Summary
Background
The clinical and public health relevance of widespread testing for asymptomatic Chlamydia trachomatis (chlamydia) infections is under debate. To address uncertainties in screening programs, we estimate reproductive tract complication risks following asymptomatic and symptomatic chlamydia infections in a long-term prospective cohort.
Methods
A cohort of 5704 reproductive-age women recruited from a chlamydia screening study was followed for up to 14 years. Chlamydia positivity was determined using screening polymerase chain reaction test results, self-reported diagnoses (with/without symptoms), and chlamydia Immunoglobulin G antibodies. Outcome data (pregnancies, pelvic inflammatory disease (PID), ectopic pregnancy, and tubal factor infertility) were collected through self-completed questionnaires. Cox regression calculated adjusted hazard ratios (aHR) with confidence intervals (CI) to compare outcomes between time-updated chlamydia groups since sexual debut.
Findings
During 104,612 person-years, 2103 (36.9%) women were chlamydia-positive and 3692 women (64.7%) had been pregnant at least once. Risks for PID, ectopic pregnancy and tubal factor infertility were 1.62 (95% CI 1.20–2.17), 1.84 (95% CI 1.14–2.95) and 2.75 (95% CI 1.53–4.94), compared to chlamydia-negatives. aHRs for PID after symptomatic and asymptomatic infections were 2.29 (95% CI 1.62–3.25) and 1.06 (95% CI 0.66–1.69), respectively. Incidence of PID, ectopic pregnancy and tubal factor infertility after symptomatic chlamydia infection remained low with rates per 1000 person-years of 5.8, 1.9, and 1.8, respectively.
Interpretation
We found a significantly higher risk of PID, ectopic pregnancy and tubal factor infertility in chlamydia-positive women compared to chlamydia-negative women, although the overall incidence rates of complications remained low. Symptomatic, but not asymptomatic, chlamydia infections were associated with PID risk, suggesting the largest disease burden of complications is in this group.
Funding
The Netherlands Organisation for Health Research and Development (ZonMW Netherlands) and Research Funding from the Ministry of Health, Welfare and Sports.
Keywords: Chlamydia trachomatis, Asymptomatic chlamydia infections, Reproductive tract complications, Prospective cohort study, Chlamydia control
Research in context.
Evidence before this study
We searched PubMed for studies on the risk of complications after chlamydia up to January 2024. The search terms were “Chlamydia trachomatis” AND “pelvic inflammatory disease” OR “ectopic pregnancy” OR “tubal factor infertility”.
Several studies assessed the risk for pelvic inflammatory disease (PID) following chlamydia infection to evaluate control efforts. One screening trial estimated untreated chlamydia infections had a 1.9% risk of PID compared to 1.3% for treated infections after 12 months. Another trial showed chlamydia screening reduced hospital-diagnosed PID, but the absolute difference was small (0.24% vs. 0.38%). Large registry based studies from Denmark, the UK, Sweden, and Australia, with sample sizes over 40,000, found that women tested positive for chlamydia had a 1.3–2.4 times increased risk of PID compared to those who tested negative. Additionally, women tested positive for chlamydia had a 30–90% increased risk of ectopic pregnancy and tubal factor infertility. However, these studies faced limitations such as limited follow-up, misclassification of chlamydia status, and gaps in data on sexual behaviour and lifestyle. Moreover, clinical indications for chlamydia testing were overlooked, leaving uncertainty about the benefits of asymptomatic chlamydia testing in preventing complications.
Added value of this study
We conducted a prospective cohort study of up to 14 years with over 5500 participants previously screened for chlamydia. Outcomes were self-reported, thus complications were estimated from different settings (e.g., inpatient and outpatient) and (time to) pregnancies in a single population. By integrating chlamydia screening records, self-reported diagnoses, and serology testing, we minimized misclassification and compared complication risks between never-diagnosed and diagnosed infections, and asymptomatic and symptomatic infections.
Implications of all the available evidence
Our results confirm that chlamydia is strongly associated with PID (relative risk (RR) 1.6; 95% confidence interval (CI), 1.2–2.2), ectopic pregnancy (RR 1.8; 95% CI, 1.1–3.0), and tubal factor infertility (RR 2.8; 95% CI, 1.5–4.9). Although chlamydia increased the risk of tubal factor infertility, the overall incidence of infertility associated with chlamydia remained low, resulting in similar overall pregnancy rates for chlamydia-positive vs. chlamydia-negative women, consistent with birth rates reported in other population-based studies. Women with symptomatic chlamydia infections were found to have over double the risk of PID compared to those without infections. However, no increased risk was seen in women with asymptomatic (presumably treated) infections or those with serology-only positive (presumably untreated) infections. These findings can inform chlamydia policy, potentially resulting in prioritizing symptomatic individuals in test-and-treatment efforts.
