Abstract
In this multicenter, non-inferiority, randomized trial, we randomly assigned 992 women undergoing in-vitro fertilization (IVF) with a good prognosis (aged 20-40, ≥3 transferrable cleavage-stage embryos) to strategies of blastocyst-stage (n = 497) or cleavage-stage (n = 495) single embryo transfer. Primary outcome was cumulative live-birth rate after up to three transfers. Secondary outcomes were cumulative live-births after all embryo transfers within 1 year of randomization, pregnancy outcomes, obstetric-perinatal complications, and livebirths outcomes. Live-birth rates were 74.8% in blastocyst-stage group versus 66.3% in cleavage-stage group (relative risk 1.13, 95%CI:1.04-1.22; Pnon-inferiority < 0.001, Psuperiority = 0.003) (1-year cumulative live birth rates of 75.7% versus 68.9%). Blastocyst transfer increased the risk of spontaneous preterm birth (4.6% vs 2.0%; P = 0.02) and neonatal hospitalization >3 days. Among good prognosis women, a strategy of single blastocyst transfer increases cumulative live-birth rates over single cleavage-stage transfer. Blastocyst transfer resulted in higher preterm birth rates. This information should be used to counsel patients on their choice between cleavage-stage and blastocyst-stage transfer (NCT03152643, https://clinicaltrials.gov/study/NCT03152643).
Subject terms: Outcomes research, Randomized controlled trials
This randomized trial assessed the effectiveness and safety of single blastocyst transfer vs single cleavage-stage embryo transfer among women with good prognosis. Here, the authors show improved cumulative live birth rates and relatively unfavorable perinatal outcomes after blastocyst transfer.
Introduction
In vitro fertilization (IVF) is the cornerstone of modern infertility treatment. More than 2 million treatment cycles are performed worldwide each year1–3. However, in the last decade, the live birth rate of IVF has been stable at 30% per transfer, resulting in cumulative live birth rates over 50%4. In an attempt to increase the success rates, extended culture of embryos from cleavage stage to blastocyst stage has been introduced5,6. It has been hypothesized that extended culture to blastocyst stage allows selection of embryos with higher implantation potential, which also facilitates elective single embryo transfer (SET) to reduce multiple gestations and associated pregnancy complications7,8.
In recent years, blastocyst transfers have become increasingly popular worldwide, whereas most countries still widely use cleavage-stage transfers, driven by the risk of no or fewer embryos available for transfer after blastocyst culture3,9,10. The evidence regarding the effectiveness and safety of the blastocyst-stage versus cleavage-stage embryo transfers is however limited11,12.
Initial studies showing that single blastocyst transfers generated higher live birth rates were halted early13. Systematic reviews show substantial study heterogeneity with conflicting results11. Some studies reported benefits of cleavage-stage transfer14, some found similar results15,16 and others gave preference to blastocyst transfer17,18. Many trials were single-center and had a small sample size, with unclear randomization and concealment methods. Only one small trial reported on cumulative live birth rates, the most important outcome from a patient perspective19. No trials reported obstetric and perinatal outcomes. Furthermore, most trials conducted a decade ago do not reflect modern IVF practice, including SET and vitrification freezing. As a consequence, the most recent Cochrane review only provides low-quality evidence and does not report on cumulative live birth outcomes, and recommends large-scale trials on the subject11.
In this work, we assessed the effectiveness and safety of single blastocyst transfer vs single cleavage-stage embryo transfer, and show improved cumulative live birth rates and relatively unfavorable perinatal outcomes after blastocyst transfer among women with good prognosis (three or more transferrable cleavage-stage embryos).
Results
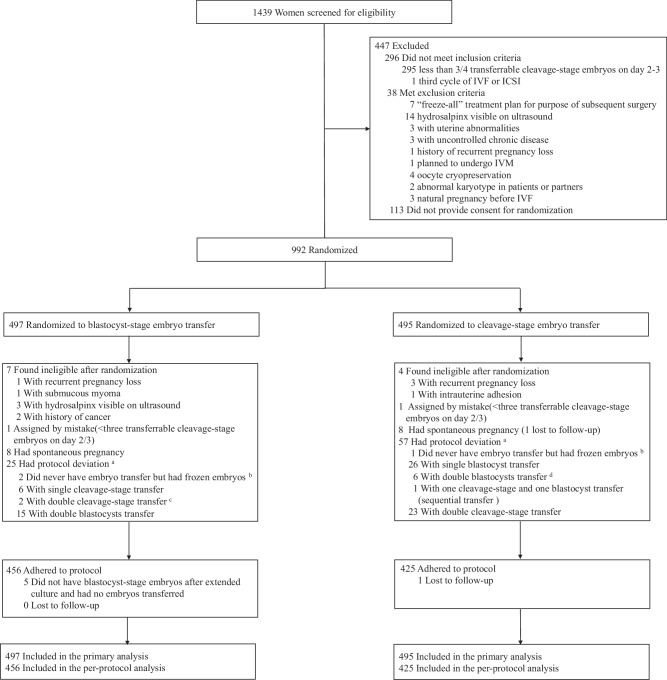
Between 8 October 2018 and 22 August 2019, we screened 1439 women, of whom 1105 were eligible, of which 113 declined to participate. Therefore, 992 women were randomized to transfer at the blastocyst stage (n = 497) or cleavage stage (n = 495) (Fig. 1). Follow-up of all live births was completed on 6 September 2021 (trial status: completed). Baseline characteristics, including details of ovarian stimulation, were comparable between the two groups (Table 1 and Table 2).
Fig. 1. Flow chart showing screening, randomization, follow-ups, and protocol deviations.
IVF, in-vitro fertilization; ICSI, intracytoplasmic sperm injection; IVM, in-vitro maturation. a Woman had protocol deviation in at least one transfer. b Two women in the blastocyst group never had embryo transfer but had frozen blastocysts for the reasons of endometrial factor and personal issues, respectively; 1 woman in the cleavage group never had embryo transfer but had frozen cleavage-stage embryos for the reason of personal issues. c Among the 2 women with double cleavage-stage transfer, 1 woman had double cleavage-stage transfer in the fresh embryo transfer cycle and double blastocyst transfer in the second frozen embryo transfer cycle. d Among the 6 women with double blastocyst transfer, 1 woman had double cleavage-stage transfer in the fresh embryo transfer cycle, and double blastocyst transfer in the subsequent frozen embryo transfer cycle; 1 woman had one blastocyst transfer in the first frozen transfer cycle, and double blastocysts transfer in the second frozen transfer cycle.
Table 1.
Baseline characteristics of trial participants (intention-to-treat analysis)
| Characteristics | Blastocyst-stage embryo transfer group (n = 497) | Cleavage-stage embryo transfer group (n = 495) |
|---|---|---|
| Female age, mean (SD), years | 29.6 (3.6) | 29.9 (3.5) |
| Female age, No. (%) | ||
| ≤30 years | 318 (64.0) | 296 (59.8) |
| 30–35 years | 150 (30.2) | 160 (32.3) |
| >35 years | 29 (5.8) | 39 (7.9) |
| Body mass index, mean (SD)a | 22.9 (3.3) | 22.8 (3.2) |
| Duration of attempt to conceive, mean (SD), years | 3.4 (2.3) | 3.5 (2.2) |
| Primary infertility, No. (%) | 255 (51.3) | 239 (48.3) |
| Number of previous IVF or ICSI cycles, No. (%) | ||
| 0 | 466 (93.8) | 449 (90.7) |
| 1 | 31 (6.2) | 46 (9.3) |
| IVF indication, No. (%) | ||
| Tubal factor | 249 (50.1) | 233 (47.1) |
| Male factor | 75 (15.1) | 79 (16.0) |
| Ovulatory dysfunction | 46 (9.3) | 32 (6.5) |
| Endometriosis | 11 (2.2) | 23 (4.6) |
| Unexplained | 27 (5.4) | 32 (6.5) |
| Combined factors | 86 (17.3) | 95 (19.2) |
| Other | 3 (0.6) | 1 (0.2) |
| Antral follicle count in both ovaries, mean (SD) | 18.0 (6.4) | 17.7 (6.9) |
| Follicle-stimulating hormone, mean (SD) [No.], IU/L | 6.6 (1.9) [495] | 6.6 (2.0) [493] |
| Luteinizing hormone, mean (SD) [No.], IU/L | 5.9 (3.8) [496] | 5.7 (3.9) [493] |
| Estradiol, mean (SD) [No.], pg/mL | 45.8 (33.8) [496] | 42.6 (28.8) [493] |
| Anti-Müllerian Hormone, mean (SD) [No.], ng/mL | 5.6 (3.9) [470] | 5.5 (3.9) [467] |
IVF in-vitro fertilization, ICSI intracytoplasmic sperm injection, SD standard deviation.
aCalculated as weight in kilograms divided by the square of the height in meters.
Table 2.
