Abstract
A study of somatic embryogenesis and rhizogenesis and their influence on production of morphinan alkaloids on two species of opium poppy is presented. We identified the ratios of auxin and cytokinin that caused somatic embryogenesis and rhizogenesis in hypocotyl and cotyledons of Papaver somniferum album and Papaver orientale splendidissimum. The hypocotyls and cotyledons both show somatic embryogenesis in Papaver somniferum album whereas only the cotyledons were embryogenic in Papaver orientale splendidissimum. For rhizogenesis, the most important response is on the cotyledons and leaves in these two species. Histology showed characteristic stages of somatic embryo: Globular, cotyledonous, and heart cotyledonary. High performance liquid chromatography analysis showed that the roots of both species synthesized codeine, thebaine, and papaverine. Morphine was only detected in aerial parts of Papaver somniferum album. Codeine and thebaine were detected in the rhizogenous but no embryonic callus. These results suggest that root organogenesis is causally related to alkaloid biosynthesis.
INTRODUCTION
Secondary metabolites can have many roles including pathogen defense, color, odor, and for some others the role of nitrogen source. For example, alkaloids are degraded to amino acids [1]. Several strategies have been used to enhance the production of certain metabolites, in particular alkaloids. Some authors have varied the culture conditions in order to find a satisfactory equilibrium between the growth of cells and their production of secondary metabolites. Thus, Becker [2] demonstrated that the composition of the culture medium, light, and temperature are factors that influence the production of metabolites by Nicotiana tabacum, Catharanthus roseus, and Peganum harmala. Increased sucrose and phytohormones concentrations can also stimulate the production of alkaloids [3, 4]. Numerous studies had shown that the production of morphinan alkaloids via in vitro cultures requires organogenesis of tissues in cultures [5, 6, 7, 8].
We studied the influence of somatic embryogenesis and rhizogenesis on the accumulation of morphinan alkaloids in opium poppy. We determined the optimum culture conditions for rhizogenous callus and somatic embryos from Papaver somniferum album and Papaver orientale splendidissimum. Organogenic callus capacity to produce morphinan alkaloids was determined.
MATERIAL AND METHODS
Plant material
Seeds (of the two species) were treated using the method of Rush et al. [9] and germinated in darkness in magenta boxes containing Linsmaier and Skoog [10] medium. Seeds were also germinated in distilled water with agar at 10 g/l for stock plants that provides explants; cotyledons, hypocotyls, leaves, and roots. Explants were cultured on Linsmaier and Skoog medium at different concentrations of plant growth regulators in Petri dishes. NAA/kinetin ratios were, respectively, 0.2/0.1, 0.5/0.1, 1/0.2, and 2/0.2 mg/l. The cell suspensions for callus of opium poppy were cultured in two media:
(1) S1 medium, which is the LS medium plus 2,4-D (0.2 mg/l) and kinetin (0.1 mg/l), and the sucrose at 30 g/l.
(2) S1PGRF medium, which is LS medium without plant growth regulators. Cultures were maintained at 25°C ± 2°C, at darkness and transferred every 3 to 4 weeks.
Extraction and dosage of morphinan alkaloids
The plant material (embryogenous and rhizogenous callus, aerial parts of plants) was lyophilized for 48 hours to eliminate water. The morphinan alkaloid extraction was from 20 mg of dried material, with a mixture of chloroform and concentrated ammonium (49/1, v/v) at boiling point under reflux for two hours and repeated. After each extraction, the chloroform and ammonium extract was vaporized at 35°C under vacuum. The dried residue was dissolved by 1 ml of HPLC buffer containing acetonitrile 8% (v/v) in which the morphinan alkaloids are soluble. The mixture was agitated during one hour at 40°C and filtered for HPLC analysis. For analysis we used reverse phase chromatography, with an inverted elution gradient.
