Dialysis as a life-sustaining therapy for irreversible kidney failure was pioneered at the University of Washington in the 1960s. Yet, more than half a century later, technical innovations that have positively enhanced or transformed the patient experience and positive outcomes have been incremental and unimpressive. Most (nearly 90%) patients continue to experience dialysis as an approximately 12-hour per week in-center treatment regimen, with substantial limitations on freedom of movement, diet, and overall quality of life.1 Current hemodialysis treatments leave patients with substantial residual uremic symptoms and are frequently associated with cardiovascular, cerebrovascular, and infectious complications. While extended treatment times and more frequent dialysis have considerable potential to improve patient outcomes, they are not feasible for most patients with current technology.
To address the lack of progress, the University of Washington Center for Dialysis Innovation (CDI) was launched in 2017 with the mission to transform dialysis care using state-of-the-art biomaterials and engineering technologies. Working closely with dialysis patients and their care partners, the CDI assembled multidisciplinary engineering project teams, focused on innovating key components of dialysis, ultimately facilitating development of patient-centered, mobile (portable/wearable) hemodialysis.2,3 These teams developed and iteratively tested a series of convergent, novel technologies to collectively create a radically different approach to dialysis. Of importance, all components of the dialysis circuit (including the vascular access, dialyzers, polyvinyl chloride tubing and the dialysis machine) must be optimized, and this process requires a true innovation ecosystem with parallel research projects. A priori, we defined three core focus areas: solute separation, blood compatibility, and blood access. Here, we focus on describing our progress in solute separation and blood compatibility.
Solute Separation
Hemodialysis relies on diffusive solute clearance from the blood for sufficient uremic toxin removal, which requires keeping very low toxin concentrations in the dialysate. This is accomplished by single-pass proportioning systems that use hundreds of liters of highly purified water to support continuous, high-flow dialysate rates for each session. The necessity for such high volumes of dialysate prevents portability of the dialysis treatment and limits mobility for patients. Moreover, the profligate use of water has a substantial environmental impact and is unlikely to be sustainable in the face of climate change, lending increasing focus on making hemodialysis more green.
Innovative efforts to create alternative approaches date back to at least the 1970s. Notably, Willem Kolff, in 1975, described the clinical use of a wearable, lightweight dialysis machine that combined a dual pump for blood and dialysate flow, a rechargeable battery, and a charcoal-based dialysate regeneration module for toxin removal. However, this experimental device still required a 20-L dialysate tub to remove urea and other low molecular weight toxins.
Urea, as the single most abundant product of protein metabolism eliminated by kidney function, has proved to be the biggest challenge for portability.4 The 1980s and 1990s saw the commercialization and clinical use of sorbent-based dialysis systems. Sorbents in the dialysate circuit can bind sufficient electrolytes and minerals to maintain electrolyte and mineral homeostasis. However, sorbent-based systems were still challenged to remove sufficient urea to maintain mass balance. The Redy and Allient dialysis systems deployed the enzyme urease to remove the required large quantities of urea.4 However, the end product of urea degradation by urease is ammonia, which is itself toxic. This obligated the use of zirconium phosphate as an ion-exchanging sorbent to bind ammonia, which introduced other downstream mineral and electrolyte-related complications. Ultimately, these sorbent-based systems dependent on urease did not succeed in the marketplace compared with reverse osmosis–based hemodialysis.
Recent efforts to create portable and wearable forms of hemodialysis have revived interest in the use of urease and sorbents for continuous pass dialysis,5 including a pilot clinical trial conducted under the auspices of the US Food and Drug Administration Innovations Pathway 2.0.6 Additional developmental efforts to commercialize urease/sorbent systems (including AWAK Technologies, Wearable Artificial Kidney Project, Renart-PD, Vicenza Wearable Artificial Kidney for Peritoneal Dialysis, SORB.com, and Eopump-based Continuous Ambulatory RRT) are currently underway. Another approach under development is the deployment of a new class of nanomaterials called MXenes as a novel sorbent that can directly bind urea to eliminate urease-related complications.7
To eliminate urea and thereby regenerate and recirculate the dialysate within a portable device, CDI investigators developed a novel photo-oxidation technology that removes urea from the dialysate by decomposing it into nitrogen gas, carbon dioxide gas, and water through photochemical oxidation on a TiO2 photoanode under ultraviolet (UV) illumination (Figure 1). Nitrogen and carbon dioxide are then degassed from the circuit. The TiO2 photoelectrochemical cell contains an anode, where UV-photoexcited TiO2 oxidizes urea on its surface while electrons are collected on an underlying conducting oxide and transported to the cathode. By a combination of nanowire TiO2 growth, an oxygen permeable cathode, and efficient 365-nm light-emitting diodes, we achieved a greater than a 100-fold improvement in energy efficiency and size reduction compared with earlier literature where a similar concept was applied to agricultural waste processing.8
Figure 1.