Ovarian stimulation and embryo transfer (intention-to-treat analysis)
| Characteristics | Blastocyst-stage embryo transfer group (n = 497) | Cleavage-stage embryo transfer group (n = 495) | P value |
|---|---|---|---|
| Type of ovarian stimulation, No. (%) | |||
| Long GnRH Agonist suppression | 72 (14.5) | 76 (15.4) | 0.96 |
| GnRH Antagonist suppression | 214 (43.1) | 210 (42.4) | |
| Agonist flare | 30 (6.0) | 27 (5.5) | |
| Depot GnRH Agonist suppression | 181 (36.4) | 182 (36.8) | |
| Duration of ovarian stimulation, mean (SD), days | 10.2 (2.1) | 10.3 (2.1) | 0.53 |
| Total gonadotropin dose, mean (SD), IU | 1812.1 (692.0) | 1808.2 (732.0) | 0.93 |
| No of oocytes retrieved, mean (SD) | 15.0 (7.9) | 14.9 (7.4) | 0.86 |
| Estradiol level on hCG trigger day, mean (SD) [No.], pg/mL | 4444.6 (3168.5) [496] | 4278.0 (2685.9) [495] | 0.37 |
| Progesterone level on hCG trigger day, mean (SD) [No.], ng/mL | 1.3 (1.4) [494] | 1.2 (0.8) [495] | 0.34 |
| ICSI for insemination, No. (%) | 125 (25.2) | 126 (25.5) | 0.91 |
| No of viable embryos on day 2–3, mean (SD) | 8.8 (4.6) | 8.5 (4.4) | 0.32 |
| No of good-quality embryos on day 2–3, mean (SD) | 6.2 (3.9) | 6.1 (3.7) | 0.73 |
| No of viable embryos on day 5–6, mean (SD) | 5.6 (3.4) | - | - |
| No of good-quality embryos on day 5–6, mean (SD) | 3.7 (2.9) | - | - |
| First transfer (fresh), No. (%) | 264 (53.1)a | 272 (54.9)b | 0.56 |
| Freeze-only, No. (%)c | 227 (45.7) | 223 (45.1) | 0.84 |
| Reasons for Freeze-only, No. (%) | |||
| Endometrial factor | 8/227 (3.5) | 15/223 (6.7) | 0.28 |
| Risk of OHSS | 167/227 (73.6) | 164/223 (73.5) | |
| High progesterone | 29/227 (12.8) | 29/223 (13.0) | |
| Other | 23/227 (10.1) | 15/223 (6.7) | |
| No embryo transfers, No. (%) | 11 (2.2) | 4 (0.8) | 0.07 |
| Reasons for no-transfer, No. (%) | |||
| No embryo available | 7/11 (63.6)d | 0 | 0.056 |
| Natural conception after oocyte retrieval | 2/11 (18.2) | 3/4 (75.0) | |
| Personal issue | 1/11 (9.1) | 1/4 (25.0) | |
| Endometrial factor | 1/11 (9.1) | 0 | |
| No of embryo transfers, No. (%)e | 727 | 875 | |
| First transfers | 486/727 (66.9) | 491/875 (56.1) | 1.44e-06 |
| Second transfers | 189/727 (26.0) | 261/875 (29.8) | |
| Third transfers | 52/727 (7.2) | 123/875 (14.1) | |
| No of embryos transferred, No. (%)e | |||
| One embryo | 709/727 (97.5) | 842/875 (96.2) | 0.14 |
| Two embryos | 18/727(2.5) | 33/875 (3.8) | |
| Stage of embryo transferred, No. (%)e | |||
| Blastocyst-stage embryo transfer | 719/727 (98.9) | 42/875 (4.8)f | 1.46e-308 |
| Cleavage-stage embryo transfer | 8/727 (1.1) | 833/875 (95.2) | |
| Moderate or severe OHSS, No. (%) | 23 (4.6) | 17 (3.4) | 0.34 |
GnRH gonadotropin-releasing hormone, hCG human chorionic gonadotropin, ICSI intracytoplasmic sperm injection, OHSS ovarian hyperstimulation syndrome, SD standard deviation.
Two-sided P values. No adjustments were made for multiple comparisons. A two-sample t-test was used for continuous variables; Chi-square test or Fisher’s Exact Test was used for categorical data.
a12 participants underwent fresh blastocyst transfer on day 6.
bOnly 7 participants underwent embryo transfer on day 2, all occurring in fresh transfer cycle at one study site, due to their work schedules.
cWomen who underwent freeze-only had all embryos frozen in the fresh cycle of ovarian stimulation for risk of OHSS or other reasons. Six participants in the blastocyst group did not have any embryos available for transfer and freezing.
dFive participants had no blastocyst-stage embryos after extended culture and no embryos transferred; 1 participant had no embryos but was randomized; 1 participant had 1 blastocyst frozen after freeze-only strategy, but the blastocyst did not survive after thawing.
eCalculated based on the total number of embryo transfer cycles.
f One participant underwent sequential transfer (transfer of one cleavage-stage and one blastocyst-stage embryo in one frozen embryo transfer cycle).
Among 497 women assigned to the blastocyst group, four women (0.8%) had frozen cleavage-stage embryos, and two of them also had frozen blastocysts. Five women (1.0%) did not have blastocyst-stage embryos after extended culture. Of 727 embryo transfer cycles in the blastocyst group, 8 (1.1%) were transferred at the cleavage stage. There were 18 double embryo transfers (2.5%) in the blastocyst group (Table 2).
Among 495 women assigned to the cleavage-stage group, nine women (1.8%) frozen blastocysts only; 130 women (26.3%) frozen both cleavage-stage and blastocyst-stage embryos, with 114 women (87.7%) freezing ≥3 cleavage-stage embryos. Of 875 embryo transfer cycles in the cleavage-stage group, 42 (4.8%) were transferred at the blastocyst stage. There were 33 double embryo transfers (3.8%) in the cleavage-stage group (Table 2).
Fewer women in the blastocyst group underwent a second or third transfer than in the cleavage-stage group (189 vs 261 for second transfer; 52 vs 123 for third transfer) (Table 2). Protocol deviations of crossover occurred in 8 of 497 women (1.6%) in the blastocyst-stage group versus 33 of 495 women (6.7%) in the cleavage-stage group (Fig. 1). Only 7 women (1.4%) in the cleavage-stage group received day 2 embryo transfer, all occurring in fresh transfer cycle at one study site due to their work schedule.
Primary outcome
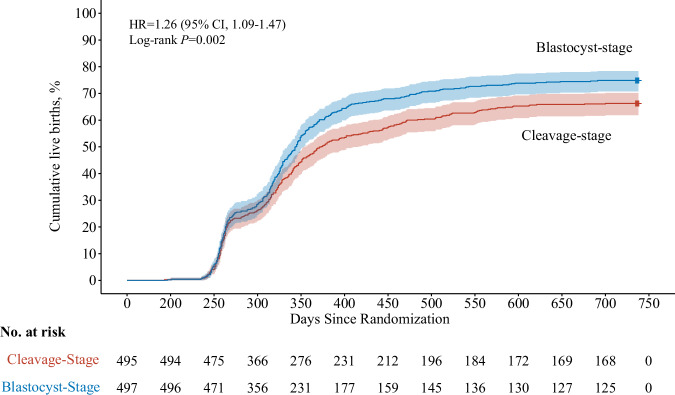
The primary outcome live birth occurred in 372 of 497 women (74.8%) in the blastocyst group versus 328 of 495 (66.3%) in the cleavage-stage group (AD 8.6% [95% CI 2.9% to 14.2%]; RR 1.13 [95% CI 1.04 to 1.22]; P-value non-inferiority <0.001; P-value superiority=0.003) (Table 3). Both non-inferiority and superiority were confirmed in the intention-to-treat population as well as in the per-protocol population (Supplementary Fig. 1 and Supplementary Table 5). The Kaplan-Meier curves for the primary outcome are shown in Fig. 2.
Table 3.
