Abstract
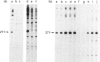
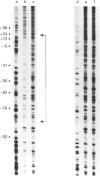
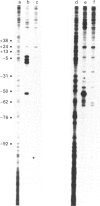
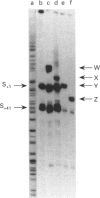
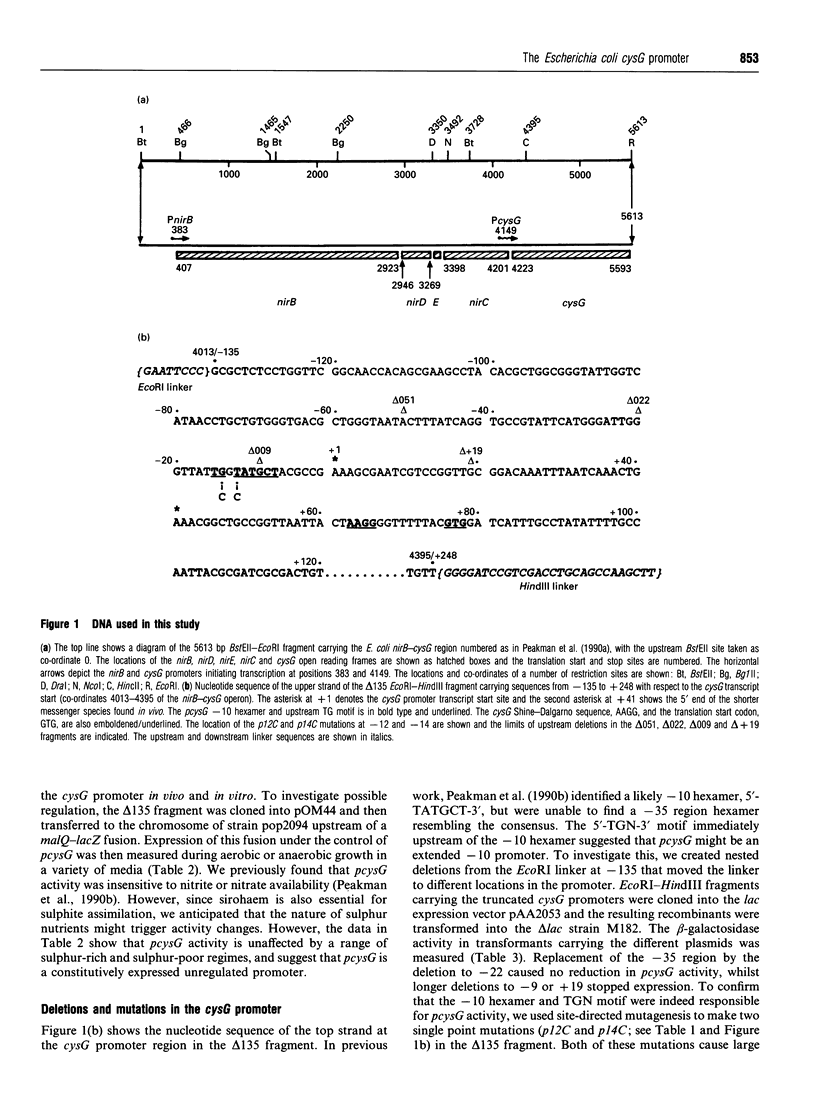
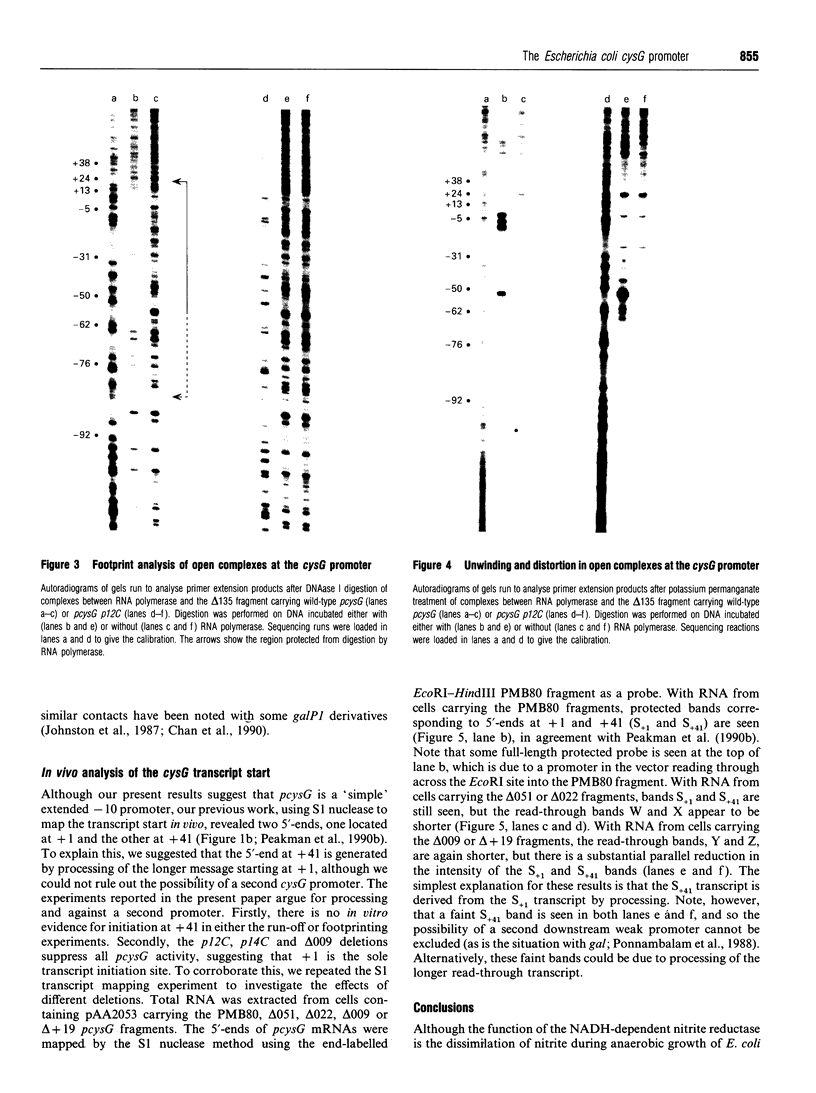
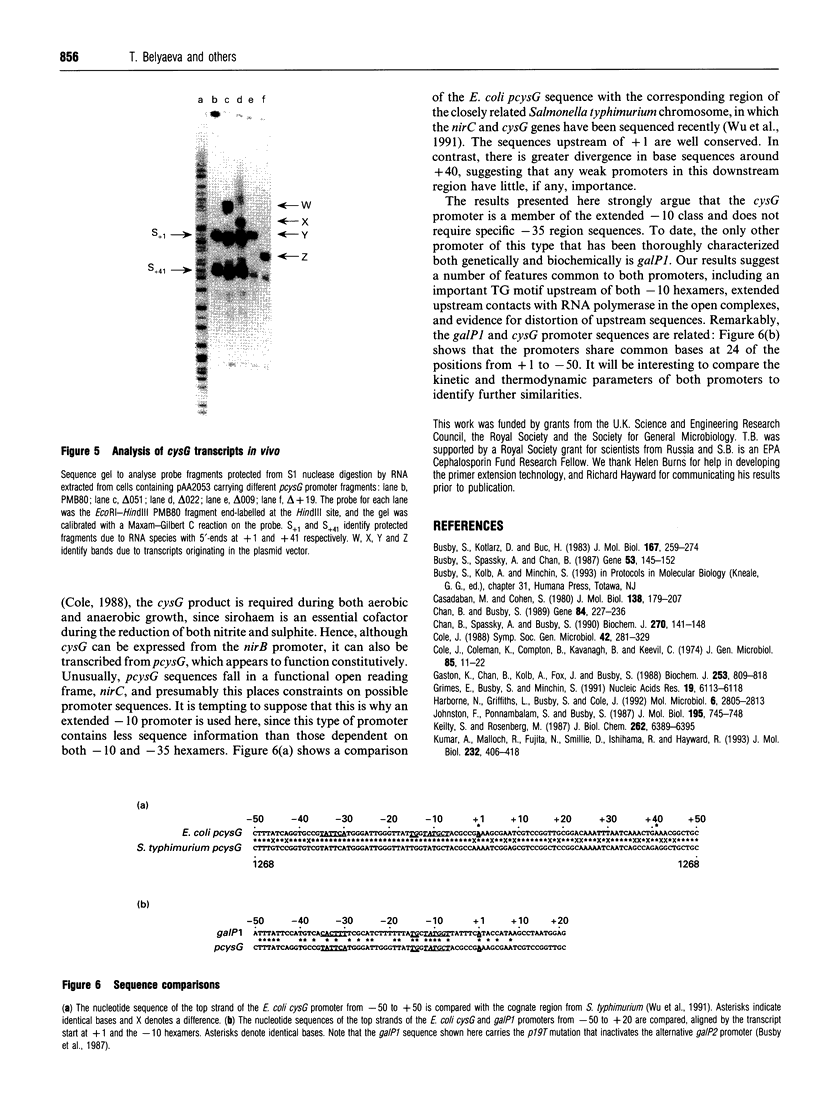
The Escherichia coli cysG promoter has been subcloned and shown to function constitutively in a range of different growth conditions. Point mutations identify the -10 hexamer and an important 5'-TGN-3' motif immediately upstream. The effects of different deletions suggest that specific sequences in the -35 region are not essential for the activity of this promoter in vivo. This conclusion was confirmed by in vitro run-off transcription assays. The DNAase I footprint of RNA polymerase at the cysG promoter reveals extended protection upstream of the transcript start, and studies with potassium permanganate as a probe suggest that the upstream region is distorted in open complexes. Taken together, the results show that the cysG promoter belongs to the 'extended -10' class of promoters, and the base sequence is similar to that of the P1 promoter of the E. coli galactose operon, another promoter in this class. In vivo, messenger initiated at the cysG promoter appears to be processed by cleavage at a site 41 bases downstream from the transcript start point.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busby S., Kotlarz D., Buc H. Deletion mutagenesis of the Escherichia coli galactose operon promoter region. J Mol Biol. 1983 Jun 25;167(2):259–274. doi: 10.1016/s0022-2836(83)80335-0. [DOI] [PubMed] [Google Scholar]
