply could meet the carbon demand for microalgal growth and attenuated the benefit of CO2 exchange offered by microalgae-bacteria co-cultivation. This finding is useful for practical applications since CO2 supply is one of the top environmental and economic burdens for large-scale microalgae cultivation2. Other studies have indicated that bacteria could produce critical growth factors and micronutrients, such as thiamine (vitamin B1) and cobalamin (vitamin B12) derivatives, to facilitate microalgal growth25,26. However, these studies did not examine the enhancement at cellular levels. Our results show that co-cultivation of microalgae and bacteria could be a promising approach with a number of benefits, not only reducing cultivation cost by providing CO2 from the co-cultivated bacteria instead of entirely relying on external CO2 supply but also enhancing the productivity of microalgal biomass and lipid, enlarging the cell size of microalgae and facilitating lipid accumulation in microalgal cells. The finding of our study is interesting because larger cells and higher lipid content are very beneficial for downstream processes of microalgal biofuel production, as particularly pertaining to microalgae harvest/concentrating and lipid extraction which are critical challenges and cost/energy burdens to the microalgal biofuel industry. Larger microalgal cells are easier to settle down and could significantly reduce harvest/concentrating cost and energy consumption. At the same time, higher lipid content requires less solvent for oil extraction, further reducing cost- and energy-demanding. Collectively, the benefits of microalgae-bacteria co-cultivation (less CO2 supply, larger microalgal cells and higher lipid content) could significantly improve the overall cost- and energy-efficiency of microalgal biofuel production.
Numerous studies have shown that CO2 is important for microalgal growth, since it is the main source of carbon for microalgal biomass. For instance, Gopalakrishnan et al.27 found that CO2 supply would significantly influence the number of algal cells in both pure Chlorella sp. culture and mixed culture (Chlorella sp. and wastewater wild algae). However, in this study CO2 was the only parameter that showed no significant impact on microalgal biomass yield for microalgae-bacteria co-cultivation. In mixed cultures of bacterial and microalgae, aerobic bacteria can oxidize organic compounds in wastewater and release CO2 that can be utilized by microalgae, reducing the need of external CO2 supply28,29. Interestingly, CO2 concentration was substantially different for optimal biomass and lipid production, 4.1% for biomass and 2.8%for lipid. High CO2 concentration could undermine lipid accumulation in algae and bacteria co-culture. One explanation is that the elevated CO2 concentration inhibits the growth of bacteria that supply micronutrients and growth factors necessary for carbon assimilation in microalgae30. Additionally, higher CO2 concentration may cause the culture medium to become more acidic, resulting in a reduction of lipid synthesis31,32. Regarding the impact of light intensity, our results suggest that there exists an optimal light intensity for microalgal growth. Increase in light intensity promotes photosynthesis, but too much of light could cause photoinhibition. Light intensity could also affect lipid accumulation. Although high light intensity could promote microalgal growth, it could lead to the usage of produced energy for cell division instead of lipid synthesis33. The optimal time to harvest algal biomass was about 10 or 11 days. Beyond this time, both cell count and lipid content began to decline rapidly. It is noteworthy that the best harvest time may vary among different studies depending on the configuration of the cultivation system.
With all the cultivation parameters (inoculum ratio of bacteria/microalgae, CO2, light intensity, and harvest time) being optimized, algal biomass and lipid productivity could reach to 1.7 × 107 cells/mL and 3.7 × 1012 Total FL/mL, corresponding to 1.10 mg/mL of dry biomass and 0.21 mg/mL of total lipid respectively (SIS3—algal biomass and lipid productivity analyses). The optimum lipid content of algal biomass would be 18.92%. This productivity is significantly higher than conventional wastewater-based algae cultivation, where algae are usually susceptible to various stresses that stunt algal growth hardly beyond a cell concentration of 0.5 mg/mL and lipid content of 10%5,6. Our results demonstrate the necessity of applying systematic approaches (such as RSM used in this study) to holistically understand the impact of different cultivation parameters on microalgal cultivation so that the design and operation of cultivation systems can be optimized for higher biomass and lipid productivity.
Conclusions
This work uses the RSM model to explore the benefit of symbiotic microalgae-bacteria culture for bioenergy feedstock production and to optimize cultivation parameters (including inoculum ratio of bacteria to microalgae, light intensity, CO2, and harvest time) within complex wastewater environments. According to the results of this research, when co-cultivating wastewater-native microalgae-bacteria cultures, the optimum conditions for maximizing microalgal biomass and lipid production were 100% (1:1) of inoculum ratio (bacteria/microalgae), 3.6% of CO2 concentration, 205.8 µmol/m2/s of light intensity, and 10.6 days of harvest time. Our results demonstrate that symbiotic microalgae-bacteria cultures can significantly improve microalgal biomass yield and lipid productivity, particularly at a lower concentration (< 3%) of CO2 supply, which could remarkably reduce the cost of CO2 supply for microalgae cultivation. Co-cultivation of bacteria and microalgae can also enlarge microalgal cell size and stimulate lipid accumulation in microalgal cells. These benefits will significantly reduce the cost- and energy-burdens of downstream processing, especially for microalgae harvesting and lipid extraction. In addition, different cultivation parameters can significantly affect the performance of microalgal-bacterial co-cultivation individually and collectively. This work provides a systematic approach with the RSM design for practical applications of wastewater-microalgae integration for cost-efficient biofuel production.
