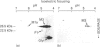Abstract
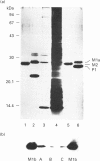
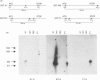
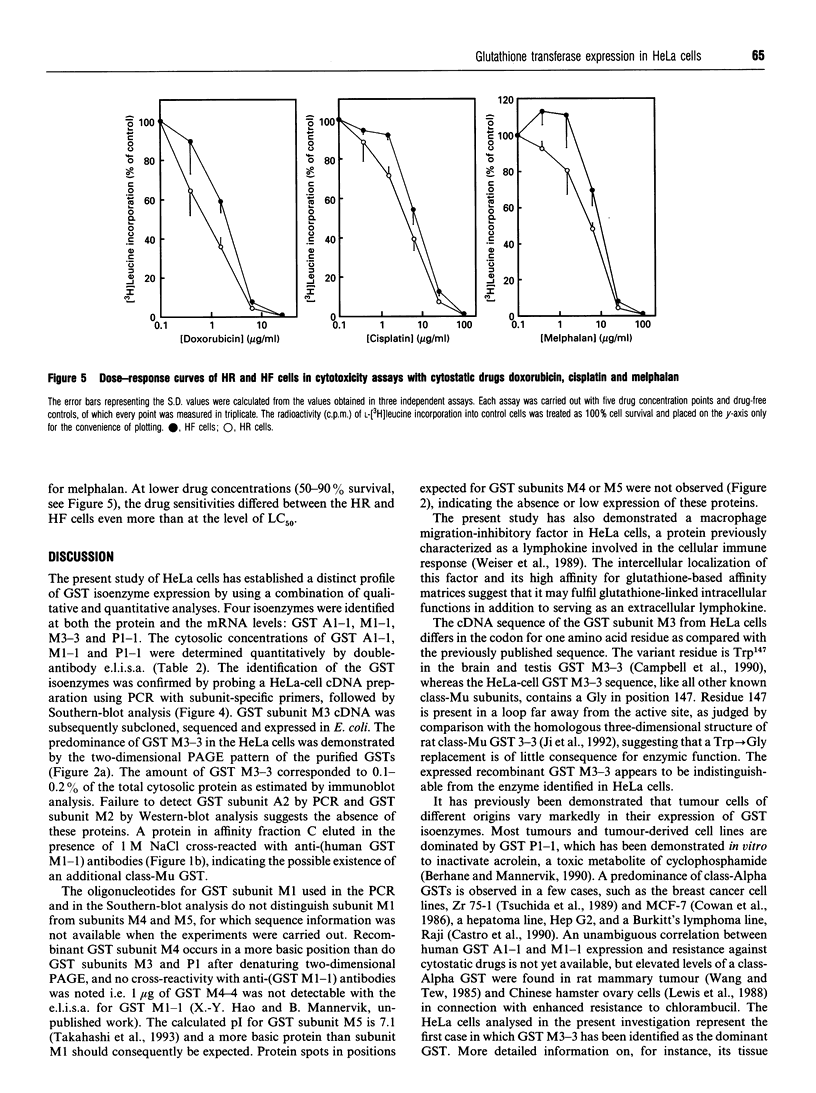
Qualitative and quantitative analyses of glutathione, glutathione transferases (GSTs) and other glutathione-linked enzymes in HeLa cells have been made in order to study their significance in cellular resistance to electrophilic cytotoxic agents. The cytosolic concentrations of three GSTs, GST M1-1 (53 +/- 9 ng/mg of cytosolic protein), GST P1-1 (11 +/- 3 ng/mg) and GST A1-1 (1.1 +/- 0.4 ng/mg) were quantified by isoenzyme-specific enzyme-linked immunoassays. Electrophoretic analysis and immunoblotting demonstrated another component, GST M3-3, which was identified by amino acid sequence analysis. GST M3-3 was quantified (1550 +/- 250 ng/mg) by slot-blot immunoanalysis and was the most abundant GST in HeLa cells. An additional cytosolic 13 kDa protein with high affinity for immobilized glutathione or S-hexyglutathione was found to be identical with a macrophage migration-inhibitory factor, previously identified as a lymphokine. Cells grown in roller bottles (HR) rather than in ordinary culture flasks contain a significantly lower concentration of all the GSTs and were found to be more sensitive to the cytostatic agents doxorubicin (2.3-fold), cisplatin (1.7-fold) and melphalan (1.4-fold). The cytosolic concentrations of glutathione reductase and glyoxalase I were also lower in HR cells, whereas the total glutathione concentration was unchanged and the glutathione peroxidase activity was increased. The results indicate that GSTs contribute to the cellular resistance phenotype.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R. N. Glutathione S-transferases: reaction mechanism, structure, and function. Chem Res Toxicol. 1991 Mar-Apr;4(2):131–140. doi: 10.1021/tx00020a001. [DOI] [PubMed] [Google Scholar]
- Berhane K., Hao X. Y., Egyházi S., Hansson J., Ringborg U., Mannervik B. Contribution of glutathione transferase M3-3 to 1,3-bis(2-chloroethyl)-1-nitrosourea resistance in a human non-small cell lung cancer cell line. Cancer Res. 1993 Sep 15;53(18):4257–4261. [PubMed] [Google Scholar]
- Berhane K., Mannervik B. Inactivation of the genotoxic aldehyde acrolein by human glutathione transferases of classes alpha, mu, and pi. Mol Pharmacol. 1990 Feb;37(2):251–254. [PubMed] [Google Scholar]
- Black S. M., Wolf C. R. The role of glutathione-dependent enzymes in drug resistance. Pharmacol Ther. 1991;51(1):139–154. doi: 10.1016/0163-7258(91)90044-m. [DOI] [PubMed] [Google Scholar]
- Board P. G., Pierce K. Expression of human glutathione S-transferase 2 in Escherichia coli. Immunological comparison with the basic glutathione S-transferases isoenzymes from human liver. Biochem J. 1987 Dec 15;248(3):937–941. doi: 10.1042/bj2480937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E., Takahashi Y., Abramovitz M., Peretz M., Listowsky I. A distinct human testis and brain mu-class glutathione S-transferase. Molecular cloning and characterization of a form present even in individuals lacking hepatic type mu isoenzymes. J Biol Chem. 1990 Jun 5;265(16):9188–9193. [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975 Jul 25;250(14):5475–5480. [PubMed] [Google Scholar]