Cumulative Live Births and Pregnancy Outcomes (Intention-to-Treat Analysis)
| Outcomes, No./total (%) | Blastocyst-stage embryo transfer group (n = 497) | Cleavage-stage embryo transfer group (n = 495) | Absolute difference (95% CI)a | Relative risk (95% CI) | P valueb |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Cumulative live birthsc | 372 (74.8) | 328 (66.3) | 8.6 (2.9 to 14.2) | 1.13 (1.04 to 1.22) | 0.003 |
| Singleton live births | 363 (73.0) | 324 (65.5) | 7.6 (1.9 to 13.3) | 1.12 (1.03 to 1.21) | 0.010 |
| All twin live births | 9 (1.8) | 4 (0.8) | 1.0 (−0.4 to 2.4) | 2.24 (0.69 to 7.23) | 0.17 |
| Monozygotic twin live births | 7 (1.4) | 2 (0.4) | 1.0 (−0.2 to 2.2) | 3.49 (0.73 to 16.70) | 0.18 |
| Secondary outcomes | |||||
| Cumulative biochemical pregnanciesd | 430 (86.5) | 399 (80.6) | 5.9 (1.3 to 10.5) | 1.07 (1.02 to 1.13) | 0.01 |
| Cumulative clinical pregnanciese | 416 (83.7) | 378 (76.4) | 7.3 (2.4 to 12.3) | 1.10 (1.03 to 1.17) | 0.004 |
| Cumulative ongoing pregnanciesf | 385 (77.5) | 336 (67.9) | 9.6 (4.1 to 15.1) | 1.14 (1.06 to 1.23) | 0.00070 |
| Cumulative pregnancy loss | |||||
| Biochemical pregnancy loss | 37 (7.4) | 36 (7.3) | 0.2 (−3.1 to 3.4) | 1.02 (0.66 to 1.59) | 0.92 |
| Clinical pregnancy loss | 54 (10.9) | 54 (10.9) | −0.0 (−3.9 to 3.8) | 1.00 (0.70 to 1.42) | 0.98 |
| Miscarriage <12 weeks gestation | 43 (8.7) | 49 (9.9) | −1.2 (−4.9 to 2.4) | 0.87 (0.59 to 1.29) | 0.50 |
| Miscarriage 12-24 weeks gestation | 11 (2.2) | 5 (1.0) | 1.2 (−0.4 to 2.8) | 2.19 (0.77 to 6.26) | 0.13 |
| Ectopic pregnancy | 7 (1.4) | 11 (2.2) | −0.8 (−2.5 to 0.8) | 0.63 (0.25 to 1.62) | 0.34 |
| Live birth after first embryo transferg | 256/486 (52.7) | 200/491 (40.7) | 11.9 (5.7 to 18.2) | 1.29 (1.13 to 1.48) | 0.00018 |
| Live birth after second embryo transfer | 89/189 (47.1) | 85/261 (32.6) | 14.5 (5.4 to 23.6) | 1.45 (1.15 to 1.82) | 0.002 |
| Live birth after third embryo transfer | 21/52 (40.4) | 36/123 (29.3) | 11.1 (−4.5 to 26.7) | 1.38 (0.90 to 2.12) | 0.15 |
| Live birth after natural conception | 6 (1.2) | 7 (1.4) | −0.2 (−1.6 to 1.2) | 0.85 (0.29 to 2.52) | 0.78 |
| Median time to livebirth since randomization, daysh | 344 (334−353)i | 373 (353 − 416)i | −31.3 (−32.2 to −30.4) | 1.26 (1.09 to 1.47)j | 0.002k |
| No. of unused frozen embryos, mean (SD) | 4.1 (3.3) | 5.9 (4.0) | −1.8 (−2.3 to −1.3) | - | 3.37e-14 |
| No. of unused frozen embryos in women with a live birth, mean (SD) | 4.7 (3.3) | 6.4 (3.8) | −1.7 (−2.2 to −1.2) | - | 7.15e-10 |
| No. of unused frozen embryos in women without a live birth, mean (SD) | 2.2 (2.6) | 4.9 (4.2) | −2.7 (−3.4 to −1.9) | - | 2.03e-09 |
| No. of women without a frozen embryo | 71 (14.3) | 23 (4.6) | 9.6 (6.0 to 13.2) | 3.07 (1.95 to 4.84) | 2.18e-07 |
| No. of women without a frozen embryo without a livebirth | 46 (9.3) | 22 (4.4) | 4.8 (1.7 to 7.9) | 2.08 (1.27 to 3.41) | 0.003 |
| No. of women without a frozen embryo with a livebirth | 25 (5.0) | 1 (0.2) | 4.8 (2.9 to 6.8) | 24.90 (3.39 to183.05) | 1.94e-06 |
SD standard deviation.
aAbsolute differences in percentages are indicated in percentage points, and absolute differences in other values are indicated in units of that value.
bAll P values are for superiority, two-sided. No adjustments were made for multiple comparison. Two-sample t-test was used for continuous variable; Chi-square test was used for categorical data.
cCumulative live births were calculated from up to the first 3 embryo transfers in 1 year after randomization (with a 3-months extension for those affected by Covid-19) from one oocyte retrieval cycle. Live birth was defined as delivery of any neonate ≥24 weeks gestation that had a heartbeat and was breathing. P = 5.60e-11 for non-inferiority (with margin = −0.1, α = 0.025, one-sided).
dBiochemical pregnancy was defined as serum human chorionic gonadotropin ≥25 IU/L 14 days after embryo transfer.
eClinical pregnancy was defined as detection of intrauterine gestation sacs at 30–35 days after embryo transfer. One woman in the cleavage-stage group was lost to follow-up after confirmation of clinical pregnancy.
fOngoing pregnancy was defined as detection of a viable fetus with heartbeat at 12 weeks’ gestation. One woman in the cleavage-stage group was lost to follow-up after confirmation of an ongoing pregnancy.
gLivebirth after the first embryo transfers included 235 livebirths from 536 fresh embryo transfer and 221 livebirths from 441 first frozen embryo transfer (freeze-only strategy).
hThe length of time from randomization to 50% of the participants who achieved a livebirth.
i95% confidence interval for median time to live birth.
jHazard ratio (95% confidence interval).
klog-rank test.
Fig. 2. Kaplan–Meier graph of time to cumulative live births by randomized group (intention-to-treat population).
HR, hazard ratio. Shaded areas indicate 95% confidence intervals (CI). Median time to live birth in the blastocyst group was 344 days (95% CI, 334–353 days); median time to live birth in the cleavage-stage group was 373 days (95% CI, 353–416 days). Hazard ratio and the associated 95% CIs were estimated by using a Cox proportional hazards model. Source data are provided as a Source Data file.
Secondary outcomes
Blastocyst transfer was associated with higher cumulative rates of biochemical, clinical, and ongoing pregnancy than was cleavage-stage transfer (Table 3). The cumulative incidence of twins and pregnancy loss did not differ significantly between the two groups (Table 3). Median time to live birth was significantly shorter in the blastocyst group versus the cleavage-stage group (344 days vs 373 days; HR 1.26[95% CI 1.09 to 1.47]; P = 0.002) (Table 3). Between-group comparisons for pregnancy outcomes of each transfer showed higher frequencies of live birth, implantation, biochemical, clinical, and ongoing pregnancy after blastocyst transfer (Supplementary Table 2–4). Post hoc analysis showed a significantly fewer number of unused frozen embryos (4.1 [SD3.3] vs 5.9 [SD4.0]; P < 0.001), while a higher number of women without a frozen embryo (14.3% vs 4.6%; P < 0.001) in blastocyst group versus cleavage-stage group (Table 3).
Safety outcomes
Blastocyst transfer was associated with a higher cumulative rate of preterm premature rupture of membrane (PPROM) (5.0% vs 1.6%; AD 3.4%[95%CI 1.2% to 5.6%]; RR 3.11 [95%CI 1.42 to 6.83]; P = 0.003), preterm birth (6.0% vs 3.6%; AD 2.4%[95%CI −0.3% to 5.1%]; RR 1.66 [95%CI 0.94 to 2.94]; P = 0.08), neonatal hospitalization >3 days (11.5% vs 6.3%; AD 5.2%[95%CI 1.7% to 8.7%]; RR 1.83 [95%CI 1.20 to 2.79]; P = 0.004), and neonatal infection (4.8% vs 2.2%; AD 2.6%[95%CI 0.3% to 4.9%]; RR 2.17 [95%CI 1.08 to 4.39]; P = 0.03); but a lower cumulative rate of preeclampsia (1.0% vs 2.8%; AD −1.8%[95%CI −3.5% to −0.1%]; RR 0.36 [95%CI 0.13 to 0.98]; P = 0.04) (Table 4). Of preterm birth, spontaneous preterm birth occurred more frequently in the blastocyst group versus the cleavage-stage group (4.6% vs 2.0%; AD 2.6% [95%CI 0.4% to 4.8%]; RR 2.29 [95%CI 1.10 to 4.76]; P = 0.02), whereas the frequency of iatrogenic preterm birth was similar. Women after blastocyst transfer had an increased risk of developing at least one of the maternal or neonatal complications compared with those after cleavage-stage transfer (50.9% vs 43.8%; AD 7.1%[95%CI 0.9% to 13.3%]; RR 1.16 [95%CI 1.02 to 1.32]; P = 0.03). In addition, more preeclampsia occurred after fresh cleavage-stage transfer (6/14 [42.9%] vs 0/5 [0.0%] for fresh cycles (Supplementary Table 11), and logistic regression analyzes showed that the risk of preeclampsia remained higher in the cleavage-stage group than in the blastocyst group after adjustment for frozen or fresh embryo transfer (Supplementary Table 12). The number of uncomplicated live birth and incidences of other obstetrical and neonatal complications including congenital anomalies were comparable (Table 4; Supplementary Table 7,8).
Table 4.
