Abstract
The peripheral nervous system consists of ganglia, nerve trunks, plexuses, and nerve endings, that transmit afferent and efferent information. Regeneration after a peripheral nerve damage is sluggish and imperfect. Peripheral nerve injury frequently causes partial or complete loss of motor and sensory function, physical impairment, and neuropathic pain, all of which have a negative impact on patients’ quality of life. Because the mechanism of peripheral nerve injury and healing is still unclear, the therapeutic efficacy is limited. As peripheral nerve injury research has processed, an increasing number of studies have revealed that biological scaffolds work in tandem with progenitor cells to repair peripheral nerve injury. Here, we fabricated collagen chitosan nerve conduit bioscaffolds together with collagen and then filled neuroepithelial stem cells (NESCs). Scanning electron microscopy showed that the NESCs grew well on the scaffold surface. Compared to the control group, the NESCs group contained more cells with bigger diameters and myelinated structures around the axons. Our findings indicated that a combination of chitosan-collagen bioscaffold and neural stem cell transplantation can facilitate the functional restoration of peripheral nerve tissue, with promising future applications and research implications.
Keywords: Chitosan, Biological scaffolds, Peripheral nerve regeneration, Immune microenvironment
Subject terms: Biotechnology, Cell biology, Stem cells, Neurology
Introduction
Peripheral nerve injury frequently results in limb dysfunction, which has a major negative impact on patients’ quality of life and presents a significant difficuly for both patients and doctors. Although nerve stump anastomosis and autologous nerve transplantation can be used to repair nerves, the long distance between nerve stumps significantly limits therapeutic options for peripheral nerve injures. Currently, one of the hottest research topics is trying to find novel ways to heal peripheral nerve. Extensive research evidence supports cell-based approaches to peripheral nerve healing1,2. The local microenvironment plays an important role in the regeneration of nerve fibers, and Schwann cells are the key to the inner process of nerve regeneration. However, broad axonal contact lose may cause it to become dysfunctional due to extracellular matrix-integrin interactions and oxidative stress. Therefore, in cell-based therapeutic approaches, various bioscaffolds have been effectively demonstrated to synergize with neural progenitor cells to achieve functional neural tissue formation3,4.
As a significant part of the extracellular matrix, collagen is crucial for the migration and regeneration of Schönwang5. Collagen is an ideal material for neural tissue engineering because of its low antigenicity and strong biocompatibility, which help to promote tissue regeneration. The drawbacks of collagen, such as its fast degradation rate and poor space maintenance abilities, limit its application as a bioscaffold alone. Crustaceans, including shrimp and crab, are rich in chitosan, a rare positively charged polymeric saccharide found in nature. Chitosancan has numerous potential applications in the event of peripheral nerve damage, including the promotion of axon regeneration and the prevent neuromas forming following injury6,7. However, the brittleness and poor toughness of pure chitosan scaffolds limit their application as carriers. In recent years, the use of collagen and chitosan composites in the treatment of diseases such as bone regeneration, spinal cord injury, and TBI has expanded8–10.
Stem cell transplantation is a promising treatment for neural repair. The collagen/chitosan scaffold is biocompatible and can stimulate nerve healing in collaboration with stem cell transplantation, including mesenchymal stem cells and periodontal membrane stem cells11,12. Studies have demonstrated that neuroepithelial stem cells may survive on chitosan fibers and convert into neurons and glial cells, both of which have high biocompatibility, making them useful in the treatment of peripheral nerve injury13,14. NESCs develop from the neural plate and neural tube in the early embryo. They are the most primordial neural stem cells, capable of self renewal and differentiation into neurons and glial cells. NESCs are useful seed cells in neural tissue engineering because of their great cell survival and low immunogenicity.
Through animal experiments, we have demonstrate that collagen-chitosan composite catheters are noticeably better than silicone catheters in promoting regeneration and functional repair of injured rat sciatic nerves15. However, it is still unclear whether NESCs attach to collagen-chitosan composite conduits in the repair of peripheral nerve injury. In this study, the collagen protein–chitosan nerve conduits were made in a 3:1 ratio by freeze-drying, and NESCs were subsequently filled with them. After the operation, the effect of the collagen protein-chitosan nerve conduits combined with NESCs on nerve regeneration were evaluated in a rat sciatic nerve transection model utilizaing immunohistochemistry, nerve retrograde tracing, and electron microscopy.