- Castro V. M., Söderström M., Carlberg I., Widersten M., Platz A., Mannervik B. Differences among human tumor cell lines in the expression of glutathione transferases and other glutathione-linked enzymes. Carcinogenesis. 1990 Sep;11(9):1569–1576. doi: 10.1093/carcin/11.9.1569. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Gesser B., Rasmussen H. H., Madsen P., Leffers H., Dejgaard K., Honore B., Olsen E., Ratz G., Lauridsen J. B. Comprehensive two-dimensional gel protein databases offer a global approach to the analysis of human cells: the transformed amnion cells (AMA) master database and its link to genome DNA sequence data. Electrophoresis. 1990 Dec;11(12):989–1071. doi: 10.1002/elps.1150111202. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Huang Y. T., Ma C. M., Chou W. Y., Lin-Chao S. Overexpression of glutathione S-transferase and elevation of thiol pools in a multidrug-resistant human colon cancer cell line. Mol Pharmacol. 1992 Jan;41(1):69–75. [PubMed] [Google Scholar]
- Chao C. C., Lee Y. L., Cheng P. W., Lin-Chao S. Enhanced host cell reactivation of damaged plasmid DNA in HeLa cells resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1991 Jan 15;51(2):601–605. [PubMed] [Google Scholar]
- Ciaccio P. J., Tew K. D., LaCreta F. P. Enzymatic conjugation of chlorambucil with glutathione by human glutathione S-transferases and inhibition by ethacrynic acid. Biochem Pharmacol. 1991 Sep 12;42(7):1504–1507. doi: 10.1016/0006-2952(91)90468-k. [DOI] [PubMed] [Google Scholar]
- Comstock K. E., Johnson K. J., Rifenbery D., Henner W. D. Isolation and analysis of the gene and cDNA for a human Mu class glutathione S-transferase, GSTM4. J Biol Chem. 1993 Aug 15;268(23):16958–16965. [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong J. L., Chang C. M., Whang-Peng J., Knutsen T., Tu C. P. The human liver glutathione S-transferase gene superfamily: expression and chromosome mapping of an Hb subunit cDNA. Nucleic Acids Res. 1988 Sep 12;16(17):8541–8554. doi: 10.1093/nar/16.17.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doostdar H., Duthie S. J., Burke M. D., Melvin W. T., Grant M. H. The influence of culture medium composition on drug metabolising enzyme activities of the human liver derived Hep G2 cell line. FEBS Lett. 1988 Dec 5;241(1-2):15–18. doi: 10.1016/0014-5793(88)81021-4. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C. Conversion of melphalan to 4-(glutathionyl)phenylalanine. A novel mechanism for conjugation by glutathione-S-transferases. Drug Metab Dispos. 1987 Mar-Apr;15(2):195–199. [PubMed] [Google Scholar]
- Epand R. F., Epand R. M., Gupta R. S., Cragoe E. J., Jr Reversal of intrinsic multidrug resistance in Chinese hamster ovary cells by amiloride analogs. Br J Cancer. 1991 Feb;63(2):247–251. doi: 10.1038/bjc.1991.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hansson J., Berhane K., Castro V. M., Jungnelius U., Mannervik B., Ringborg U. Sensitization of human melanoma cells to the cytotoxic effect of melphalan by the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991 Jan 1;51(1):94–98. [PubMed] [Google Scholar]