Cumulative Obstetric and Perinatal Outcomes (Intention-to-Treat Analysis)
| Outcomes, No./total (%) | Blastocyst-stage embryo transfer group (n = 497) | Cleavage-stage embryo transfer group (n = 495) | Absolute differencea(95% CI) | Relative risk (95% CI) | P value |
|---|---|---|---|---|---|
| Live births outcomes | |||||
| Gestational age, mean (SD), weeks | 38.8 (1.7) | 38.9 (1.7) | −0.1 (−0.4 to 0.1) | - | 0.40 |
| Birthweight, mean (SD), grams | |||||
| Singleton | |||||
| No of observations | 363 | 324 | |||
| Mean weight | 3357.6 (505.0) | 3333.7 (526.5) | 23.9 (−53.4 to 101.2) | - | 0.54 |
| Twin | |||||
| No of observations | 18 | 8 | |||
| Mean weight | 2388.3 (650.5) | 2088.8 (622.6) | 299.6 (−263.9 to 863.0) | - | 0.28 |
| Low birth weightb | 18 (3.6) | 18 (3.6) | −0.0 (−2.3 to 2.3) | 1.00 (0.52 to 1.89) | 0.99 |
| Very low birth weightc | 5 (1.0) | 4 (0.8) | 0.2 (−1.0 to 1.4) | 1.24 (0.34 to 4.61) | 1.00 |
| Macrosomiad | 33 (6.6) | 36 (7.3) | −0.6 (−3.8 to 2.5) | 0.91 (0.58 to 1.44) | 0.70 |
| Small for gestational agee | 23 (4.6) | 20 (4.0) | 0.6 (−1.9 to 3.1) | 1.15 (0.64 to 2.06) | 0.65 |
| Large for gestational agef | 63 (12.7) | 54 (10.9) | 1.8 (−2.2 to 5.8) | 1.16 (0.83 to 1.63) | 0.39 |
| Sex ratio | |||||
| Male | 206/381(54.1) | 164/332(49.4) | 4.7 (−2.7 to 12.0) | 1.09 (0.95 to 1.26) | 0.21 |
| Female | 175/381(45.9) | 168/332(50.6) | |||
| Live birth without a complication | 130 (26.2) | 118 (23.8) | 2.3 (−3.1 to 7.7) | 1.10 (0.88 to 1.36) | 0.40 |
| Maternal complications | |||||
| Gestational diabetes mellitus | 54 (10.9) | 55 (11.1) | −0.2 (−4.1 to 3.6) | 0.98 (0.69 to 1.39) | 0.90 |
| Preeclampsia or eclampsia | 5 (1.0) | 14 (2.8) | −1.8 (−3.5 to −0.1) | 0.36 (0.13 to 0.98) | 0.04 |
| Gestational hypertension | 20 (4.0) | 17 (3.4) | 0.6 (−1.8 to 2.9) | 1.17 (0.62 to 2.21) | 0.62 |
| Preterm premature rupture of membraneg | 25 (5.0) | 8 (1.6) | 3.4 (1.2 to 5.6) | 3.11 (1.42 to 6.83) | 0.003 |
| Premature rupture of membrane | 51 (10.3) | 37 (7.5) | 2.8 (−0.7 to 6.3) | 1.37 (0.92 to 2.06) | 0.12 |
| Preterm birthh | 30 (6.0) | 18 (3.6) | 2.4 (−0.3 to 5.1) | 1.66 (0.94 to 2.94) | 0.08 |
| Spontaneous preterm birthi | 23 (4.6) | 10 (2.0) | 2.6 (0.4 to 4.8) | 2.29 (1.10 to 4.76) | 0.02 |
| Iatrogenic preterm birthj | 7 (1.4) | 8 (1.6) | −0.2 (−1.7 to 1.3) | 0.87 (0.32 to 2.38) | 0.79 |
| Very preterm birth ( < 32 Weeks)k | 5 (1.0) | 4 (0.8) | 0.2 (−1.0 to 1.4) | 1.24 (0.34 to 4.61) | 1.00 |
| Placenta previa | 8 (1.6) | 2 (0.4) | 1.2 (−0.0 to 2.4) | 3.98 (0.85 to 18.67) | 0.11 |
| Placental abruption | 3 (0.6) | 2 (0.4) | 0.2 (−0.7 to 1.1) | 1.49 (0.25 to 8.90) | 1.00 |
| Placental accreta | 12 (2.4) | 6 (1.2) | 1.2 (−0.5 to 2.9) | 1.99 (0.75 to 5.27) | 0.16 |
| Other placental abnormality | 11 (2.2) | 8 (1.6) | 0.6 (−1.1 to 2.3) | 1.37 (0.56 to 3.38) | 0.49 |
| Postpartum hemorrhage | 10 (2.0) | 17 (3.4) | −1.4 (−3.4 to 0.6) | 0.59 (0.27 to 1.27) | 0.17 |
| Neonatal complications | |||||
| Therapeutic abortion or fetal reduction due to fetal congenital anomalies during 12 to 28 weeks of gestationl | 6 (1.2) | 4 (0.8) | 0.4 (−0.8 to 1.6) | 1.49 (0.42 to 5.26) | 0.76 |
| Stillbirth | 8 (1.6) | 4 (0.8) | 0.8 (−0.6 to 2.2) | 1.99 (0.60 to 6.57) | 0.25 |
| Neonatal hospitalization > 3 days | 57 (11.5) | 31 (6.3) | 5.2 (1.7 to 8.7) | 1.83 (1.20 to 2.79) | 0.004 |
| Neonatal jaundice | 119 (23.9) | 101 (20.4) | 3.5 (−1.6 to 8.7) | 1.17 (0.93 to 1.48) | 0.18 |
| Neonatal infection | 24 (4.8) | 11 (2.2) | 2.6 (0.3 to 4.9) | 2.17 (1.08 to 4.39) | 0.03 |
| Neonatal death among live newborns | 1 (0.2) | 2 (0.4) | −0.2 (−0.9 to 0.5) | 0.50 (0.05 to 5.47) | 1.00 |
| Congenital anomalyl | 16 (3.2) | 10 (2.0) | 1.2 (−0.8 to 3.2) | 1.59 (0.73 to 3.48) | 0.24 |
| Participants with at least one of the maternal or neonatal complicationsm | 253 (50.9) | 217 (43.8) | 7.1 (0.9 to 13.3) | 1.16 (1.02 to 1.32) | 0.03 |
SD standard deviation. Two-sided P values. No adjustments were made for multiple comparisons. Two-sample t-test was used for continuous variable; Chi-square test or Fisher’s Exact Test was used for categorical data.
aAbsolute differences in percentages are indicated in percentage points, and absolute differences in other values are indicated in units of that value.
bLow birth weight was defined as a value of <2500 g.
cVery low birth weight was defined as a value of <1500 g.
dMacrosomia was defined as a value of >4000 g.
eBirthweight lower than the 10th percentile.
fBirthweight higher than the 90th percentile.
gPreterm premature rupture of membrane in the table refers to premature rupture of membrane before 37 weeks gestational age.
hPreterm birth was defined as delivery at less than 37 weeks gestational age.
iSpontaneous preterm birth was defined as preterm delivery due to spontaneous labor resulting from preterm premature rupture of membranes, cervical factors and other reasons.
jIatrogenic preterm birth was defined as a preterm birth resulting from a planned delivery due to maternal and/or fetal complications, including labor induction or cesarean section.
kVery preterm birth was defined as delivery at less than 32 weeks gestational age.
lDetails of congenital anomaly were listed in Supplementary Table 7.
mMaternal complications include gestational diabetes mellitus, preeclampsia or eclampsia, gestational hypertension, premature rupture of membrane, preterm birth, placenta previa, placental abruption, placental accreta, other placental abnormality, and postpartum hemorrhage. Neonatal complications include therapeutic abortion or fetal reduction due to fetal congenital anomalies during 12 to 28 weeks of gestation, stillbirth, neonatal hospitalization > 3 days, neonatal jaundice, neonatal infection, neonatal death among live newborns, congenital anomaly, low birth weight, macrosomia, small for gestational age, and large for gestational age.
Sensitivity Analyzes
The results of the per-protocol analyzes (Supplementary Table 5–6) and full analysis set as well as embryo transfers within 1 year of randomization and all embryo transfers within the study period (Supplementary Table 9–10) were consistent with those of the intention-to-treat analysis for the rates of live birth, pregnancy, and perinatal outcomes. The results for the primary outcomes remained robust after controlling for centers.
Post Hoc Subgroup Analyzes
Hyper-responders ( > 15 oocytes retrieved) benefitted more from blastocyst transfer with regard to the primary outcome than poor or normal responders (P-value for interaction = 0.03). For women with estradiol on hCG day at the highest and medium tertiles, there seemed to be a benefit of blastocyst transfer (P-value for interaction = 0.01). There were no differential effects of treatment on other subgroups (Supplementary Fig. 2).
Post Hoc Analyzes of long-term follow-up outcomes
When analyzing follow-ups of embryo transfers from day of randomization to July 28th, 2023, cumulative live birth rate was not significantly higher in the blastocyst group than in the cleavage-stage group (80.9% [402/497] vs 77.6% [384/495]; AD, 3.3% [95%CI −1.7 to 8.4]; RR 1.04 [95%CI 0.98 to 1.11]; P = 0.199) (Table 5). Among the deviations that occurred after the study period (1 year after randomization), 41.3% of transfers in the cleavage-stage group were blastocyst transfers, whereas all transfers in the blastocyst group were blastocyst transfers (Supplementary Table 13). Furthermore, 48.8% of women in cleavage-stage group obtained an extra live birth through blastocyst transfers (Supplementary Table 14).