Materials and methods
Preparation of the collagen protein-chitosan scaffold
The collagen protein-chitosan scaffold was prepared in accordance with published methods. Following the removal of fascia and adipose tissue, the cattle tendons were frozen and cut into 1–2 mm slices. After sterilization and drying, the sections were placed in 0.1% trypsin, incubated for 16 h at 37 °C, and then dried at room temperature. The sections were immersed in 80 ml of saline and 0.2 ml of 3% hydrogen peroxide for 20 min, then washed with physiological saline and dried at room temperature. The samples were soaked in 200 ml of 0.5 mmol/l hydrochloric acid for 24 h. After pulverizing the mixture, the viscosity was adjusted with 100–150 ml of acetic acid at the same concentration to remove eliminate any undissolved matter. Homogenates were prepared and stored at 0–4 °C until needed.
Chitosan was provided by Shandong Univeristy’s Institute of Physical Chemistry, School of Pharmacy. Chitosan was dissolved in 3% acetic acid and stirred in 50 °C water to create a 3% solution. The collagen protein and chitosan were mixed at a mass ratio of 3:1. The mixture was centrifuged at 3000 r/min for 15 min, frozen at − 35 °C for 2 h and it was then put right into a freeze dryer that had been precooled. The scaffold made of chitosan and collagen protein was completed forty hours later.
Isolation, culture, and identification of NESCs
Pregnant 11-day-old Wistar rats were provided by the Laboratory Animal Center of Shandong University. NESCs were isolated and cultured in accordance with the literature 12. In brief, 11-day-old pregnant Wistar rat embryos were removed under sterile conditions, with the neural tube isolated under a dissecting microscope and placed in D-Hank’s solution. The separated neural tube tissue was placed in a centrifuge tube, digested with 0.25% trypsin at 37 °C for 10 min, centrifuged at 800 r/min for 5 min, and suspended in DMEM/F12 media containing 10 ng/ml bFGF and 1:50 B27. The cells were incubated at 37 °C in 5% CO2 at a density of 106/ml. The basic medium was refreshed every 3 days. After 3–5 days, NESCs hung and formed neurospheres, which were mechanically dissected for passage. Second-generation neurospheres were inoculated into 24-well plates, and Nestin staining was performed after 4 h. A fluorescence microscope was used to monitor and photograph the cells.
NESCs cultured on collagen protein-chitosan scaffolds
The collagen protein-chitosan film was sliced into 12 mm × 8 mm pieces and sterilized using γ-rays. After three washes with D-Hanks, the films were coated in 24-well plates. The second passage of NESCs was planted on the collagen-chitosan film at a density of 107/ml and incubated at 37 °C in an incubator with 5% CO2 and 95% relative humidity. The cells were examined using a scanning electron microscope (SEM) 48 h after plating.
Preparation of sciatic nerve defect models and NESC transplantation
All male Wistar rats weighing between 220 and 250 g were provided by the Laboratory Animal Center at Shandong University. Wistar rats were randomly assigned to one of two groups: NESCs-collagen-chitosan group (NESCs group) and collagen-chitosan group (control group). Each group had 16 animals.
Rats’ right sciatic nerves was exposed after being deeply anesthetized with sodium pentobarbital (40 mg/kg body weight, i.p.). The sciatic nerve segment 5 mm distal to the inferior border of the piriformis was removed, resulting in a 10 mm long lesion after nerve terminal retraction. The collagen-chitosan membrane was formed into a single-layer cylindrical collagen-chitosan composite porous scaffold measuring approximately 1 mm, 12 mm long and 1.2 mm in diameter. Nerve defects were bridged using NESCs-collagen or collagen-chitosan scaffolds. Both the proximal and distal stumps of the nerves were inserted into the scaffolds at 1.0 mm and sutured using three needles. All animals were kept in temperature and humidity-controlled rooms under standard laboratory conditions, with unlimited access to food and water.
Electrophysiological evaluation
Nerve conduction latency was assessed 14 weeks following surgery using an electromyography device (Dantec Keypoint, Denmark). The stimulating electrode was placed in the sciatic nerve’s superficial section, between the sciatic tubercle and the greater trochanter, while the recording electrode was positioned in the triceps calf. The nerve conduction latency, distance between electrodes, and amplitude of nerve action potentials were all recorded and calculated.