- He M., Wilde A., Kaderbhai M. A. A simple single-step procedure for small-scale preparation of Escherichia coli plasmids. Nucleic Acids Res. 1990 Mar 25;18(6):1660–1660. doi: 10.1093/nar/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Kolm R. H., Sroga G. E., Mannervik B. Participation of the phenolic hydroxyl group of Tyr-8 in the catalytic mechanism of human glutathione transferase P1-1. Biochem J. 1992 Jul 15;285(Pt 2):537–540. doi: 10.1042/bj2850537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Awasthi Y. C., Board P. G., Hayes J. D., Di Ilio C., Ketterer B., Listowsky I., Morgenstern R., Muramatsu M., Pearson W. R. Nomenclature for human glutathione transferases. Biochem J. 1992 Feb 15;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Gilmore K. S., Harris J. M., Hartley J. A., Ketterer B. Chlorambucil-monoglutathionyl conjugate is sequestered by human alpha class glutathione S-transferases. Br J Cancer. 1992 Sep;66(3):433–438. doi: 10.1038/bjc.1992.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Cowan K. H. Elevation of pi class glutathione S-transferase activity in human breast cancer cells by transfection of the GST pi gene and its effect on sensitivity to toxins. Mol Pharmacol. 1989 Jul;36(1):22–28. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. C., Kelley S. L., Henner W. D. Identification of an anionic form of glutathione transferase present in many human tumors and human tumor cell lines. Cancer Res. 1988 Feb 1;48(3):527–533. [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Evans C. G., Doane-Setzer P., Castro V. M., Tahir M. K., Mannervik B. Denitrosation of 1,3-bis(2-chloroethyl)-1-nitrosourea by class mu glutathione transferases and its role in cellular resistance in rat brain tumor cells. Cancer Res. 1989 May 15;49(10):2621–2625. [PubMed] [Google Scholar]
- Speth P. A., Linssen P. C., Boezeman J. B., Wessels H. M., Haanen C. Cellular and plasma adriamycin concentrations in long-term infusion therapy of leukemia patients. Cancer Chemother Pharmacol. 1987;20(4):305–310. doi: 10.1007/BF00262581. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Campbell E. A., Hirata Y., Takayama T., Listowsky I. A basis for differentiating among the multiple human Mu-glutathione S-transferases and molecular cloning of brain GSTM5. J Biol Chem. 1993 Apr 25;268(12):8893–8898. [PubMed] [Google Scholar]
- Tew K. D., Bomber A. M., Hoffman S. J. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res. 1988 Jul 1;48(13):3622–3625. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. J., Tu C. P., Cowan K. H. Expression of human mu or alpha class glutathione S-transferases in stably transfected human MCF-7 breast cancer cells: effect on cellular sensitivity to cytotoxic agents. Mol Pharmacol. 1992 Feb;41(2):230–236. [PubMed] [Google Scholar]
- Tsuchida S., Sekine Y., Shineha R., Nishihira T., Sato K. Elevation of the placental glutathione S-transferase form (GST-pi) in tumor tissues and the levels in sera of patients with cancer. Cancer Res. 1989 Sep 15;49(18):5225–5229. [PubMed] [Google Scholar]
- Vandenberghe Y., Foriers A., Rogiers V., Vercruysse A. Changes in expression and "de novo" synthesis of glutathione S-transferase subunits in cultured adult rat hepatocytes. Biochem Pharmacol. 1990 Feb 15;39(4):685–690. doi: 10.1016/0006-2952(90)90146-c. [DOI] [PubMed] [Google Scholar]
- Vorachek W. R., Pearson W. R., Rule G. S. Cloning, expression, and characterization of a class-mu glutathione transferase from human muscle, the product of the GST4 locus. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4443–4447. doi: 10.1073/pnas.88.10.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]
- Weiser W. Y., Temple P. A., Witek-Giannotti J. S., Remold H. G., Clark S. C., David J. R. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]