Table 5.
Non-prespecified outcomes for cumulative live births including the long-term follow-up cohort (Intention-to-Treat Analysis)a
| Outcomes, No./total (%) | Blastocyst-stage embryo transfer group (n = 497) | Cleavage-stage embryo transfer group (n = 495) | Absolute difference (95% CI)b | Relative risk (95% CI) | P valuec |
|---|---|---|---|---|---|
| Cumulative live births | 402 (80.9%) | 384 (77.6%) | 3.3% (−1.7 to 8.4) | 1.04 (0.98 to 1.11) | 0.199 |
| Singleton live births | 391 (78.7%) | 375 (75.8%) | 2.9% (−2.3 to 8.1) | 1.04 (0.97 to 1.11) | 0.274 |
| All twin live births | 11 (2.2%) | 9 (1.8%) | 0.4% (−1.4 to 2.1) | 1.22 (0.51 to 2.91) | 0.658 |
| No. of unused frozen embryos, mean (SD) | 3.9 (3.4) | 5.2 (4.1) | −1.3 (−1.8 to −0.9) | - | 3.87e-08 |
| No. of unused frozen embryos in women with a live birth, mean (SD) | 4.5 (3.3) | 5.9 (4.0) | −1.4 (−1.9 to −0.9) | - | 1.03e-07 |
| No. of unused frozen embryos in women without a live birth, mean (SD) | 1.2 (1.9) | 2.8 (3.8) | −1.6 (−2.4 to −0.7) | - | 0.00035 |
| No. of women without a frozen embryo | 89 (17.9%) | 65 (13.1%) | 4.8% (0.3 to 9.3) | 1.36 (1.02 to 1.83) | 0.038 |
| No. of women without a frozen embryo without a livebirth | 58 (11.7%) | 53 (10.7%) | 1.0% (−3.0 to 4.9) | 1.09 (0.77 to 1.55) | 0.631 |
| No. of women without a frozen embryo with a livebirth | 31 (6.2%) | 12 (2.4%) | 3.8% (1.3 to 6.3) | 2.57 (1.34 to 4.95) | 0.003 |
Non-inferiority P value = 1.16e-07 for cumulative live births (with margin = −0.1, α = 0.025, one-sided).
SD standard deviation.
aThis is a secondary, post-hoc analysis of the long-term follow-ups from randomization day to July 28th, 2023, and treatment after the study period (1 year of randomization) did not follow our prespecified protocol, reflecting real-world practice.
bAbsolute differences in percentages are indicated in percentage points, and absolute differences in other values are indicated in units of that value.
cAll P values are for superiority, two-sided. No adjustments were made for multiple comparisons. Two-sample t-test was used for continuous data; Chi-square test was used for categorical data.
Discussion
In this multicenter randomized clinical trial, we found that among infertile women with good prognosis ( ≥ 3 transferrable cleavage-stage embryos), single blastocyst-stage transfer was non-inferior and even superior to single cleavage-stage transfer for improving cumulative live birth rates, with a shorter time to live birth. From a perinatal perspective, blastocyst transfer was associated with a higher cumulative rate of PPROM, spontaneous preterm birth and neonatal hospitalization >3 days, and a lower rate of preeclampsia than the cleavage-stage transfer.
Blastocyst or cleavage-stage embryo transfers are both widely used in current IVF practices. Although blastocyst transfer has become popular in some regions, the limited quality of the available evidence has prevented a shift in practice in other areas11. This resulted in a call for better quality data by the most recent Cochrane review and European IVF Monitoring Consortium for European Society of Human Reproduction and Embryology3,11. As our large trial provides reports of clearly higher cumulative live birth rates after single blastocyst transfer in women with good prognosis, this could support a shift to single blastocyst transfer in this population.
A recent Cochrane systematic review comparing blastocyst and cleavage-stage transfers concluded that live birth rate after fresh blastocyst transfer was higher than fresh cleavage-stage transfer, but the evidence was graded as low quality11. This review included five relatively small, single-center trials conducted at the early years (632 women in total, mainly with a good prognosis) reporting cumulative pregnancy rates with considerable heterogeneity11. Moreover, none of the previous trials reported on the cumulative live birth rate20. One additional pilot trial that reported higher cumulative live birth rate after blastocyst transfer in oocyte recipients did not apply SET and was terminated after reaching half of the planned sample size18. Our trial shows that the cumulative live birth rate after three single blastocyst transfers is higher than that after three cleavage-stage transfers, which might be hypothesized based on previous reports of higher live birth rates after one fresh blastocyst transfer11. Since the depletion of embryos by blastocyst culture leads to a reduction in the number of embryos, data are needed to confirm whether blastocyst transfer really improves the cumulative outcomes in couples undergoing IVF.
Our large trial, which directly compared cumulative live birth rate after SET and vitrification cryopreservation in women with good prognosis, showed that single blastocyst transfer resulted in an 8.6% absolute increase in cumulative live birth rates from conceptions with 12 months after randomization. As the number of women in the blastocyst group without live birth and with no frozen embryos left was 4.8% higher than in the cleavage-stage group, it is unlikely for cleavage-stage transfer to catch up with blastocyst transfer in cumulative live birth rate, due to long timeframe of transfers required to make up 8.6% lower cumulative live birth rate. Furthermore, our data show that blastocyst transfer results in a shorter time to live birth despite similar number of freeze-all cycles in both groups.
Extended embryo culture to blastocyst stage is likely to self-select the most viable embryos in vitro13, yield a lower risk of aneuploidy embryos21, have better embryo-endometrial synchronization by mimicking the natural in vivo embryo implantation process7; and therefore, increase the chances of having a baby. In view of 8.6% higher cumulative live birth rate with only 4.8% more couples without extra embryos in blastocyst group, we speculate that apart from better selection the day 5 culture itself results in higher live birth rates. In our trial, the higher implantation rates after each single blastocyst transfer translated into higher live birth rates in women with good prognosis. The cumulative live birth rates of blastocyst culture were not compromised by reduced number of embryos in our study population. Since more frozen cleavage-stage embryos were left than frozen blastocysts in women who did not achieve a live birth (4.9 vs 2.2), we continued the follow-ups and will conduct a life-course analysis to reveal the results in real-world practice.
We conducted a secondary, post-hoc analysis of the long-term follow-ups from randomization day to July 28th, 2023, and found similar cumulative live birth rate between the two group. However, treatment after the study period (1 year of randomization) did not follow our prespecified protocol, as 41.3% of transfers in the cleavage-stage group were blastocyst transfers. Therefore, it is difficult to determine whether the catch-up in live birth rates among women in the cleavage-stage group is due to crossover to blastocyst transfer, more embryo transfer cycles, or both. Therefore, this analysis cannot be used as a basis for conclusions. In addition, the number of frozen embryos remaining in the cleavage-stage group, among women who have not achieved a live birth, was higher than in the blastocyst group (2.8 vs 1.2). The number and quality of embryos derived from an oocyte-retrieval cycle are key determinants of cumulative pregnancy and live birth rates. Thus, mathematically, the cumulative live birth rate might in the end be the same in both treatment groups, assuming that women in the cleavage-stage group would continue to return for embryo transfers. Of note, our results of increased cumulative live birth rate and reduced time to live birth after blastocyst transfer should be applied in the context of a maximum of the first three SETs and embryo transfers within 1 year of randomization in good-prognosis patients. From the perspective of cumulative transfers, extended embryo culture to blastocyst may negatively affect the pregnancy outcomes due to poor laboratory performance or the fact that most embryos are arrested between cleavage and blastocyst stages in certain subgroups of women (e.g., women with low prognosis), which would produce a pregnancy if transferred at cleavage stage. Therefore, we should not perform routine blastocyst transfer on everybody, similar to the recommendations for the utilization of PGT-A.
Our results showed that blastocyst transfer was associated with a higher rate of spontaneous preterm birth, consistent with previous observational reports22,23, and a higher risk of PPROM might be the main cause for preterm birth. The underlying mechanism linking blastocyst culture to preterm birth and PPROM remains unknown but may involve altered placentation and trophoblast function through epigenetic changes24. Of note, rate of preterm birth after single blastocyst transfer was much lower than the transfer of two cleavage-stage embryos (15.5%)25, which emphasizes the importance of SET in compliance with European and American guidelines8,26,27. Additionally, the higher rates of prolonged neonatal hospitalization and neonatal infection warrant attention. Our results showed that women after blastocyst transfer had a 7.1% higher absolute risk and 16% higher relative risk of developing at least one obstetrical-perinatal complication, in contrast to an 8.6% absolute and 13% relatively higher cumulative live birth rates. We evaluate the cumulative obstetrical-perinatal complications because each complication has a low frequency. Patients should be well informed of the information before deciding on an embryo transfer strategy. We will conduct a cost-effectiveness analysis after this original publication to further explore the benefit-risk ratio of blastocyst transfer in our study population.