Histological study
Callus, roots, or somatic embryos tissue were suspended in one drop of gelose, and put in a fixer AAF (Alcohol Acetic/Formol) for 48 hours at room temperature [11]. Dehydratation was with ethanol, at 100% (v/v), which permitted water extraction from the samples [12]. Embedded tissue was sliced with a microtome, deparaffinated, and colored with Shiff Periodic Acid (SPA) and Naphtol Blue Black (NBB). We monted slices on slides using the EUKIT resin. The sections were dried and observed under a photonic microscope.
RESULTS AND DISCUSSION
Somatic embryogenesis
There are two common origins for somatic embryos. Direct, in which the embryo developed upon the explant, and indirect where the embryo arises from a callus [13].
We studied somatic embryogenesis in opium poppy from two different systems: A cell suspension and different explants in liquid and solid media.
Somatic embryogenesis from a cell suspension
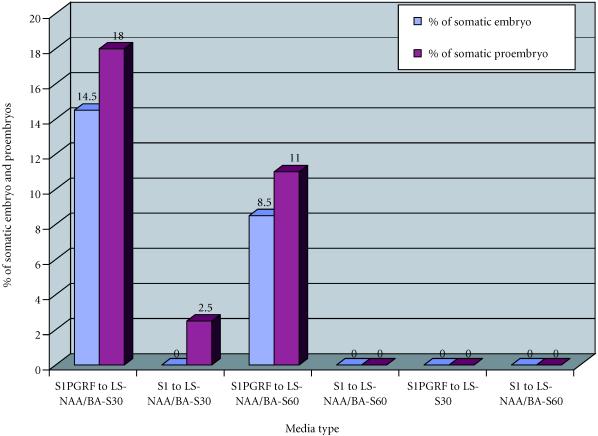
From an embryogenic suspension of Papaver somniferum album cultivated on liquid media S1 and S1PGRF, small black callus were transferred to solid media containing 0.2 mg/l of NAA and 0.1 mg/l of kinetin and two different concentrations of sucrose (LS-NAA/BA-S30 and LS-NAA/BA-S60). These small black callus were also transferred to solid medium LS-S30 without plant growth regulators.
We observed that 14.5% of explants derived from callus grown in liquid medium S1HF gave somatic embryo and 18% of somatic proembryo when transferred to solid medium LS-NAA/BA-S30. When transferred to solid medium LS-NAA/BA-S60, however, callus derived from liquid medium S1 produced no somatic embryos but only 2.5% of somatic proembryos after transfer to solid media LS-NAA/BA-S30 and LS-NAA/BA-S60. We also observed that callus derived from S1HF and from S1 produced no embryonic structures when transferred to medium LS-S30 without plant growth regulators (Figure 1). The presence of some small proembryos on solid medium LS-NAA/BA-S30 after transfer of callus from the medium S1 (containing 2,4-D and kinetin) confirmed that removal from plant growth regulators improves somatic embryogenesis.
Figure 1.
Somatic embryogenesis from a cell suspension of Papaver somniferum album cultivated on liquid media S1 (2,4-D 0.2 mg/l and kinetin 0.1 mg/l) and S1PGRF (plant growth regulators free). NAA, naphtaleneacetic acid; BA, benzyladenine; LS, Linsmaier and Skoog medium.
We observed the first somatic embryo after four weeks of culture in liquid suspension S1PGRF. The somatic embryos were white masses (Figure 2, photo 1) within which we observed different stages of somatic embryogenesis: Globular, cordiforme, and cotyledonous.
Figure 2.
Somatic embryos of Papaver somniferum album. (1) Somatic proembryo and embryo in globular stage after transfer to solid medium LS-NAA/BA-S60 (NAA/BA = 0.2/0.1 mg/l) (× 10), (2) Nonembryogenous suspensions after transfer to solid medium LS-S30 (× 10), (3) Two somatic embryos of 20 days old, from cotyledon, on solid medium LS-NAA/kinetin = 0.2/0.1 mg/l (× 10), (4) Proembryo of 20 days old, from cotyledon, on solid medium LS-NAA/kinetin = 0.2/0.1 mg/l (× 10), (5) Plant of 5 weeks from a cotyledon on solid medium LS-NAA/kinetine = 0.2/0.1 mg/l (× 10), (6) Rhizogenous callus from a cotyledon on solid medium LS-NAA/kinetine = 0.2/0.1 mg/l (× 10). Scale: 1 cm = 1 mm.