Retrograde neuronal tracing
To determine whether dorsal root ganglion (DRG) neurons and spinal neurons had regenerated through the nerve scaffold, a retrograde neuronal labeling technique was employed. The retrograde neuronal labeling technique using Lumafluor fluorescent tracer (Lumafluor, USA) was used to measure neural route reconstruction 14 weeks following transplantation, as described by the manufacturer. After exposing the sciatic nerve, 100 nl of Lumafluor fluorescent tracer was injected into the inside of the sciatic epineurum for 5–10 min at a distance of 3 mm from the regenerated nerve. After 48 h of recovery, the animals were reanesthetized and 4% paraformaldehyde was perfused through the ascending aorta. The spinal cord from the enlarged lumbar segment was removed, fixed with 4% paraformaldehyde for 12 h, and immmersed in 30% sucrose in 0.1 M PBS overnight. After freezing at − 20 °C, serial lateral 20 μm thick frozen slices were taken from the DRGs via a freezing microtome. The sections were collected and examined using a fluorescence microscope. The sciatic nerve was traced using the above method.
Transmission electron microscopy (TEM) and toluidine blue staining
At 14 weeks after surgery, toluidine blue staining and TEM were utilized to observe 5 mm of the proximal end of the regenerated nerve. The specimens were fixed by immersion in 2.5% glutaraldehyde for 24 h before being fixed in 1% osmium tetroxide at 4 °C for 2 h. They were then dehydrated in a graded ethanol series (50%, 70%, 90%, 100%) and acetone for 15 min at each concentration. They were then infiltrated with resin and polymerized for 48 h at 60 °C. Eight semithin slices were cut from each specimen and stained with toluidine blue for light microscopy analysis. Images of the toluidine blue-stained semithin nerve sections were digitized and analyzed using Image-Pro Plus 6.0 software. The specimens were cut into ultrathin sections and stained with uranyl acetate and lead citrate for 2 min before TEM.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using the SPSS 26.0 software package. Significant differences between two groups were analyzed by t tests. Differences were considered statistically significant at p < 0.05.
Results
The amplification and identification of NESCs
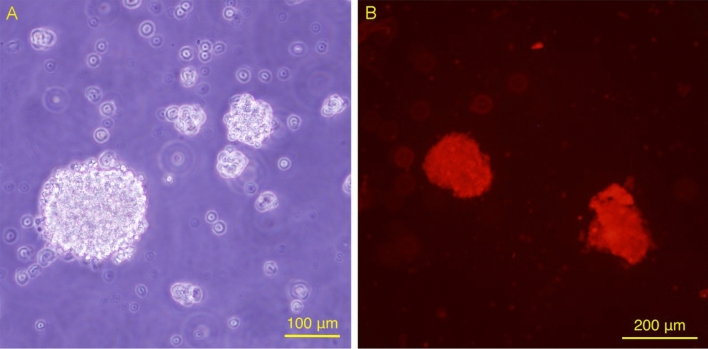
After 2 days of culture in serum-free medium, the extracted NESCs formed suspension neurospheres composed of several or a dozen cells and grew into neurospheres composed of dozens or even hundreds of cells on the 5th day (Fig. 1A). Immunocytochemical staining revealed that the neurospheres were positive for Nestin (red), indicating that the amplified cells were NESCs (Fig. 1B).
Fig. 1.
The proliferation and identification of NESCs. (A) Suspended growth neurospheres formed when NESCs were cultured in vitro for 5 days. (B) Neurosphere Nestin immunofluorescence staining was positive, showing that the amplified cells were NESCs.
NESCs can survive on collagen-chitosan membranes
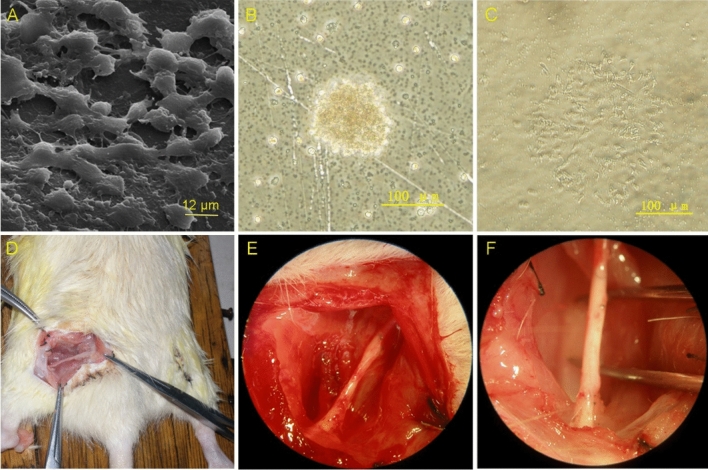
Two days after the NESCs were seeded on the collagen-chitosan scaffolds, the NESCs were observed to attach to the scaffold surface, with protrusions growing and connecting to a network (as shown in Fig. 2A–C). Figure 2D shows that we separated the sciatic nerve. Fourteen weeks after the operation, the collagen chitosan protein conduit had degraded, and the sciatic nerve was connected. (as shown in Fig. 2E,F).