Our study found more preeclampsia after fresh cleavage-stage transfer, however the mechanism is unclear. Although frozen embryo transfer may be a confounder for the increased incidence of pre-eclampsia, the risk of pre-eclampsia remained higher in the cleavage-stage group after adjustment for frozen embryo transfer. The higher rate of monozygotic twins in blastocyst group is consistent with previous findings7, although not statistically significant in our study. Furthermore, in contrast to previous studies22, the incidence of large for gestational age infants did not differ between the two groups (RR1.16 [95%CI 0.83 to 1.63]), which was defined based on a Chinese reference population consisting of natural conceptions28. The discrepant results may be attributed to different study populations. In addition, the long-term impact on the infants born from blastocyst transfers warrants further study with large maternal and neonatal cohorts, as a recent study reported the possible implications of blastocyst transfer on shortened leukocyte telomeres which predicts a reduction in lifespan29.
Our post-hoc subgroup analysis suggested the benefit of blastocyst transfer appeared to decrease with increasing age. Patients with younger age ( ≤ 30 years), representing subgroups of women with very good prognosis, benefitted from single blastocyst transfer. Conversely, women with older age, diminished ovarian reserve and fewer oocyte retrieved did not appear to have between-group differences in cumulative livebirth rates10,12. Given our study was not powered for post-hoc subgroup analysis and the majority of participants were ≤35 years, we cannot draw definitive conclusions on treatment effects in other subgroups. Further studies of specific subgroups with sufficient power are needed to support our exploratory findings in the use of blastocyst transfer in different populations, especially in women with older age or poor prognosis.
To our knowledge, this is the largest randomized controlled trial to date and the first to provide robust data on cumulative live birth and obstetrical-perinatal outcomes of the two embryo transfer strategies. The strengths of this study include its large sample size, the low loss-to-follow-up rate, randomized allocation in multiple cycles over the course of a year, the multicenter and pragmatic design that improves the generalizability of our results, and strict adherence to SETs in both groups, that ensures the comparable number of embryos between groups. In addition, our study informs the discussion on blastocyst versus cleavage-stage transfer and the design of such studies. We use both absolute and relative terms in expressing success rates and risks, which strongly contributes to the clinical message conveyed to clinicians and patients. Furthermore, our study had for pragmatic reasons a follow-up period of 1 year after randomization. While this might favor blastocyst transfer, as the cleavage stage group has more unused embryos left, we also think that a 1-year follow-up reflects the reality of clinical practice.
Our study has several limitations. First, we include women with a good prognosis of no less than three cleavage-stage embryos and a mean age of 29.8 years, with the age distribution ≤35 years accounting for 93% (924) of the women. As shown in Supplementary fig. 2, the benefits of blastocyst transfer appear to diminish with advancing age. Therefore, our results may not be generalizable to other populations including women with older age, fewer oocytes retrieved and less than three cleavage-stage embryos available. However, our trial fills the research gap concerning cumulative live birth outcomes after blastocyst versus cleavage-stage transfer, which has been an important questionable debate for decades and has significant practice value on shift to blastocyst transfer in our study population3,11. Our study provides exploratory results for future studies evaluating whether other populations would benefit from blastocyst transfer. Second, there were protocol deviations, mainly in the cleavage-stage group, where 6.7% of participants received at least one blastocyst transfer. However, the results did not change in the per-protocol analysis. Third, our study was not adequately powered to detect the differences in pregnancy and perinatal complications. A future meta-analysis pooling all the evidence might answer these questions. Fourth, open-label design has the potential to introduce treatment bias, including crossovers and double embryo transfer, thereby underestimating the effects. However, except for stages of embryo transferred, all interventions were strictly adhered to the same standard protocol and patient management in both groups. Moreover, regular investigator meetings and monitoring were conducted to ensure compliance with the study protocol.
Finally, we calculated a maximum of the first three SETs as the primary outcome and all embryo transfers within the study period as the secondary outcome. Ideally, the “true” cumulative live birth rate would be obtained after all embryos have been transferred. However, considering that the first three SETs may achieve the most pregnancies, as well as the feasibility and applicability of the trial to real-world clinical practice, we studied the live births from a maximum of the first three SETs as the primary outcome, which happened in the first year after randomization, ensuring equal number of embryos transferred in both groups, to reveal the efficacy and safety of the two strategies.
In conclusion, among infertile women undergoing IVF with good prognosis ( ≤ 40 years with at least three cleavage-stage embryos), single blastocyst transfer was non-inferior and even superior to single cleavage-stage transfer in improving cumulative live birth rates and reducing time to live birth. However, the increased risk of preterm premature rupture of membranes, preterm birth and neonatal hospitalization after blastocyst transfer need to be fully informed of patients before deciding on an embryo transfer strategy. The cost-effectiveness of blastocyst transfer in this population and the long-term impact on the infants warrants further studies.
Methods
Trial design, Oversight and Governance
This is a multicenter, open-label, non-inferiority, randomized clinical trial conducted at 11 academic clinical centers throughout China. The aim of the trial was to assess the effectiveness and safety of blastocyst-stage vs cleavage-stage embryo transfer in IVF/ICSI treatment cycle, taking into account subsequent vitrified embryo transfers. This trial was approved by the ethics committee at each study site (including Ethics Committee at First Affiliated Hospital of Nanjing Medical University, Ethics Committee of Hospital for Reproductive Medicine Affiliated to Shandong University, Ethics Committee for Reproductive Medicine of Ren Ji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Ethics Committee for Reproductive Medicine of First Affiliated Hospital of Anhui Medical University, Medical Ethics Committee of Maternal and Child Health Hospital/Obstetrics and Gynecology hospital of Guangxi Zhuang Autonomous Region, Ethics Committee of Shengjing Hospital of China Medical University, Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University, Ningxia Medical University General Hospital Scientific Research Ethics Committee, Ethics Committee for Reproductive Medicine of Suzhou Municipal Hospital, Ethics Committee for Reproductive Medicine of Henan Provincial People’s Hospital, Ethics Committee of The Third Affiliated Hospital of Zhengzhou University), and performed in accordance with principles of Good Clinical Practice and Declaration of Helsinki. All participants provided written informed consent. The study protocol including statistical analysis plan are available in Supplementary note. The trial was registered at ClinicalTrial.gov, NCT03152643 (https://clinicaltrials.gov/study/NCT03152643). A data and safety monitoring board oversaw the study. The data in this trial was collected using a Web-based data management system at https://www.medresman.org.cn/login.aspx.
Participants
We enrolled infertile women who met the following inclusion criteria: aged 20 to 40 years, undergoing their first or second IVF or intracytoplasmic sperm injection (ICSI) cycle, and with the number of transferrable cleavage embryos ≥3. The exclusion criteria for this study are as follows: women diagnosed with uterine abnormalities (confirmed by three-dimensional ultrasonography or hysteroscopy, including uterus unicornis, septate or duplex uterus, submucous myoma, or intrauterine adhesions); women planned for in vitro maturation, or preimplantation genetic testing (PGT); women with hydrosalpinx visible on ultrasound; women who had experienced recurrent pregnancy loss, defined as 2 or more previous pregnancy losses; women who planned “freeze-all” treatment for purpose of subsequent surgery, such as salpingectomy due to hydrosalpinx after oocytes retrieval. We also excluded women with contraindications to assisted reproductive technology and/or pregnancy, such as uncontrolled hypertension, symptomatic heart disease, uncontrolled diabetes, undiagnosed liver disease or dysfunction (based on serum liver enzyme test results), undiagnosed renal disease or abnormal renal function, severe anemia, history of deep venous thrombosis, pulmonary embolus or cerebrovascular accident, history of or suspicious for cancer, undiagnosed vaginal bleeding. Sex was self-reported and confirmed by transvaginal ultrasound with female reproductive organs, and mostly karyotype result of 46, XX.
Couples were counseled by local investigators at the office visit at the time of their decision to undergo IVF or ICSI treatment; both female and male partners of the infertile couple provided written informed consent prior to participation after completing all tests in preparation for IVF or ICSI. Actual randomization was performed on day 2 or 3 after oocyte retrieval when the presence of ≥3 transferrable embryos was confirmed. Enrollment began on 8 October 2018 and ended on 22 August 2019. Follow-up of all live births was completed on 6 September 2021.
Interventions, randomization, and follow-up
Controlled ovarian stimulation was performed with Gonadotropin-releasing hormone (GnRH) agonist protocols or GnRH antagonist protocol according to local investigator’s preference. The long GnRH agonist protocol included the use of a short-acting GnRH agonist starting at the luteal phase or a long-acting GnRH agonist on days 1–2 of the menstrual cycle or during the luteal phase. When pituitary down-regulation was achieved, recombinant follicular stimulating hormone was initiated with a dose of 75 to 225 IU per day. Short GnRH agonist regimen and antagonist regimen were as reported previously30,31. When at least two follicles reached 18 mm or three follicles reached 17 mm in mean diameter, oocyte maturation was achieved by administration of human chorionic gonadotropin (hCG) or GnRH agonist or both. Oocyte retrieval was performed 36 to 37 hours later.