Increased sucrose concentration in the culture medium inhibited the development of somatic proembryos to somatic embryos. The results contrast with observations in Panax genseng, embryogenous tissues cultivated on Gamborg medium with a 100 mg/l of sucrose at high temperature produced numerous somatic embryos [14].
Somatic embryogenesis and root formation occurs simultaneously in cell suspensions or cultures established on solid media. This is remarkable because these cells were simultaneously embryogenic and organogenic. No previous data on somatic embryogenesis in opium poppy [15, 8] shows the roduction of roots and somatic embryo, from the same culture media. We observed similar results in Papaver orientale splendidissimum, however, the frequency of somatic embryogenesis was lower than in Papaver somniferum album.
Somatic embryogenesis from different explants
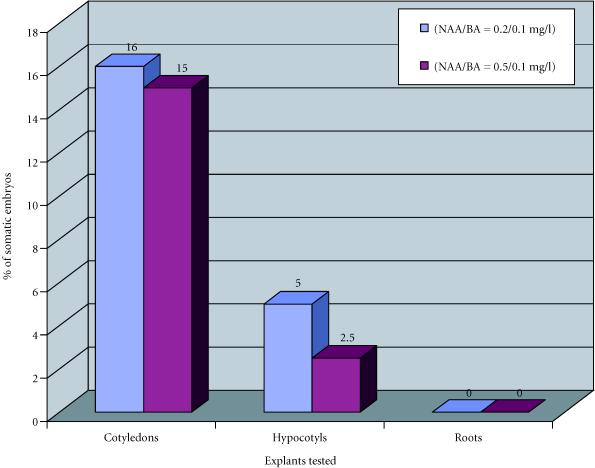
In order to study somatic embryogenesis as a function of explant type and plant growth regulator combinations, we cultivated cotyledons, hypocotyls, and roots on LS medium with different ratios NAA/kinetin.
We observed that after four months of culture, cotyledon was the explant that gave the highest percentages of somatic embryos, 16% with NAA/kinetin = 0.2/0.1 mg/l and 15% with NAA/kinetin = 0.5/0.1 mg/l. Embryogenesis from hypocotyl occurs in 5% of explants with NAA/kinetin = 0.2/0.1 mg/l and 1.5% with NAA/kinetin = 0.5/0.1 mg/l. Roots did not produce any somatic embryos. For both cotyledons and hypocotyls NAA/kinetin = 0.2/0.1 mg/l and 0.5/0.1 mg/l were the optimal plant growth regulator combinations (Figure 3).
Figure 3.
Somatic embryogenesis obtained from roots, cotyledons, and hypocotyls of Papaver somniferum album, with two plant growth regulator combinations (NAA/kinetin = 0.2/0.1 and 0.5/0.1 mg/l), after four months of culture.
On solid medium we observed somatic proembryos, somatic embryo in the heart cotyledonary stage and small plants developed from somatic embryos born directly from cotyledons (Figure 2, photos 3, 4, and 5).
The absence of somatic embryos in the presence of highest ratios of plant growth regulator combinations maybe due to the high concentration of kinetin, inhibiting embryo induction. We observed that kinetin did not promote somatic embryogenesis when the concentration passed beyond 0.2 mg/l. During some transfers, a portion of somatic embryo was lost. The mineral solution used, or inadequate transfers frequency may have caused these losses [16].