Supplementary Information
Acknowledgements
This work was supported by Wayne State University Research Enhancement Program. We thank the Great Lakes Water Authority, the Detroit Water Resource Recovery Facility, and Mr. Michael Jurban for assisting in wastewater sampling.
Author contributions
K.G. designed and conducted all the experiments, collected and analyzed all experimental data, wrote the main manuscript text and prepared all figures. Y.W. developed the research idea, mentored Dr. Gopalakrishnan’s research work, provided research resources for this work, and revised the manuscript. J.R. assisted with some data collection and analysis.
Funding
This study was supported by Wayne State University Research Enhancement Program.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70033-1.
References
- 1.de Mendonca, H. V. et al. Microalgae in a global world: New solutions for old problems?. Renew. Energy165, 842–862 (2021). 10.1016/j.renene.2020.11.014 [DOI] [Google Scholar]
- 2.Colosi, L. M., Zhang, Y., Clarens, A. F. & White, M. A. Will algae produce the green? Using published life cycle assessments as a starting point for economic evaluation of future algae-to-energy systems. Biofuels3(2), 129–142 (2012). 10.4155/bfs.12.4 [DOI] [Google Scholar]
- 3.Liu, J.-Z. et al. Enhancements of lipid productivity and phosphorus utilization efficiency of Chlorella pyrenoidosa by iron and acetate supplements in actual municipal wastewater. Renew. Energy170, 927–935 (2021). 10.1016/j.renene.2021.01.148 [DOI] [Google Scholar]
- 4.Miyawaki, B. et al. Microalgae derived biomass and bioenergy production enhancement through biogas purification and wastewater treatment. Renew. Energy163, 1153–1165 (2021). 10.1016/j.renene.2020.09.045 [DOI] [Google Scholar]
- 5.Chen, G., Zhao, L. & Qi, Y. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review. Appl. Energy137, 282–291 (2015). 10.1016/j.apenergy.2014.10.032 [DOI] [Google Scholar]
- 6.Kazamia, E., Aldridge, D. C. & Smith, A. G. Synthetic ecology–A way forward for sustainable algal biofuel production?. J. Biotechnol.162(1), 163–169 (2012). 10.1016/j.jbiotec.2012.03.022 [DOI] [Google Scholar]
- 7.Johnson, K. R. & Admassu, W. Mixed algae cultures for low cost environmental compensation in cultures grown for lipid production and wastewater remediation. J. Chem. Technol. Biotechnol.88(6), 992–998 (2013). 10.1002/jctb.3943 [DOI] [Google Scholar]
- 8.Unnithan, V. V., Unc, A. & Smith, G. B. Mini-review: A priori considerations for bacteria–algae interactions in algal biofuel systems receiving municipal wastewaters. Algal Res.4, 35–40 (2014). 10.1016/j.algal.2013.11.009 [DOI] [Google Scholar]
- 9.Wang, H., Hill, R. T., Zheng, T., Hu, X. & Wang, B. Effects of bacterial communities on biofuel-producing microalgae: Stimulation, inhibition and harvesting. Crit. Rev. Biotechnol.36(2), 341–352 (2016). 10.3109/07388551.2014.961402 [DOI] [PubMed] [Google Scholar]
- 10.Xu, K., Zou, X., Xue, Y., Qu, Y. & Li, Y. The impact of seasonal variations about temperature and photoperiod on the treatment of municipal wastewater by algae-bacteria system in lab-scale. Algal Res.54, 102175 (2021). 10.1016/j.algal.2020.102175 [DOI] [Google Scholar]
- 11.Vergara, C., Muñoz, R., Campos, J., Seeger, M. & Jeison, D. Influence of light intensity on bacterial nitrifying activity in algal-bacterial photobioreactors and its implications for microalgae-based wastewater treatment. Int. Biodeterior. Biodegrad.114, 116–121 (2016). 10.1016/j.ibiod.2016.06.006 [DOI] [Google Scholar]
- 12.Saravanan, A. et al. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere271, 129540 (2021). 10.1016/j.chemosphere.2021.129540 [DOI] [PubMed] [Google Scholar]
- 13.Zhou, X. et al. Enhancement of productivity of Chlorella pyrenoidosa lipids for biodiesel using co-culture with ammonia-oxidizing bacteria in municipal wastewater. Renew. Energy151, 598–603 (2020). 10.1016/j.renene.2019.11.063 [DOI] [Google Scholar]
- 14.Hallenbeck, P. C., Grogger, M., Mraz, M. & Veverka, D. The use of design of experiments and response surface methodology to optimize biomass and lipid production by the oleaginous marine green alga, Nannochloropsis gaditana in response to light intensity, inoculum size and CO2. Bioresour. Technol.184, 161–168 (2015). 10.1016/j.biortech.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 15.Ghaedi, M., Khafri, H. Z., Asfaram, A. & Goudarzi, A. Response surface methodology approach for optimization of adsorption of Janus Green B from aqueous solution onto ZnO/Zn (OH) 2-NP-AC: Kinetic and isotherm study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.152, 233–240 (2016). 10.1016/j.saa.2015.06.128 [DOI] [PubMed] [Google Scholar]