Fig. 2.
NESCs grow on collagen-chitosan membranes. (A) SEM image (× 2500) of NESCs attached to a collagen-chitosan membrane with protrusions growing and connecting into a network. (B) Neurospheres extended to the surroundings. (C) NESCs grow with protrusions. (D) Mode diagram of surgical operation. (E) The right sciatic nerve was exposed, and the injury was approximately 10 mm long. (F) The injured sciatic nerve was repaired after 14 weeks.
Electrophysiological results of regenerated nerves
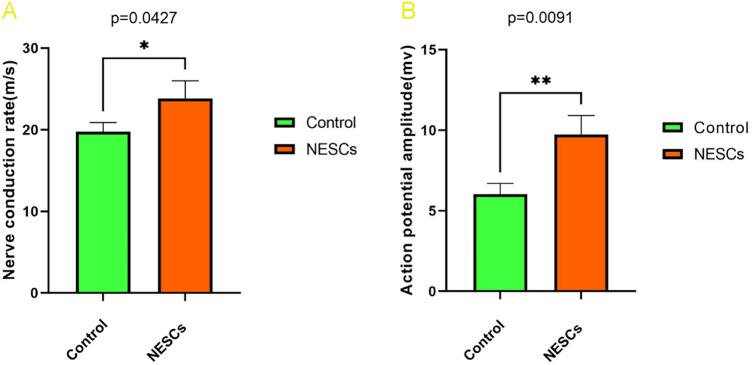
At 14 weeks after the operation, the nerve conduction latency and amplitude of the animals in each group were detected by electromyography, and the nerve conduction latency rate was calculated. The results showed that compound muscle action potentials were detected in both groups of animals, and the nerve conduction latency rate (23.84 ± 2.14 m/s) and amplitude (9.74 ± 1.18 mv) in the NESCs group were significantly greater than those in the control group (19.75 ± 1.12 m/s and 6.03 ± 0.67 m/v, respectively) (as shown in Table 1), and the differences were statistically significant (as shown in Fig. 3A,B).
Table 1.
Comparison of nerve conduction between the NESC group and the CTRL group.
| Group | Nerve conduction latent rate (m/s) | Amplitude value (mv) |
|---|---|---|
| Collagen-chitosan scaffolds group (control group) | 19.75 ± 1.12 | 6.03 ± 0.67 |
| NESCs-collagen-chitosan scaffolds group (NESCs group) | 23.84 ± 2.14* | 9.74 ± 1.18** |
Fig. 3.
Comparison of nerve conduction between the NESCs group and the CTRL group. (A) Comparison of the nerve conduction latency rates between the two groups. (B) Comparison of nerve conduction amplitude between the two groups. *p < 0.05, **p < 0.01 for the NESCs group vs the CTRL group.