The photo-oxidative urea removal strategy. (A) A patient is dialyzed by a conventional KRT dialysis strategy. (B) The spent dialysate, which would be flushed down the drain in convention hemodialysis, is passed through a FO membrane that transports urea and ions, but excludes glucose, amino acids, and other larger molecules. Small volume sorbent cartridges are used to remove low levels of other soluble uremic toxins and undesirable byproducts of the photo-oxidation. (C) The urea transporting through the FO membrane is converted to CO2 and N2 by UV-catalyzed photo-oxidation on a TiO2 catalyst. FO, forward osmosis; UV, ultraviolet.
Eliminating urea into nontoxic end products enables a hemodialysis system that runs continually on a 2-liter bag of prepackaged, sterile dialysate, without the need for an external water connection. Extensive bench testing has demonstrated proof of concept that a photo-oxidation–based prototype can remove the average daily amount of urea and other uremic toxins typically produced by the body from recirculating dialysate, thus enabling a truly portable/wearable dialysis system. This system has been extensively bench tested and is awaiting large animal model studies. By adding small amounts of sorbents and binders to achieve mineral and electrolyte homeostasis and activated charcoal to bind other uremic toxins in the circuit,9 a fully functional, low-water hemodialysis system is enabled. Design and prototyping are in preparation for first in human trials.
Blood Compatibility
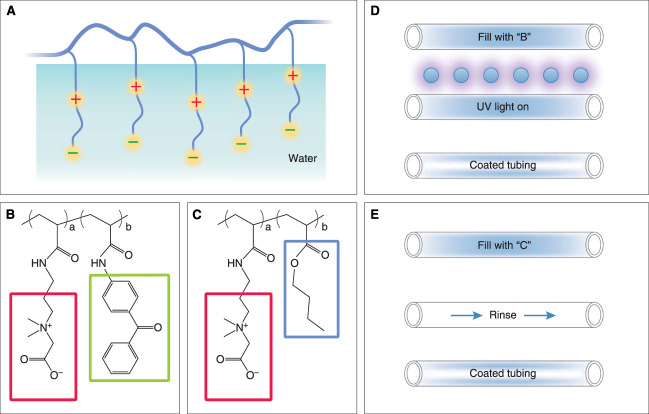
The CDI has developed novel approaches to improve blood compatibility during hemodialysis (Figure 2). Recognizing that the future of hemodialysis will be patient-centered, portable, and home-based, the longer and more frequent hemodialysis treatments may exacerbate complications, such as blood coming into contact with artificial surfaces leading to increased clotting risk, inflammation, and also more exposure to plasticizer leaching from polyvinyl chloride tubing. The extracorporeal hemodialysis circuit (Figure 1) includes three large surface area components: a dialyzer (semipermeable membrane, approximately 2 m2 of surface), a forward osmosis hollow fiber device, and tubing (approximately 0.5 m2 of inner surface). Although the conversion from unmodified cellulosic to modified cellulosic and subsequently synthetic membranes has improved blood compatibility, the direct contact with blood still demonstrates limited blood compatibility. For example, repetitive complement activation can promote inflammation and clotting, infection, and cardiovascular events.10
Figure 2.
Two strategies for surface modification of dialysis components to reduce thrombogenicity, prevent biofilm formation, minimize complement activation, and inhibit plasticizer leaching. (A) The generalized structure of the polymerized zwitterionic structure that tightly bonds water at the interface. (B) The polyacrylate backbone polymer has side groups of zwitterionic CB (>70%) (red outline) and a benzophenone groups (green outline) facilitating photo-immobilization. (C) The polyacrylate backbone polymer has side groups of zwitterionic CB (>70%) (red outline) and butyl methacrylate groups (blue outline) facilitating hydrophobic immobilization to hydrophobic component surfaces. (D) UV light treatment of surfaces to bond the CB to the surface. (E) Hydrophobic, noncovalent bonding of butyl-derivatized CB to hydrophobic surfaces. CB, carboxybetaine.
To address this problem, various modification techniques have been explored to improve the blood compatibility of extracorporeal hemodialysis circuits. These include chemical immobilization of functional groups on a surface by grafting, layer-by-layer chemical attachment of species, covalent attachment of super-hydrophilic hydrogels, mixed matrix membrane, and base polymer modification (blending). Surface modification through grafting (glow discharge plasma or UV-induced, for example) and adsorptive attachment are common and generally recognized as effective methods for improving the chemical structure of the outer layer of dialyzer membranes.