- 16.Pandey, A., Gupta, A., Sunny, A., Kumar, S. & Srivastava, S. J. R. E. Multi-objective optimization of media components for improved algae biomass, fatty acid and starch biosynthesis from Scenedesmus sp. ASK22 using desirability function approach. Renew. Energy150, 476–486 (2020). 10.1016/j.renene.2019.12.095 [DOI] [Google Scholar]
- 17.Anahas, A. M. P. & Muralitharan, G. J. R. E. Central composite design (CCD) optimization of phytohormones supplementation for enhanced cyanobacterial biodiesel production. Renew. Energy130, 749–761 (2019). 10.1016/j.renene.2018.06.110 [DOI] [Google Scholar]
- 18.Gopalakrishnan, K., Roostaei, J. & Zhang, Y. Mixed culture of Chlorella sp. and wastewater wild algae for enhanced biomass and lipid accumulation in artificial wastewater medium. Front. Environ. Sci. Eng.12(4), 14 (2018). 10.1007/s11783-018-1075-2 [DOI] [Google Scholar]
- 19.Feng, Y., Li, C. & Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol.102(1), 101–105 (2011). 10.1016/j.biortech.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 20.Atta, M., Idris, A., Bukhari, A. & Wahidin, S. Intensity of blue LED light: A potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour. Technol.148, 373–378 (2013). 10.1016/j.biortech.2013.08.162 [DOI] [PubMed] [Google Scholar]
- 21.Khalili, A., Najafpour, G. D., Amini, G. & Samkhaniyani, F. Influence of nutrients and LED light intensities on biomass production of microalgae Chlorella vulgaris. Biotechnol. Bioprocess Eng.20(2), 284–290 (2015). 10.1007/s12257-013-0845-8 [DOI] [Google Scholar]
- 22.Metsoviti, M. N., Papapolymerou, G., Karapanagiotidis, I. T. & Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants9(1), 31 (2020). 10.3390/plants9010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumin, J. et al. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels8(1), 42 (2015). 10.1186/s13068-015-0220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roostaei, J., Zhang, Y., Gopalakrishnan, K. & Ochocki, A. J. Mixotrophic microalgae biofilm: A novel algae cultivation strategy for improved productivity and cost-efficiency of biofuel feedstock production. Sci. Rep.8(1), 12528 (2018). 10.1038/s41598-018-31016-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuentes, J. L. et al. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs14(5), 100 (2016). 10.3390/md14050100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins, B. T. et al. Cofactor symbiosis for enhanced algal growth, biofuel production, and wastewater treatment. Algal Res.17, 308–315 (2016). 10.1016/j.algal.2016.05.024 [DOI] [Google Scholar]
- 27.Gopalakrishnan, K., Roostaei, J. & Zhang, Y. Mixed culture of Chlorella sp. and wastewater wild algae for enhanced biomass and lipid accumulation in artificial wastewater medium. Front. Environ. Sci. Eng.12, 1–16 (2018). 10.1007/s11783-018-1075-2 [DOI] [Google Scholar]
- 28.Arita, C. E. Q., Peebles, C. & Bradley, T. H. Scalability of combining microalgae-based biofuels with wastewater facilities: A review. Algal Res.9, 160–169 (2015). 10.1016/j.algal.2015.03.001 [DOI] [Google Scholar]
- 29.Moreira, D. & Pires, J. C. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol.215, 371–379 (2016). 10.1016/j.biortech.2016.03.060 [DOI] [PubMed] [Google Scholar]
- 30.Teplitski, M., Rajamani, S. Signal and Nutrient Exchange in the Interactions Between Soil Algae and Bacteria, Biocommunication in Soil Microorganisms 413–426 (Springer, 2011).
- 31.Yung, K.-H. & Mudd, J. J. P. P. Lipid synthesis in the presence of nitrogenous compounds in Chlorella pyrenoidosa. Plant Physiol.41(3), 506–509 (1966). 10.1104/pp.41.3.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu, S.-Y. et al. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol.100(2), 833–838 (2009). 10.1016/j.biortech.2008.06.061 [DOI] [PubMed] [Google Scholar]
- 33.Cheirsilp, B. & Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol.110, 510–516 (2012). 10.1016/j.biortech.2012.01.125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.