- Busby S., Spassky A., Chan B. RNA polymerase makes important contacts upstream from base pair -49 at the Escherichia coli galactose operon P1 promoter. Gene. 1987;53(2-3):145–152. doi: 10.1016/0378-1119(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chan B., Busby S. Recognition of nucleotide sequences at the Escherichia coli galactose operon P1 promoter by RNA polymerase. Gene. 1989 Dec 14;84(2):227–236. doi: 10.1016/0378-1119(89)90496-4. [DOI] [PubMed] [Google Scholar]
- Chan B., Spassky A., Busby S. The organization of open complexes between Escherichia coli RNA polymerase and DNA fragments carrying promoters either with or without consensus -35 region sequences. Biochem J. 1990 Aug 15;270(1):141–148. doi: 10.1042/bj2700141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Coleman K. J., Compton B. E., Kavanagh B. M., Keevil C. W. Nitrite and ammonia assimilation by anaerobic continuous cultures of Escherichia coli. J Gen Microbiol. 1974 Nov;85(1):11–22. doi: 10.1099/00221287-85-1-11. [DOI] [PubMed] [Google Scholar]
- Gaston K., Chan B., Kolb A., Fox J., Busby S. Alterations in the binding site of the cyclic AMP receptor protein at the Escherichia coli galactose operon regulatory region. Biochem J. 1988 Aug 1;253(3):809–818. doi: 10.1042/bj2530809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes E., Busby S., Minchin S. Different thermal energy requirement for open complex formation by Escherichia coli RNA polymerase at two related promoters. Nucleic Acids Res. 1991 Nov 25;19(22):6113–6118. doi: 10.1093/nar/19.22.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne N. R., Griffiths L., Busby S. J., Cole J. A. Transcriptional control, translation and function of the products of the five open reading frames of the Escherichia coli nir operon. Mol Microbiol. 1992 Oct;6(19):2805–2813. doi: 10.1111/j.1365-2958.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Johnston F., Ponnambalam S., Busby S. Binding of Escherichia coli RNA polymerase to a promoter carrying mutations that stop transcription initiation. J Mol Biol. 1987 Jun 5;195(3):745–748. doi: 10.1016/0022-2836(87)90194-x. [DOI] [PubMed] [Google Scholar]