Introduction
Chlamydia trachomatis (chlamydia) is the most reported bacterial sexually transmitted infection worldwide, with about 130 million annual new diagnoses.1 Many countries have implemented widespread testing for (asymptomatic) chlamydia infections to reduce population prevalence and burden of the reproductive tract complication pelvic inflammatory disease (PID), that can result in tubal factor infertility, and ectopic pregnancy.2
Randomized controlled trials found that chlamydia screening can reduce PID risk at the individual level,2, 3, 4 however it has been shown that it is difficult to achieve a sizable reduction in population level rates of chlamydia and PID,5,6 and there is no evidence of a protective effect on tubal factor infertility and ectopic pregnancy.2,4 The risk and preventable fraction of genital chlamydia for these complications remain uncertain, especially in asymptomatic infections, which account for around 70% of diagnoses in women.7,8 Modelling studies have identified further knowledge gaps about the timing, symptoms and role of treatment in progression from chlamydia infection to complications.7,9
Previously, increased risks were observed for PID, ectopic pregnancy and tubal factor infertility after chlamydia infection.10, 11, 12, 13, 14, 15 Data collection in these studies was through registry linkage, which is prone to misclassification. Historical chlamydia tests have different sensitivity, and complications have been identified either from primary care records11 or hospital and emergency room records.10,12, 13, 14, 15 In particular PID diagnosis is challenging due to variability in severity and symptoms resulting in differences in reporting methods and documentation systems.16 A more accurate approach would be capturing chlamydia status prospectively and updating it, along with clinical indications for testing. By evaluating pregnancy data alongside tubal factor infertility, a more real-world representation of the impact on female fertility can be achieved, while overcoming diagnostic misclassification challenges related to tubal factor infertility.17
In this study, we aimed to estimate chlamydia-associated risks for late complications (PID, ectopic pregnancy, and tubal factor infertility) and the impact on (time to) pregnancy in a long-term prospective cohort. Additionally, we estimated risks of complications after asymptomatic and symptomatic chlamydia infections to inform diagnostic and treatment guidelines and policy on chlamydia.
Methods
Study design and participants
The design of the Netherlands chlamydia cohort study (NECCST) has been described elsewhere.18,19 In brief, NECCST is a prospective cohort of 5704 persons assigned female at birth (hereafter referred to as “women”) of reproductive-age (aged 20–44 years) recruited from a chlamydia screening implementation study (CSI) between 2008–11 and followed up until 2022. The CSI involved yearly chlamydia polymerase chain reaction (PCR) test screening in women (aged 16–29 years), across four study rounds. Screening-positive women were provided treatment and partner notification.19 Women who were chlamydia PCR tested at least once and consented to further research were invited for NECCST in 2015/16. Online questionnaires were distributed in 2015/16, 2017/18, 2019/20 and 2021/22. Questionnaires collected retrospective data on chlamydia infections, pregnancies, and complications, including event timing. The initial questionnaire requested information from sexual debut onward. Included participants who did not actively withdraw were re-invited each round (Figure S1). These data were merged with prior CSI data on self-reported chlamydia infections and chlamydia PCR.
Laboratory methods
Participants received a kit in 2015/16 and 2021/22 to self-collect a capillary blood sample via finger-prick, which they returned via postal mail to the laboratory (a validated process18). The serum samples were stored at −20 °C until serological measurements in 2022. In the first round 4024/5704 (70.5%) and in the last round 2147/2798 (76.7%) women returned blood samples. For 3661/5704 (64.2%) women sufficient serum was collected to perform the antibody test on at least one of the samples.
Chlamydia Immunoglobulin G (IgG) antibodies were determined using an enzyme-linked immunosorbent assay based on conserved, serovar-independent and species-specific, recombinant Major Outer Membrane Protein (MOMP) antigen domains of Chlamydia trachomatis (Serion Immunologics, Würzburg, Germany). Assay sensitivity was established at 70.2% at a specificity of 98% in comparison to a composite reference standard for anti-C. trachomatis antibody status.20
Serological outcomes were classified as positive (>15 AU/ml), negative (<10 AU/ml), or grey-zone (10–15 AU/ml). Grey-zone outcomes underwent retesting according to manufacturers’ instructions (grey-positive assigned positive; grey–grey assigned negative; grey-negative assigned negative).
Definitions and outcomes
Exposure status
Chlamydia-positive was defined as either one or a combination of: 1) a positive PCR-test outcome in the CSI (screening-PCR+), 2) a self-reported chlamydia infection (self-reported+), or 3) presence of chlamydia antibodies in serum (serology+). If women tested negative by screening-PCR, did not self-report a chlamydia infection, and were not serology positive, they were classified as chlamydia-negative.
