Abstract
The N-methyl-D-aspartate R1 (NMDAR1) and NMDAR2A subunits were expressed transiently either alone or in combination in human embryonic kidney (HEK) 293 cells. The biochemical and pharmacological properties of the cloned receptors were compared with those of adult rat brain NMDA receptors using both immunological methods with a newly developed anti-NMDAR2A-(1435-1445) antibody and [3H]MK801 radioligand binding activity. Anti-NMDAR2A-(1435-1445) antibodies recognized specifically four immunoreactive species with M(r)s of 180,000, 122,000, 97,000 and 54,000 in rat brain, but only a single band of M(r) 180,000 in HEK 293 cells singly transfected with plasmid pCISNMDAR2A. N-deglycosylation of HEK cell membranes yielded a 165,000-M(r) immunoreactive species, which is in agreement with the size predicted from the cDNA sequence for the mature NMDAR2A subunit. Co-expression of NMDAR1 and NMDAR2A subunits in HEK 293 cells resulted in cell death. Thus conditions were established for the optimum expression of heteromeric receptors in viable cells, including a requirement for DL-2-amino-5-phosphonopentanoic acid (AP5) in the culture medium post-transfection. Cells transfected with pCISNMDAR1 and pCISNMDAR2A combined yielded a 10-fold increase in the number of [3H]MK801 binding sites compared with single subunit expression. MK801 had similar affinity for the expressed receptors as for those found in adult rat and mouse brain. These results demonstrate that the NMDAR1 and NMDAR2A receptor subunits co-assemble to form a heteromeric complex with properties similar to those of the native receptors of adult mammalian forebrain. Furthermore, the conditions reported for maximal transient expression provide a basis for further structure-activity studies.
Full text
PDF



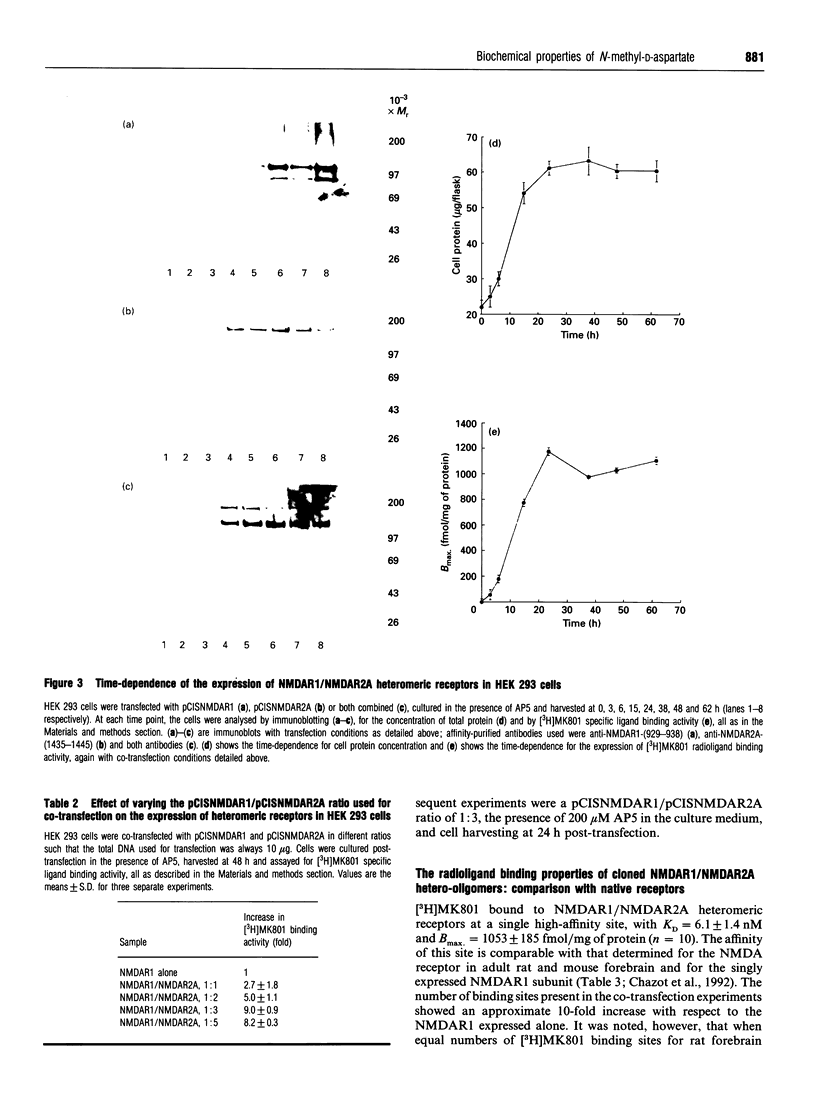


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benke D., Marti T., Heckendorn R., Rehm H., Künzi R., Allgeier H., Angst C., Mohler H. Photoaffinity labeling of the NMDA receptor. Eur J Pharmacol. 1993 Jul 15;246(2):179–180. doi: 10.1016/0922-4106(93)90096-r. [DOI] [PubMed] [Google Scholar]
- Chazot P. L., Cik M., Stephenson F. A. Immunological detection of the NMDAR1 glutamate receptor subunit expressed in embryonic kidney 293 cells and in rat brain. J Neurochem. 1992 Sep;59(3):1176–1178. doi: 10.1111/j.1471-4159.1992.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Chazot P. L., Fotherby A., Stephenson F. A. Evidence for the involvement of a carboxyl group in the vicinity of the MK801 and magnesium ion binding site of the N-methyl-D-aspartate receptor. Biochem Pharmacol. 1993 Feb 9;45(3):605–610. doi: 10.1016/0006-2952(93)90133-h. [DOI] [PubMed] [Google Scholar]
- Duggan M. J., Pollard S., Stephenson F. A. Immunoaffinity purification of GABAA receptor alpha-subunit iso-oligomers. Demonstration of receptor populations containing alpha 1 alpha 2, alpha 1 alpha 3, and alpha 2 alpha 3 subunit pairs. J Biol Chem. 1991 Dec 25;266(36):24778–24784. [PubMed] [Google Scholar]
- Duggan M. J., Stephenson F. A. Biochemical evidence for the existence of gamma-aminobutyrateA receptor iso-oligomers. J Biol Chem. 1990 Mar 5;265(7):3831–3835. [PubMed] [Google Scholar]
- Ebert B., Wong E. H., Krogsgaard-Larsen P. Identification of a novel NMDA receptor in rat cerebellum. Eur J Pharmacol. 1991 Sep 12;208(1):49–52. doi: 10.1016/0922-4106(91)90050-r. [DOI] [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Sonders M. S., Barmettler P., Lee J. A., Kitahara Y., Keana J. F., Weber E. A novel photoaffinity ligand for the phencyclidine site of the N-methyl-D-aspartate receptor labels a Mr 120,000 polypeptide. J Biol Chem. 1990 Apr 25;265(12):6776–6781. [PubMed] [Google Scholar]
- Stern P., Béhé P., Schoepfer R., Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992 Dec 22;250(1329):271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Sugihara H., Moriyoshi K., Ishii T., Masu M., Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992 Jun 30;185(3):826–832. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- Tingley W. G., Roche K. W., Thompson A. K., Huganir R. L. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993 Jul 1;364(6432):70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Yokotani N., Doi K., Wada K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J Biol Chem. 1992 Jan 5;267(1):501–507. [PubMed] [Google Scholar]
- Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990 Jun;11(6):216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]