Toluidine blue staining and TEM analysis
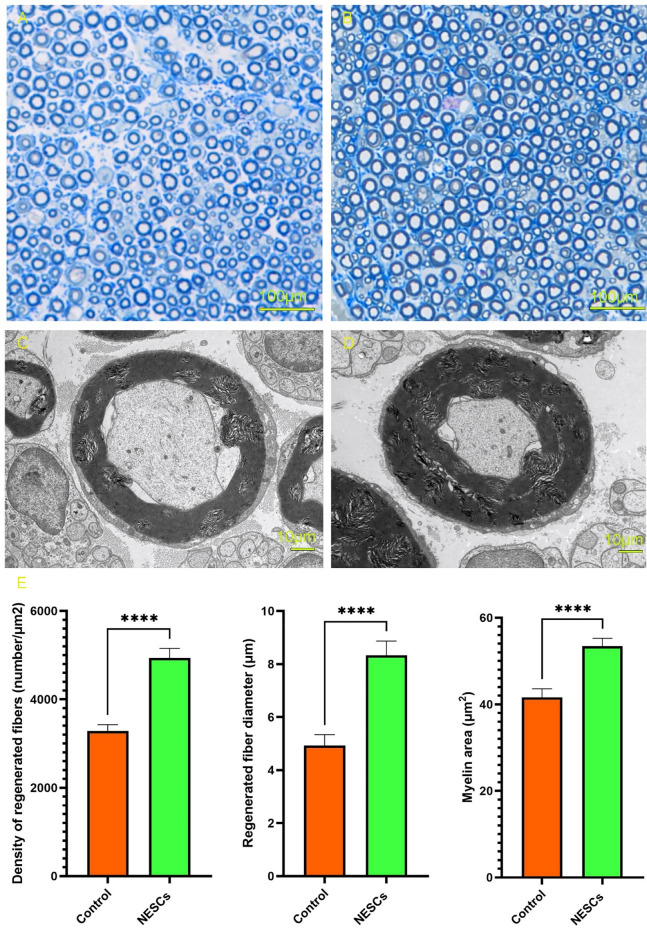
Toluidine blue staining and TEM were used to compare the structural changes in axons in the proximal regenerated sciatic nerve at 14 weeks after surgery between the two groups of animals. The results of toluidine blue staining showed that compared with those in the control group, the regenerated axons in the NESCs group had more obvious myelination, more myelinated fibers, and a larger diameter (as shown in Fig. 4A,B), and the difference was statistically significant. The TEM results showed that compared with those in the control group, the number of regenerated fibers in the NESCs group was greater, the diameter was greater (as shown in Table 2), and some Schwann cells could form the myelin sheath structure around the axon (as shown in Fig. 4C,D); these differences were statistically significant (as shown in Fig. 4E).
Fig. 4.
Toluidine blue staining of nerve fibers and TEM analysis of the two groups. (A) Toluidine blue staining of the CTRL group (× 100). (B) Toluidine blue staining of the NESCs group (× 100). (C) Nerve fibers of the CTRL group by TEM (× 7500). (D) Nerve fibers of the NESCs group by TEM (× 7500). (E) Comparison of regenerated nerve fiber density, regenerated nerve fiber diameter and myelin sheath thickness between the two groups. **p < 0.01, ****p < 0.0001, the NESC group vs the CTRL group.
Table 2.
Comparisons of regenerated nerve fiber density, regenerated nerve fiber diameter and myelin sheath thickness between the two groups.
| Density of regenerated fibers (number/mm2) | Regenerated fiber diameter (μm) | Myelin area (μm2) | |
|---|---|---|---|
| Collagen-chitosan scaffolds group (Control group) | 33.25 ± 13.4 | 4.648 ± 0.243 | 40.44 ± 1.25 |
| NESCs-collagen-chitosan scaffolds group (NESCs group) | 48.62 ± 15.8 | 5.484 ± 0.312 | 52.24 ± 1.07 |
Number of retrogradely labeled DRG neurons and sciatic nerves
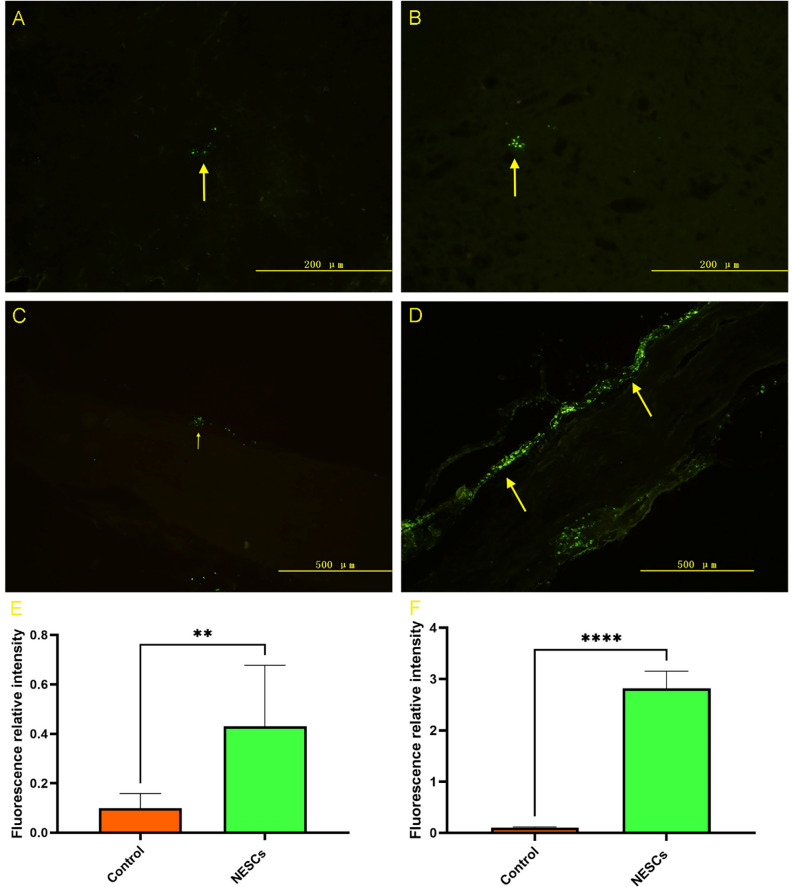
To detect the reconstruction of neural pathways, we used a Lumafluor fluorescent tracer to detect the neural pathway in experimental animals 14 weeks after the operation. Lumafluor-labeled motor neurons showing green fluorescence could be detected in the anterior horn of the spinal cord in both groups of animals. The number of labeled-positive cells in the NESCs group was significantly greater than that in the control group (Fig. 5A,B). The number of labeled sciatic nerves in the NESCs group was obviously greater than that in the control group (Fig. 5C,D). These results were statistically significant (Fig. 5E). All these results indicated that the neural pathway was mainly reconstructed (Table 2).
Fig. 5.