After oocyte retrieval, the quality of embryos was assessed by morphological criteria based mainly on the number and regularity of blastomeres as well as percentage fragmentation32. On day 2 or 3, women with ≥3 transferrable cleavage-stage embryos were randomly assigned to undergo blastocyst-stage or cleavage-stage embryo transfer in a 1:1 ratio with block randomization (variable block size of four, six or eight), and stratified by study sites. Allocation concealment was ensured through use of an online central randomization system with a randomization sequence generated by an independent statistician in the data coordinating center. Allocation was done by trained coordinators using password-protected accounts. Since it was impractical to conduct masking and since all outcomes were objective indicators, the trial was not blinded after randomization. Investigators, participants and trial coordinators were aware of the allocation after randomization.
For women assigned to blastocyst-stage group, embryos were cultured to day 5 or 6. A single fresh blastocyst of best quality was transferred after oocyte retrieval, using sequential media for blastocyst culture and with a preference for day 5 over day 6 transfer. Blastocyst quality was evaluated with the Gardner morphological criteria, according to blastocyst expansion, inner cell mass, and trophectoderm development33,34. For women assigned to the cleavage-stage group, a single fresh cleavage-stage embryo of best quality was transferred after oocyte retrieval.
For both groups, surplus embryos (to be transferred within the study period) were vitrified for future frozen embryo transfer as per the allocation group. When a patient was unable to undergo fresh transfer for risk of ovarian hyperstimulation syndrome (OHSS) or other reasons, all embryos were cryopreserved by vitrification. Frozen embryo transfer was initiated on the second menstrual cycle after oocyte retrieval, and a single frozen embryo with best morphology score was transferred first. Day 6 frozen transfers were performed on the same day as the day 5 transfer.
For the first three embryo transfers within 1 year after randomization (with a 3-month extension for those unable to undergo transfers due to COVID-19), SET was required. For transfers beyond the third attempt within the intervention period, SET was no longer mandatory26,27. If the initial embryo transfer did not result in a live birth, patients went through cryopreserved cycles within the study period and pregnancy was followed up.
Luteal phase support for fresh embryo transfer was vaginal progesterone gel 90 mg per day plus oral dydrogesterone 10 mg twice daily, starting on the day of oocyte retrieval and continuing until 10 weeks’ gestation if the pregnancy was achieved. For frozen transfers, endometrial preparation including natural cycle, minimal stimulation cycle or hormone replacement cycle was performed based on local routine as previously reported25,35.
Outcomes
The primary outcome was the cumulative live birth rate for a maximum of the first three embryo transfers resulting from one oocyte retrieval cycle, as long as these transfers happened in the first year after randomization (or 1 year and 3 months in case of delays due to COVID-19). Live birth was defined as the delivery of any neonate ≥24 weeks gestation that had a heartbeat and was breathing. The cumulative live birth rate was calculated by dividing the number of participants obtaining their first live birth by a number of randomized participants.
Secondary outcomes included biochemical pregnancy, clinical pregnancy, implantation, ongoing pregnancy, live birth, pregnancy loss, birth weight and sex ratio. The safety outcomes included moderate or severe OHSS, ectopic pregnancy, multiple pregnancies, obstetric and perinatal complications, and congenital anomalies. The definitions of secondary outcomes are listed in Supplementary Table 1. Outcomes from all embryo transfers within 1 year of randomization were followed up for the occurrence of live birth until two years after randomization as the secondary outcome.
Post hoc secondary outcomes included the number of embryo transfers, the number of unused frozen embryos, women without a frozen embryo and live birth without a complication. The non-prespecified outcome of cumulative live birth rate was also calculated, including follow-up of embryo transfers from day of randomization to July 28th, 2023. The treatments after the study period (1 year of randomization) did not follow our prespecified protocol.
Sample size calculations
We hypothesized that the cumulative live birth rate of blastocyst-stage transfers is non-inferior to that of cleavage-stage transfers. Assuming that a cumulative live birth rate of 52%36, a minimum sample size of 392 subjects per treatment arm would provide 80% power to show the non-inferiority of blastocyst transfer to cleavage-stage transfer at one-sided significance level of 0.025, with a non-inferiority margin of 10% for the lower 95% confidence interval (CI) for the difference in cumulative live birth rates between the two groups. Considering a withdrawal, cross-over and lost-to-follow-up rate of 20%, we planned to enroll 980 participants. The non-inferiority margin of 10% was agreed to be clinically meaningful by the study leadership of reproductive endocrinologists.
Statistical Analysis
The primary and secondary analysis were performed according to the intent-to-treat principle (ITT) including all subjects who were randomly allocated into the treatment groups. Cumulative outcomes after up to the first three SETs within the study period were analyzed. Between-group difference in cumulative live births and its 95% CI were estimated using the Newcombe-Wilson method. If the lower limit of one-sided 95% CI for absolute difference (AD) in the cumulative live birth rates was larger than the prespecified non-inferiority margin (−10%), the blastocyst group was considered non-inferior to the cleavage-stage group. If non-inferiority would be demonstrated, a superiority test would be performed.
Time to cumulative live birth rates were estimated using Kaplan-Meier methods and analyzed with log-rank tests. Hazard ratio (HR) with 95% CIs were estimated by using a Cox proportional hazards model. Categorical data were represented as a frequency and percentage, and assessed by the Chi-square analysis or Fisher’s Exact Test. Continuous data were expressed as mean and standard deviation, with Student’s t-test for testing between-group differences.
The AD and relative risks, as well as their 95% CI, are presented. To ensure robustness of the results, analyses for per-protocol population and full analysis set were conducted in participants who fully complied with the protocol and those who did not meet major entry criteria and lacked any post-randomization data, respectively. The sensitivity analyzes of cumulative live birth rate for a maximum of the first three transfers without 3-month extension were also performed, as well as secondary analyzes of cumulative outcomes from all transfers within the study period.
We did post-hoc subgroup analyses to test the treatment effect at different maternal ages, previous conception, previous IVF, ovarian reserve, ovarian response based on number of oocytes retrieved, estradiol and progesterone on hCG day.
For the non-inferiority test of the primary outcome, a one-sided p-value of less than 0.025 was considered statistically significant, whereas for all other analyzes, a two-sided p-value of less than 0.05 was statistically significant. All analyzes were performed using SAS software (version 9.4; SAS institute, Cary, NC).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We thank all couples who consented to participate in this study, all research staff in study sites, and staff of Resman central randomization platform. We are thankful to Daimin Wei and Tianxiang Ni at the Center for Reproductive Medicine, Shandong University for their technical support; Lijuan Lin, Xuan Wang and Qian Ye at the Department of Biostatistics, School of Public Health, Nanjing Medical University for statistical support; Richard S. Legro at Penn State College of Medicine, Hershey, PA for guiding the design, conduct and data interpretation of the trial as the steering committee member; and the members of the data and safety monitoring committee: Robert Rebar (chair), Tin Chiu Li, Jun Zhang, Xiuqing Wang, and Yan Liu. They received no compensation for their contributions. This work was supported by the Key Program of National Natural Science Foundation of China (81730041(J.L.)), Shandong Provincial Key Research and Development Program (2020ZLYS02(Z-J.C.)), the National Key Research and Development Program of China (2021YFC2700404(J.L.)), Innovative research team of high-level local universities in Shanghai (SHSMU-ZLCX20210200(Z-J.C.)), and Jiangsu Provincial Science and Technology Department Social Development Project (BE2021743(X.M.)). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
X.M., J.W., Y.S., J.T., Y.G., Y.S., B.Z. and J.Z. contributed equally as first authors to this work. J.L. and Z-J.C. contributed equally as corresponding authors. Senior author (J.L., Z-J.C., H.Z., Z.H., B.M.) jointly directed this work. X.M., J.W., Y.S., H.Z., Z-J.C. and J.L. were responsible for the study conception and design, and wrote the study protocol. X.M., J.W., Y.S., J.T., Y.G., Y.S., B.Z., J.Z., JQ.L., Y.C., H.L., C.Z., Z.W., X.X., P.K., Y.L., L.H., Y.Y., HY.L., C.L., Z-J.C. and J.L. were involved in recruitment of patients and acquisition of the data. F.C., Z.H., H.Z., Z-J.C. and J.L. supervised the study. J.W., B.M., H.Z., F.C., X.M., Z-J.C. and J.L. drafted the manuscript. X.M., J.W., F.C., B.M., Z.H., H.Z., Z-J.C. and J.L. contributed analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. J.W., F.C., H.Y. and H.Z. did the statistical analysis. All authors read and approved the final draft of the report.