Rhizogenesis
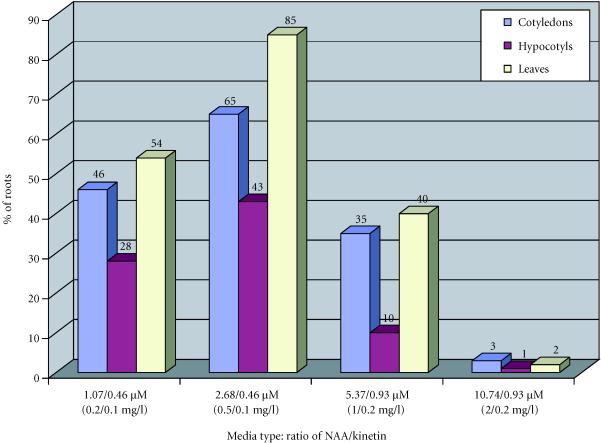
In Papaver somniferum album, the plant growth regulators combinations most favorable to rhizogenesis were NAA/kinetin = 0.5/0.1 mg/l and 0.2/0.1 mg/l. The explants that were rhizogenic were cotyledons and leaves (Figure 4).
Figure 4.
Percentages of roots obtained from callus of Papaver somniferum album as a function of plant growth regulator combinations, after four months of culture.
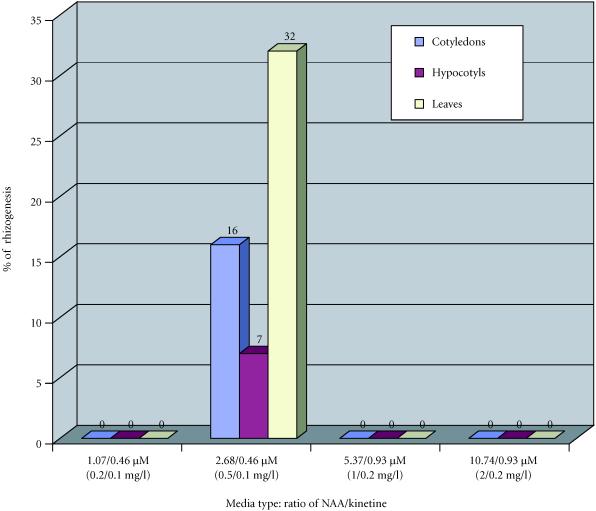
In Papaver orientale splendidissimum, rhizogenesis was only obtained with NAA/kinetin = 0.5/0.1 mg/l. The percentages of rhizogenic explants were lower than in Papaver somniferum album. As with Papaver somniferum album, leaves and cotyledons were the explants that produces the most rhizogenesis (Figure 5).
Figure 5.
Percentages of roots obtained from callus of Papaver orientale splendidissimum in function of plant growth regulator combinations NAA/kinetin after four months of culture.
Root formation from in vitro cultures pass through two stages: Induction and initiation. The stages are inversely related to endogenous auxin concentration [17].
Root initiation, in Papaver somniferum, started after the passage of explants to a medium containing low concentrations of auxin. When the exogenous concentration of auxin increases, the percentage of rhizogenesis can be reduced (ratios NAA/kinetin = 1/0.2 mg/l and 2/0.2 mg/l).
HPLC analysis of morphinane alkaloids
Roots of plants cultivated in vitro
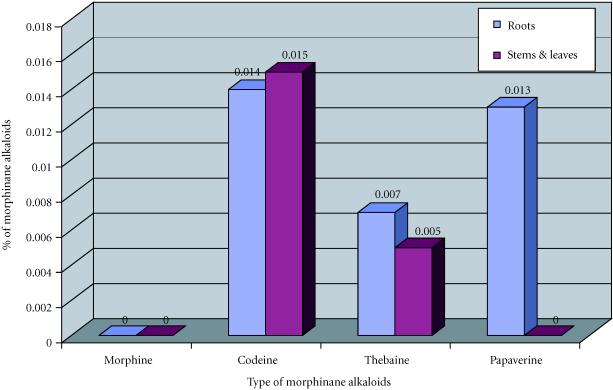
Plants roots of Papaver somniferum album contained codeine (0.031%, 0.031 mg of codeine per 100 mg of dried material), thebaine (0.012%) and papaverine (0.01%) (Figure 7). The roots of plants of Papaver orientale splendidissimum contained the same morphinan alkaloids in lower quantities (codeine (0.014%), thebaine (0.007%), and papaverine (0.013%)) (Figure 8). The roots of both species did not contain any morphine. However, morphine is preponderant in young roots of plants of opium poppy in field [18]. The accumulation of codeine is different from the accumulation of morphine in plant organs.