The results of the Lumafluor fluorescent tracer. (A) Cells in the anterior horn of the spinal cord in the CTRL group. (B) Positive cells labeled with group NESCs in the anterior horn of the spinal cord. (C) Retrogradely labeled sciatic nerves in the CTRL group. (D) Retrograde-labeled sciatic nerves in the NESC group. (E) Comparison of fluorescent tracer-labeled positive cells between the two groups. *p < 0.05, ****p < 0.0001 for the comparison between the NESC group and the CTRL group.
Discussion
The objective of this study was to investigate the potential of delivering NESCs to a collagen protein-chitosan nerve conduit to promote peripheral nerve regeneration. Our data indicate that NESCs grafted to collagen-chitosan scaffolds can improve the regeneration and function of the sciatic nerve after a transected lesion in vivo. The results presented in this study have implications for future therapeutic applications using neuroepithelial stem cells delivered in a collagen protein-chitosan nerve conduit to enhance peripheral nerve repair.
Peripheral nerve injury is a common and frequently occurring disease in the clinic. The regeneration and repair of nerve injury is a hot research topic for scholars at home and abroad16. By means of nerve stump anastomosis, nerve transplantation, or bridging nerve stumps by implanting a nerve guiding catheter, a good microenvironment can be provided for nerve regeneration. Recently, it has been reported that chitosan-polyethoxylate nerve grafts combined with bone marrow monocytes have achieved good clinical results in repairing median nerve damage17. However, the transplant process has risks of complications such as rejection and infection. Biological graft materials with few rejection reactions, good biocompatibility and degradability can provide a good solution for peripheral nerve injury. Nerve conduits play a role in supporting and guiding nerve fiber regeneration in the repair of peripheral nerve injury, providing a good local regeneration microenvironment for nerve repair.
The collagen-chitosan composite catheter used in this study was prepared by combining collagen and chitosan at a 3:1 ratio. The composite scaffold provides a suitable microenvironment for nerve cell-surrounding interactions to facilitate tissue regeneration. The collagen-chitosan catheter was completely degraded at 14 weeks after surgery in vivo, and the surface of the material had good degradability and biocompatibility, providing a suitable time and space for the regeneration of nerve axons13. Different mass ratios of collagen/chitosan affect the degradation time and biocompatibility of the composites. A recent study demonstrated that a collagen/chitosan scaffold with a mass ratio of 1:8 was completely degraded 6 months after transplantation and was the most suitable material for traumatic brain injury repair5. As the collagen/chitosan ratio decreased, the degradation time of the composites gradually increased. The degradation rate of the desired scaffold should match the time required for tissue repair.