- Keilty S., Rosenberg M. Constitutive function of a positively regulated promoter reveals new sequences essential for activity. J Biol Chem. 1987 May 5;262(13):6389–6395. [PubMed] [Google Scholar]
- Kumar A., Malloch R. A., Fujita N., Smillie D. A., Ishihama A., Hayward R. S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an "extended minus 10" promoter. J Mol Biol. 1993 Jul 20;232(2):406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- Lavigne M., Kolb A., Buc H. Transcription activation by cAMP receptor protein (CRP) at the Escherichia coli gal P1 promoter. Crucial role for the spacing between the CRP binding site and the -10 region. Biochemistry. 1992 Oct 13;31(40):9647–9656. doi: 10.1021/bi00155a018. [DOI] [PubMed] [Google Scholar]
- Macdonald H., Cole J. Molecular cloning and functional analysis of the cysG and nirB genes of Escherichia coli K12, two closely-linked genes required for NADH-dependent nitrite reductase activity. Mol Gen Genet. 1985;200(2):328–334. doi: 10.1007/BF00425444. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Minchin S., Busby S. Location of close contacts between Escherichia coli RNA polymerase and guanine residues at promoters either with or without consensus -35 region sequences. Biochem J. 1993 Feb 1;289(Pt 3):771–775. doi: 10.1042/bj2890771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman T., Busby S., Cole J. Transcriptional control of the cysG gene of Escherichia coli K-12 during aerobic and anaerobic growth. Eur J Biochem. 1990 Jul 31;191(2):325–331. doi: 10.1111/j.1432-1033.1990.tb19126.x. [DOI] [PubMed] [Google Scholar]