Figure 7.
Concentrations of morphinane alkaloids (mg/100 mg of dryed material) obtained in function of explants tested, in Papaver orientale splendidissimum.
Figure 8.
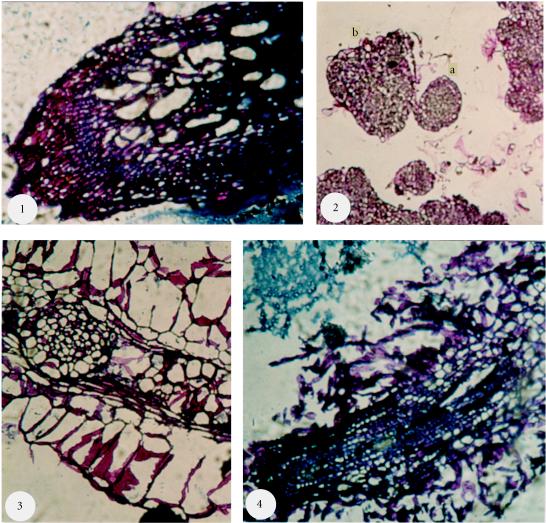
Histologic cuts of Papaver somniferum album cultures. (1) Roots of plants, at 3 months, cultivated in soil (× 10), (2) Embryogenous callus (× 10): 2a, somatic embryo in globular stage; 2b, somatic embryo in heart cotyledonary stage, (3) Rhizogenous callus (× 10): Presence of a meristematic zone that shows the depart of a secondary root, (4) Rhizogenous callus (× 10): Presence of vessels in helix. Scale: 1 cm = 1 mm.
Aerial parts of plants cultivated in vitro (stems and leaves)
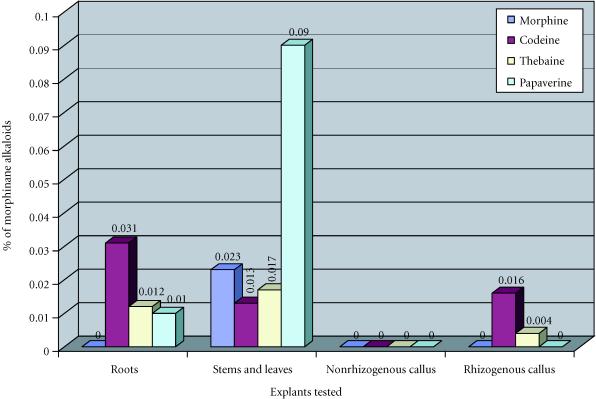
The aerial parts of plants of Papaver somniferum album contained morphine (0.023%), codeine (0.013%), thebaine (0.017%), and papaverine (0.09%) (Figure 6). The aerial parts of plants of Papaver orientale splendidissimum did not contain morphine and papaverine but did contain codeine (0.015%), less thebaine (0.005%) (Figure 7).
Figure 6.
Concentrations of morphinane alkaloids (mg/100 mg of dryed material) obtained in function of explants tested, in Papaver soniferum album.
With Papaver somniferum, a low level of thebaine was detected after the 10th day of culture whereas codeine and morphine were detected after the 15th and 20th days of culture, respectively [18]. Thebaine was the principal morphinan alkaloid synthesized by Papaver somniferum cultures followed by codeine. Morphine was present in certain cases but with the lowest level, it is absent in general [19, 20, 21, 9].