Our findings show that the collagen-chitosan composite is biocompatible with NESCs and that inoculated NESCs not only survive well but also grow protrusions. Studies on the repair of peripheral nerve injury have demonstrated that chitosan promotes peripheral nerve regeneration by supporting axonal regeneration and reducing scar tissue formation18,19. The combination of appropriate materials with chitosan can be used as a nerve guiding catheter for cell therapy for the treatment of peripheral nerve injury18,20. NESCs are neural stem cells with multidifferentiation potential that play important roles as seed cells in the repair of nervous system diseases and peripheral nerve damage21,22. In this study, after 5 days of culture, NESCs obtained from early neural tubes grew into neurospheres composed of dozens or even hundreds of cells, indicating that these cells have a very strong ability to proliferate. After 48 h, the adherent cells grew out of the protrusions and connected with each other, indicating that the NESCs had good compatibility with the collagen-chitosan scaffolds. Previous studies showed that NESCs can survive well on chitosan fibers and differentiate into neurons, astrocytes and a few Schwann-like cells expressing S-10013. Electrophysiological tests revealed that the motor conduction potential rate and amplitude of the regenerated nerves in the NESCs group were significantly better than those in the control group, indicating that the motor conduction function recovery of the regenerated nerve in the NESCs group was better than that in the simple collagen-chitosan conduit group.
This finding was confirmed by toluidine blue staining and TEM analysis. The results of toluidine blue staining and TEM analysis showed that the neural fiber regeneration and diameter of myelinated fibers in the NESCs group were greater than those in the control group. Retrograde tracing revealed labeled neurons in the anterior horn of the spinal cord in both groups of animals at 14 weeks after the operation, indicating that the proximal end of the defective nerve had reached the distal end through regeneration and that axonal transport was restored. The number of labeled neurons in the NESCs group was greater than that in the collagen-chitosan group. The number of labeled cells in sciatic nerves in the NESCs group was much greater than that in the collagen-chitosan conduit group. These findings prove that NESCs can more effectively promote the regeneration and repair of damaged nerves. All these results indicated that the neural pathway was mainly reconstructed and that NESCs exhibited superior nerve regeneration characteristics.
The mechanism by which the NESCs-collagen-chitosan scaffold promotes peripheral nerve regeneration is multifaceted and comprehensive. The growth and maturation of regenerated axons are largely limited by local microenvironmental factors during nerve regeneration, such as cells, the extracellular matrix, and various cytokines. Transplanted neural stem cells can locally proliferate and induce differentiation into specific cells to compensate for damaged neurons. Transplanted cells inhibit neurodegeneration or promote nerve regeneration by releasing neurotransmitters such as dopamine, acetylcholine, and various neurotrophic factors23,24. Studies have shown that neural stem cells can secrete GDNF, BDNF, nerve growth factor and other nutritional factors, which can be detected not only quantitatively in vitro, but also at a high level after being implanted in rats with spinal cord injury25. In addition, Llado et al. reported that NSCs can secrete NTFs to promote the survival of injured motor nerve cells and reduce the toxic damage to nerve cells by antagonizing excitatory glutamate26. However, the mechanism by which NESCs promote peripheral nerve regeneration is complex, and further research on the mechanism by which transplanted cells promote nerve regeneration is needed.
Conclusion
In conclusion, we used NESCs as seed cells combined with chitosan-collagen to construct tissue engineering bridges, providing a favorable regenerative microenvironment for peripheral nerves. In addition to the effects of NESCs, an unknown mechanism, for example, cytokine or inflammation, might be involved in enhancing nerve regeneration. This study demonstrated that it can promote peripheral nerve repair and has good application prospects and potential research value. In addition, through “therapeutic cloning”, that is, cloning early embryos and extracting stem cells through somatic cell nuclear transfer technology, autologous differentiated cells are generated for therapeutic purposes. Therefore, with the further development of science and technology in the future, the source of neural stem cells may be commercialized and widely used in clinical treatment.
Acknowledgements
This work was supported by the Shandong Provincial Medical and Health Science and Technology Development Plan (202104040051) and the Youth Science Fund Cultivation Grant Program of Shandong First Medical University (Shandong Academy of Medical Sciences) (No. 202201-106).
Author contributions
Y-CP and L-W: conception, analysis and writing of the manuscript; Q-BM, Q-YJ and X-HC: methodology, analysis and validation; X-T and L-T: discussed the final edition and was responsible for the overall content; all the authors have read and approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Affiliation 1, which was incorrectly given as ‘Department of Neurosurgery, The Third Affiliated Hospital of Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, 250117, Shandong, China’. The correct affiliation is: ‘Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan 250117, Shandong, China’.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chenping Yun and Wei Li.