Zwitterionic compounds have been widely explored for surface modification of medical devices because these highly hydrophilic, nonfouling materials are known to minimize blood protein adsorption to synthetic surfaces and thus reduce human platelet activation and complement activation.11 The CDI is developing polycarboxybetaine (PCB)-based zwitterionic materials that show imperceptible protein adsorption (<0.3 ng/cm2) toward 100% human serum or plasma and have been explored in numerous biomedical applications without triggering adverse physiological responses. We have tested two PCB-based copolymers (Figure 2), each specifically designed for coating separate components of an extracorporeal circuit.11 Coating techniques by UV irradiation and hydrophobic self-attachment facilitate surface modification of tubing and dialyzers, respectively. In vitro testing of coated and uncoated extracorporeal circuits with human blood, assessing activation of both the intrinsic and extrinsic coagulation systems, consistently show reduced platelet, clotting cascade, and complement activation in the coated system, indicating that PCB-based zwitterionic coatings can enhance blood compatibility (manuscript in preparation). Plans are in progress for in vivo clinical trials testing PCB-coated versus PCB-uncoated surfaces of dialysis components.
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/JSN/E624.
Funding
J. Himmelfarb is supported by NIH grants NCATS UH3TR003288, NIDDK U01DK133090, NIDDK U24DK114886, NIDDK R01DK130815, and NIDDK 1 R01DK133177. The Center for Dialysis Innovation (CDI) has been supported by a generous grant from the Northwest Kidney Centers, NIH 1R41DK137677, and generous gifts from the Mount Baker Foundation and Dialysis Clinic, Inc. The CDI has also received funding from KidneyX prize competitions.
Author Contributions
Conceptualization: Jonathan Himmelfarb, Buddy D. Ratner.
Data curation: Jonathan Himmelfarb, Buddy D. Ratner.
Formal analysis: Jonathan Himmelfarb.
Funding acquisition: Jonathan Himmelfarb, Buddy D. Ratner.
Investigation: Jonathan Himmelfarb, Buddy D. Ratner.
Methodology: Jonathan Himmelfarb, Buddy D. Ratner.
Resources: Buddy D. Ratner.
Supervision: Jonathan Himmelfarb.
Writing – original draft: Jonathan Himmelfarb.
Writing – review & editing: Jonathan Himmelfarb, Buddy D. Ratner.
References
- 1.Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573–585. doi: 10.1038/s41581-020-0315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramada DL de Vries J Vollenbroek J, et al. Portable, wearable and implantable artificial kidney systems: needs, opportunities and challenges. Nat Rev Nephrol. 2023;19(8):481–490. doi: 10.1038/s41581-023-00726-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivara MB, Himmelfarb J. From home to wearable hemodialysis: barriers, progress, and opportunities. Clin J Am Soc Nephrol. 2024. Epub ahead of print. doi: 10.2215/CJN.0000000000000424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenkrantz MJ Gordon A Roberts M, et al. Applications of the Redy sorbent system to hemodialysis and peritoneal dialysis. Artif Organs. 1979;3(3):230–236. doi: 10.1111/j.1525-1594.1979.tb01054.x [DOI] [PubMed] [Google Scholar]
- 5.van Gelder MK Jong JAW Folkertsma L, et al. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials. 2020;234:119735. doi: 10.1016/j.biomaterials.2019.119735 [DOI] [PubMed] [Google Scholar]
- 6.Gura V Rivara MB Bieber S, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1(8):e86397. doi: 10.1172/jci.insight.86397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng F Seredych M Chen C, et al. MXene sorbents for removal of urea from dialysate: a step toward the wearable artificial kidney. ACS Nano. 2018;12(10):10518–10528. doi: 10.1021/acsnano.8b06494 [DOI] [PubMed] [Google Scholar]
- 8.Shao G Tang H Ren S, et al. Dialysate regeneration via urea photodecomposition with TiO2 nanowires at therapeutic rates. Artif Organs. 2023 Jul;47(7):1174–1183. doi: 10.1111/aor.14514 [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Sirich TL, Blanco IJ, Plummer NS, Meyer TW. Removal of uremic solutes from dialysate by activated carbon. Clin J Am Soc Nephrol. 2022;17(8):1168–1175. doi: 10.2215/CJN.01610222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poppelaars F Faria B Gaya da Costa M, et al. The complement system in dialysis: a forgotten story? Front Immunol. 2018;9:71. doi: 10.3389/fimmu.2018.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X Wu K Zhou Q, et al. Photoreactive carboxybetaine copolymers impart biocompatibility and inhibit plasticizer leaching on polyvinyl chloride. ACS Appl Mater Inter. 2020;12(37):41026–41037. doi: 10.1021/acsami.0c09457 [DOI] [PubMed] [Google Scholar]