Rhizogenous and nonrhizogenous callus
In nonrhizogenous callus derived from cotyledons of Papaver somniferum album no morphinan alkaloids were detected. However, in rhizogenous callus we detected a low amount of codeine (0.016%) and thebaine (0.004%) (Figure 6).
Hsu and Pack [20] had shown that cellular extracts from callus obtained from hypocotyls of Papaver somniferum, contained thebaine and a low level of codeine but morphine was not detected.
Our studies on Papaver somniferum, and others, shows that this species is the most able to produce morphinan alkaloids to in vitro culture.
Histological study
In order to confirm the presence of different stages of somatic embryos observed in opium poppy, we expected to observe laticifer cells that maybe connected to synthesis and accumulation of morphinan alkaloids in opium poppy tissues cultivated in vitro [19, 20, 21, 9]. We observed embryonic structures in globular and heart cotyledonary stages (Figure 8, photo 2). We observed several different characteristic stages of somatic embryo observed under binocular wen (Figure 8, photos 3 and 4). We also observed plant lets from somatic embryo that derives from cotyledons, and roots from rhizogenous callus (Figure 2, photos 5 and 6).
Laticifer cells were not observed in the roots of plants of Papaver somniferum album, Papaver orientale splendidissimum cultivated in vitro, in plants of Papaver somniferum album obtained from the field, in embryogenous callus or in rhisogenous callus of Papaver somniferum album. The structures observed were the conductor vessel helixes (Figure 8, photos 1 and 4) and the meristematic zones that show secondary roots departing from the principal roots (Figure 8, photos 2 and 3).
CONCLUSION
We observed somatic embryogenesis and rhizogenesis in two species of opium poppy. We provided evidence that the ratios of NAA/kinetin = 0.2/0.1 and 0.5/0.1 mg/l were the most favorable for somatic embryogenesis and rhizogenesis (2.5% to 18% for somatic embryogenesis and 7% to 85% for rhizogenesis). Hypocotyls and cotyledons were the two explants that produced most direct somatic embryogenesis (2.5% to 18%). We also demonstrated that cotyledons and leaves gave the highest percentage of rhizogenesis.
Transfer of somatic embryos to a medium without plant growth regulators may help to optimize the survival of somatic embryos and their development in plants [17].
The HPLC analysis of morphinan alkaloids content of these cultures shows the importance of differentiation and organogenesis (roots) for the biosynthesis of these alkaloids. We observed that roots are the cardinal sites of biosynthesis of codeine but also of thebaine and papaverine. Codeine is an important molecule used in prescription in medicine, and it will be interesting to optimize its production.
ABBREVIATIONS
NAA α-Naphtaleneacetic acid
BA benzyladenine
LS Linsmaier and Skoog medium
2,4-D dichlorophenoxyacetic acid
HPLC high performance liquid chromatography
Acknowledgments
ACKNOWLEDGEMENTS
I would like to thank Dr Dominique Lourain for her help and her advises during this work, Dr David Antony Lightfoot for his corrections and critics of this paper.