- Peakman T., Crouzet J., Mayaux J. F., Busby S., Mohan S., Harborne N., Wootton J., Nicolson R., Cole J. Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coli K-12 chromosome. Eur J Biochem. 1990 Jul 31;191(2):315–323. doi: 10.1111/j.1432-1033.1990.tb19125.x. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S., Chan B., Busby S. Functional analysis of different sequence elements in the Escherichia coli galactose operon P2 promoter. Mol Microbiol. 1988 Mar;2(2):165–172. doi: 10.1111/j.1365-2958.1988.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S., Webster C., Bingham A., Busby S. Transcription initiation at the Escherichia coli galactose operon promoters in the absence of the normal -35 region sequences. J Biol Chem. 1986 Dec 5;261(34):16043–16048. [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Footprinting protein-DNA complexes in vivo. Methods Enzymol. 1991;208:146–168. doi: 10.1016/0076-6879(91)08012-7. [DOI] [PubMed] [Google Scholar]
- Spassky A., Busby S., Buc H. On the action of the cyclic AMP-cyclic AMP receptor protein complex at the Escherichia coli lactose and galactose promoter regions. EMBO J. 1984 Jan;3(1):43–50. doi: 10.1002/j.1460-2075.1984.tb01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. J., Stolowich N. J., Santander P. J., Roessner C. A., Sowa B. A., Scott A. I. Enzymatic synthesis of dihydrosirohydrochlorin (precorrin-2) and of a novel pyrrocorphin by uroporphyrinogen III methylase. FEBS Lett. 1990 Feb 12;261(1):76–80. doi: 10.1016/0014-5793(90)80640-5. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Siegel L. M., Kredich N. M. High-level expression of Escherichia coli NADPH-sulfite reductase: requirement for a cloned cysG plasmid to overcome limiting siroheme cofactor. J Bacteriol. 1991 Jan;173(1):325–333. doi: 10.1128/jb.173.1.325-333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]