Peer review
Peer review information
Nature Communications thanks Norbert Gleicher and Daniel Brison, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The study protocol is available as Supplementary Note in the Supplementary Information. Clinical data are not publicly available due to the privacy of patients. Deidentified participant data, including specified dataset and a data dictionary that defines each field in the set, will be provided one year after publication of the primary manuscript for research purposes to the corresponding author(jyliu_nj@126.com). Analyzes with a written protocol including analysis plan and signed data sharing/access agreement are required. Request for data sharing will be handled in line with the regulations for data access and sharing of Human Genetic Resource Administration of China, and approved by publication committee of the trial. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
Code availability
No custom code was used for statistical analysis in this study.
Competing interests
B.M. reported that he received Investigator grant support from the National Health and Medical Research Council (NHMRC) (GNT1176437) and consultancy, travel support and research funding from Merck. The remaining authors report no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiang Ma, Jing Wang, Yuhua Shi, Jichun Tan, Yichun Guan, Yun Sun, Bo Zhang, Junli Zhao.
Contributor Information
Zi-Jiang Chen, Email: chenzijiang@hotmail.com.
Jiayin Liu, Email: jyliu_nj@126.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-52008-y.
References
- 1.Sunderam, S. et al. Assisted reproductive technology surveillance - United States, 2018. MMWR Surveill. Summ.71, 1–19 (2022). 10.15585/mmwr.ss7104a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai, F. et al. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum. Reprod.35, 446–452 (2020). 10.1093/humrep/dez245 [DOI] [PubMed] [Google Scholar]
- 3.European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE), et al. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open2022, hoac022 (2022). [DOI] [PMC free article] [PubMed]
- 4.Smith, A., Tilling, K., Nelson, S. M. & Lawlor, D. A. Live-birth rate associated with repeat in vitro fertilization treatment cycles. JAMA314, 2654–2662 (2015). 10.1001/jama.2015.17296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner, D. K. et al. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil. Steril.69, 84–88 (1998). 10.1016/S0015-0282(97)00438-X [DOI] [PubMed] [Google Scholar]
- 6.Gardner, D. K. et al. Single blastocyst transfer: a prospective randomized trial. Fertil. Steril.81, 551–555 (2004). 10.1016/j.fertnstert.2003.07.023 [DOI] [PubMed] [Google Scholar]
- 7.Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology. Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril110, 1246-1252 (2018). [DOI] [PubMed]
- 8.Practice Committee of the Society for Reproductive Endocrinology and Infertility, Quality Assurance Committee of the Society for Assisted Reproductive Technology, and the Practice Committee of the American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: a committee opinion. Fertil Steril117, 498-511 (2022).
- 9.Saket, Z., Kallen, K., Lundin, K., Magnusson, A. & Bergh, C. Cumulative live birth rate after IVF: trend over time and the impact of blastocyst culture and vitrification. Hum. Reprod. Open3, hoab021 (2021). 10.1093/hropen/hoab021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleicher, N., Kushnir, V. A. & Barad, D. H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open2019, hoz017 (2019). 10.1093/hropen/hoz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glujovsky, D. et al. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst. Rev.5, CD002118 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron, N. J., Bhattacharya, S. & McLernon, D. J. Cumulative live birth rates following blastocyst- versus cleavage-stage embryo transfer in the first complete cycle of IVF: a population-based retrospective cohort study. Hum. Reprod.35, 2365–2374 (2020). 10.1093/humrep/deaa186 [DOI] [PubMed] [Google Scholar]
- 13.Papanikolaou, E. G. et al. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N. Engl. J. Med354, 1139–1146 (2006). 10.1056/NEJMoa053524 [DOI] [PubMed] [Google Scholar]
- 14.Rienzi, L. et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum. Reprod.17, 1852–1855 (2002). 10.1093/humrep/17.7.1852 [DOI] [PubMed] [Google Scholar]
- 15.Emiliani, S. et al. Similar delivery rates in a selected group of patients, for day 2 and day 5 embryos both cultured in sequential medium: a randomized study. Hum. Reprod.18, 2145–2150 (2003). 10.1093/humrep/deg394 [DOI] [PubMed] [Google Scholar]
- 16.Van der Auwera, I. et al. A prospective randomized study: day 2 versus day 5 embryo transfer. Hum. Reprod.17, 1507–1512 (2002). 10.1093/humrep/17.6.1507 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Shaw, S., Cercas, R., Brana, C., Villas, C. & Pons, I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. J. Assist Reprod. Genet32, 177–184 (2015). 10.1007/s10815-014-0387-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clua, E. et al. Blastocyst versus cleavage embryo transfer improves cumulative live birth rates, time and cost in oocyte recipients: a randomized controlled trial. Reprod. Biomed. Online44, 995–1004 (2022). 10.1016/j.rbmo.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 19.Harbin Consensus Conference Workshop, G. Improving the Reporting of Clinical Trials of Infertility Treatments (IMPRINT): modifying the CONSORT statement. Fertil Steril102, 952-959 e915 (2014). [DOI] [PubMed]
- 20.Cornelisse, S. et al. Women’s preferences concerning IVF treatment: a discrete choice experiment with particular focus on embryo transfer policy. Hum. Reprod. Open2022, hoac030 (2022). 10.1093/hropen/hoac030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staessen, C. et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum. Reprod.19, 2849–2858 (2004). 10.1093/humrep/deh536 [DOI] [PubMed] [Google Scholar]
- 22.Marconi, N., Allen, C. P., Bhattacharya, S. & Maheshwari, A. Obstetric and perinatal outcomes of singleton pregnancies after blastocyst-stage embryo transfer compared with those after cleavage-stage embryo transfer: a systematic review and cumulative meta-analysis. Hum. Reprod. Update28, 255–281 (2022). 10.1093/humupd/dmab042 [DOI] [PubMed] [Google Scholar]
- 23.Alviggi, C. et al. Influence of cryopreservation on perinatal outcome after blastocyst- vs cleavage-stage embryo transfer: systematic review and meta-analysis. Ultrasound Obstet. Gynecol.51, 54–63 (2018). 10.1002/uog.18942 [DOI] [PubMed] [Google Scholar]
- 24.Rivera, R. M. et al. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum. Mol. Genet17, 1–14 (2008). 10.1093/hmg/ddm280 [DOI] [PubMed] [Google Scholar]
- 25.Shi, Y. et al. Transfer of fresh versus frozen embryos in ovulatory women. N. Engl. J. Med378, 126–136 (2018). 10.1056/NEJMoa1705334 [DOI] [PubMed] [Google Scholar]
- 26.Practice Committee of the American Society for Reproductive Medicine, the Practice Committee for the Society for Assisted Reproductive Technologies. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril116, 651-654 (2021).
- 27.Eshre Guideline Group on Good Practice in IVF Labs, et al. Revised guidelines for good practice in IVF laboratories (2015). Hum Reprod31, 685-686 (2016). [DOI] [PubMed]
- 28.Dai, L. et al. Birth weight reference percentiles for Chinese. PLoS One9, e104779 (2014). 10.1371/journal.pone.0104779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, C. et al. Leukocyte telomere length in children born following blastocyst-stage embryo transfer. Nat. Med28, 2646–2653 (2022). 10.1038/s41591-022-02108-3 [DOI] [PubMed] [Google Scholar]
- 30.Yan, J. et al. Live birth with or without preimplantation genetic testing for aneuploidy. N. Engl. J. Med385, 2047–2058 (2021). 10.1056/NEJMoa2103613 [DOI] [PubMed] [Google Scholar]
- 31.Chen, Z. J. et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N. Engl. J. Med375, 523–533 (2016). 10.1056/NEJMoa1513873 [DOI] [PubMed] [Google Scholar]
- 32.Puissant, F., Van Rysselberge, M., Barlow, P., Deweze, J. & Leroy, F. Embryo scoring as a prognostic tool in IVF treatment. Hum. Reprod.2, 705–708 (1987). 10.1093/oxfordjournals.humrep.a136618 [DOI] [PubMed] [Google Scholar]
- 33.Gardner, D. K., Lane, M., Stevens, J., Schlenker, T. & Schoolcraft, W. B. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil. Steril.73, 1155–1158 (2000). 10.1016/S0015-0282(00)00518-5 [DOI] [PubMed] [Google Scholar]
- 34.Wei, D. et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet393, 1310–1318 (2019). 10.1016/S0140-6736(18)32843-5 [DOI] [PubMed] [Google Scholar]
- 35.Ghobara, T., Gelbaya, T. A. & Ayeleke, R. O. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst. Rev.7, CD003414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vos, A. et al. Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Hum. Reprod.31, 2442–2449 (2016). 10.1093/humrep/dew219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is available as Supplementary Note in the Supplementary Information. Clinical data are not publicly available due to the privacy of patients. Deidentified participant data, including specified dataset and a data dictionary that defines each field in the set, will be provided one year after publication of the primary manuscript for research purposes to the corresponding author(jyliu_nj@126.com). Analyzes with a written protocol including analysis plan and signed data sharing/access agreement are required. Request for data sharing will be handled in line with the regulations for data access and sharing of Human Genetic Resource Administration of China, and approved by publication committee of the trial. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
No custom code was used for statistical analysis in this study.