Change history
11/20/2024
A Correction to this paper has been published: 10.1038/s41598-024-79830-0
Contributor Information
Tao Xu, Email: 307040527@qq.com.
Tao Li, Email: litao9654@sdfmu.edu.cn.
References
- 1.Min, Q., Parkinson, D. B. & Dun, X. P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia69, 235–254 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Lavorato, A. et al. Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: Systematic review. Int. J. Mol. Sci.22, 572 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao, L. & DeBrot, A. Preparation of bioscaffolds delivering stem cells for neural regeneration. Methods Mol. Biol.2155, 63–70 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Kaminska, A., Radoszkiewicz, K., Rybkowska, P., Wedzinska, A. & Sarnowska, A. Interaction of neural stem cells (NSCs) and mesenchymal stem cells (MSCs) as a promising approach in brain study and nerve regeneration. Cells11, 1464 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng, D. et al. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol.13, 1039241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boecker, A., Daeschler, S. C., Kneser, U. & Harhaus, L. Relevance and recent developments of chitosan in peripheral nerve surgery. Front. Cell Neurosci.13, 104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun, Y. et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J. Biomed. Mater. Res. A107, 1898–1908 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Guo, S. et al. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed.31, 155–168 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Shagdarova, B. et al. Collagen/chitosan gels cross-linked with genipin for wound healing in mice with induced diabetes. Materials15, 15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, X. et al. Integrated printed BDNF/collagen/chitosan scaffolds with low temperature extrusion 3D printer accelerated neural regeneration after spinal cord injury. Regener. Biomater.8, 047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, X. et al. Three-dimensional-printed collagen/chitosan/secretome derived from HUCMSCs scaffolds for efficient neural network reconstruction in canines with traumatic brain injury. Regener. Biomater.9, 043 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammouda, H., Farag, M., El Deftar, M., Abdel-Gabbar, M. & Mohamed, B. Effect of Ce-doped bioactive glass/collagen/chitosan nanocomposite scaffolds on the cell morphology and proliferation of rabbit’s bone marrow mesenchymal stem cells-derived osteogenic cells. J. Genet. Eng. Biotechnol.20, 33 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang, P. et al. Survival and differentiation of neuroepithelial stem cells on chitosan bicomponent fibers. Chin. J. Physiol.53, 208–214 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Yan, F. et al. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural Regener. Res.14, 1780–1786 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao, W. et al. Rapid sciatic nerve regeneration of rats by a surface modified collagen-chitosan scaffold. Injury44, 941–946 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Modrak, M., Talukder, M. A. H., Gurgenashvili, K., Noble, M. & Elfar, J. C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res.98, 780–795 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, X. et al. Combined use of chitosan-PGLA nerve grafts and bone marrow mononuclear cells to repair a 50-mm-long median nerve defect combined with an 80-mm-long ulnar nerve defect in the human upper arm. Curr. Stem Cell Res. Ther.17, 389 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Poongodi, R. et al. Bio-scaffolds as cell or exosome carriers for nerve injury repair. Int. J. Mol. Sci.22, 13347 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, N. et al. Modulating cationicity of chitosan hydrogel to prevent hypertrophic scar formation during wound healing. Int. J. Biol. Macromol.154, 835–843 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Pop, N. L. et al. Chitosan functionalized magnetic nanoparticles to provide neural regeneration and recovery after experimental model induced peripheral nerve injury. Biomolecules11, 376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taverna, E. & Huttner, W. B. The golgi apparatus in polarized neuroepithelial stem cells and their progeny: Canonical and noncanonical features. Results Probl. Cell Differ.67, 359–375 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Binh, N. T. et al. Proliferation and differentiation of dopaminergic neurons from human neuroepithelial stem cells obtained from embryo reduction following in vitro fertilization. Med. Arch.75, 280–285 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson, K. et al. Behavioral context improves optogenetic stimulation of transplanted dopaminergic cells in unilateral 6-OHDA rats. Behav. Brain Res.441, 114279 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Gori, S. et al. Acetylcholine-treated murine dendritic cells promote inflammatory lung injury. PLoS ONE14, e0212911 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu, P., Jones, L. L., Snyder, E. Y. & Tuszynski, M. H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol.181, 115–129 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Lladó, J., Haenggeli, C., Maragakis, N. J., Snyder, E. Y. & Rothstein, J. D. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol. Cell. Neurosci.27, 322–331 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.