References
- Vickery Margaret L, Vickery Brian. The Macmillan Press Ltd; London and Basingstoke: 1981. Secondary plant metabolism ; pp. 1–6. [Google Scholar]
- Becker H. Regulation of secondary metabolism in plant cell cultures. Plant tissues and cell culture. Plant biology . In: Green CE, Somers DA, Hackett WP, Biesboer DD, editors. Plant tissues and cell culture Plant biology. Vol. 3. Alan R. Liss Inc; NewYork: 1987. pp. 199–212. [Google Scholar]
- Merillon JM, Chenieux JC, Rideau M. Cinetique de croissance, evolution du metabolisme glucido-azote et accumulation alcaloidique dans une suspension cellulaire de Catharantus roseus . Planta medica. 1983;47:169–176. doi: 10.1055/s-2007-969979. [DOI] [PubMed] [Google Scholar]
- Morris P. Regulation of product synthesis in cell cultures of Catharantus roseus. II. Comparison of production media. Planta medica. 1986;47:169–176. [Google Scholar]
- Kamo KK, Kimoto W, Hsu AF, Mahlberg PG, Bellis DD. Morphinan alkaloids in cultured tissues and Redifferenciated organs of Papaver somniferum . Phytochemistry. 1982;21(1):219–222. [Google Scholar]
- Parr AJ, Robins RG, Rhodes MJC. Alkaloid transport in Cinchona spp. Cell culture. Physilogie vegetale. 1986;24:419–429. [Google Scholar]
- Renaudin JP, Guern J. Transport and vacuolar storage of secondary metabolites in plant cell cultures. Secondary products from Plant Tissues Culture . In: Chrwood BV, Rhodes MJC, editors. Secondary products from Plant Tissues Culture. Oxford Science Publications; 1990. pp. 59–78. [Google Scholar]
- Schuchmann R, Willmann E. Somatic embryogenesis of tissues cultures of Papaver somniferium and Papaver orientale and its relationship to alkaloid and lipid metabolism. Plant Cell Reports. 1983;2(2):88–91. doi: 10.1007/BF00270173. [DOI] [PubMed] [Google Scholar]
- Rush MD, Kutchan TM, Coscia CJ. Correlation of the appearance of morphinan alkaloids and laticifer cells in germinating Papaver bracteatum seedlings . Plant Cell Reports. 1985;4:237–240. doi: 10.1007/BF00269366. [DOI] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of Tobacco tissue cultures. Physiologia Plantarum. 1965;18:100–127. [Google Scholar]
- Langeron M. Precis de microscopie. Collection de precis medicaux. 1949. p. 1430.
- Paul H. [PhD thesis] University of Picardie; Amiens, France: 1994. Regeneration par embryogenese somatique et cryoconservation d'apex auxilliaires de vitroplants et d'embryons somatiques de Pommier . [Google Scholar]
- Margara J. INRA; Paris: 1982. Bases de la multiplication vegetative. Les meristemes et l'organogenese ; pp. 118–129. [Google Scholar]
- Asaka I, Ichio I, Hirotani, Asada Y, Furuya T. Mass production of ginseng (Panax ginseng) embryoids on media containing high concentrations of sugar. Planta medica. 1994;60:146–148. doi: 10.1055/s-2006-959438. [DOI] [PubMed] [Google Scholar]
- Nessler CL. Somatic embryogenesis in the opium poppy, Papaver somniferum . Physiol Plant. 1982;55:453–458. [Google Scholar]
- Vidalie H, Auge R, Beauchesne G, et al. Les biotechnologies en horticulture: possibilites et perspectives. In: Boccon-Gibod J, Jalouzot R, editors. La culture in vitro et ses applications horticoles. 3ème éd revue. 1989. pp. 91–131. [Google Scholar]
- Zryd JP, Brettel R, Derreudre J, et al. 1ère éd. Press Polytechnique Romande; 1988. Tissues et Organes Vegetaux ; pp. 119–134. [Google Scholar]
- Williams RD, Ellis BE. Age and tissues distribution of alkaloids in Papaver somniferum . Phytochemistry. 1989;28(8):2085–2088. [Google Scholar]
- Griffing LR, Fowk LC, Constabel F. Radioimmunoassay of morphinan alkaloids in Papaver somniferum Hypocotyls, Callus and suspensions cultures. J Plant Physiol. 1989;134:645–650. [Google Scholar]
- Hsu AF, Pack J. Metabolism of 14C-codein in cell culture of Papaver somniferum . Phytochemistry. 1989;28(7):1879–1881. [Google Scholar]
- Kutchan TM, Ayabes S, Krueger RG, Coscia CJ. Cytodifferenciation and alkaloids Accumulation in cultured cells of Papaver somniferum . Plant Cell Reports. 1983;2:281–284. doi: 10.1007/BF00270181. [DOI] [PubMed] [Google Scholar]