For subgroup analyses chlamydia status was grouped into:
-
1)
Diagnosed chlamydia infections. For each self-reported chlamydia infection, symptoms were recorded: abnormal vaginal discharge, bleeding between periods, painful sexual intercourse/urination and lower abdominal pain. Based on the aforementioned, chlamydia status was grouped as: a) symptomatic (screening-PCR+ and/or self-reported+ with symptoms), b) asymptomatic (screening-PCR+ and/or self-reported + without symptoms, and c) serology+ only (those with chlamydia antibodies tested in study without screening-PCR + or self-reported + diagnosis).
-
2)
Repeat chlamydia infections. Categorized as: a) single (self-reported + or screening-PCR+), b) multiple (2 or more) (self-reported+ and/or screening-PCR+), and c) serology+ only. Serology+ only was considered a separate category as it was not possible to infer successive reinfections and elapsed time since exposure for this group.
This study used the following self-reported outcomes:
-
1)
PID defined as an episode of inflammation of the fallopian tubes, ovaries, and/or uterus, diagnosed by a medical professional. We asked if women had been hospitalized due to PID.
-
2)
Ectopic pregnancy in women with current or past pregnancies.
-
3)
Tubal factor infertility resulting from tubal abnormalities (potentially combined with other infertility causes), diagnosed by a medical professional. Although self-reported tubal factor infertility was found a reliable measure in the Netherlands,21 we validated a subset of cases in medical records. We calculated substantial agreement between self-reported tubal factor infertility and the medical records (Kappa = 0.72) (Table S1).
-
4)
Pregnancy defined as any pregnancy including miscarriages and abortions.
-
5)
Time to pregnancy defined by the reported number of months women reported that they (had) attempted to conceive. This included women who had become pregnant intentionally, those who ever attempted to conceive and those who were still trying.
To assess the risk of outcomes 1–4 for chlamydia-positive compared to chlamydia-negative women, exposure time was defined as years since sexual debut. From sexual debut women were both “at risk” for chlamydia infection (exposure) and complications and/or pregnancy (outcomes). Follow-up time ended when the last questionnaire was returned or when the first event of an outcome occurred.
Time to pregnancy was defined as the number of months that women attempted to conceive until their first planned pregnancy, until they stopped trying, or until the last data collection.
Statistical analysis
Population characteristics were compared between chlamydia groups using student's t-test, Mann–Whitney U-test, and chi-squared tests.
Incidence rates were calculated as the number of new outcomes divided by the total person-years-at-risk (py). Associations between chlamydia and study outcomes were assessed through Kaplan–Meier curves and Cox proportional hazards (PH) regression models. Risks were expressed as hazard ratio (HR) and adjusted hazard ratio (aHR) with corresponding confidence intervals (CI). The PH assumption was checked by assessing log–log plots and testing Schoenfeld residuals. Violations of the PH assumption were resolved by stratification.
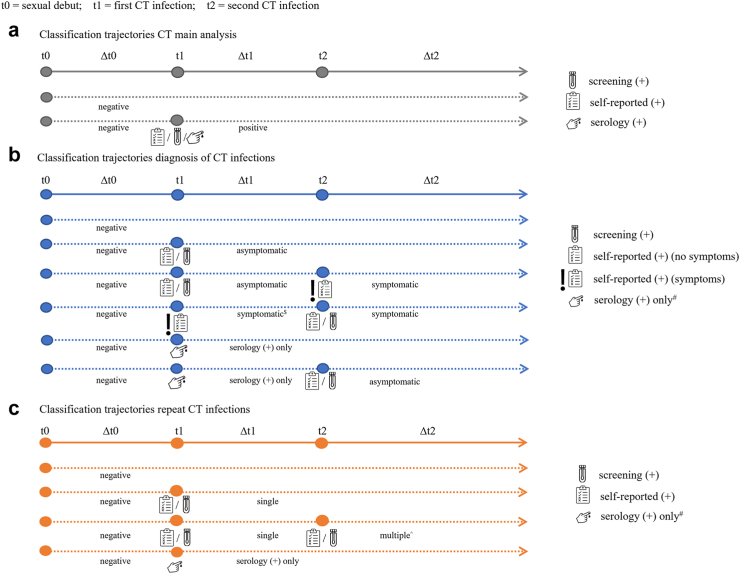
Chlamydia status was a time-dependent variable, whereby once tested positive for chlamydia, women remained chlamydia-positive for the further study period. The same applied for chlamydia subgroup analyses; for example, women could initially be classified as chlamydia-negative, then as having an asymptomatic infection and after reporting a symptomatic infection switch to that classification for the remaining study period (Fig. 1). In time-to-event analyses we used multivariate imputation by chained equations to estimate infection time for women with unknown year of infection, creating 15 simulation datasets based on available data from women with known infection year.
Fig. 1.
Methodology for classification of chlamydia groups based on time-varying chlamydia status. Circles represent chlamydia infections (t1–t2). Dotted lines represent hypothetical follow-up period for an individual (Δt0–Δt2). Follow-up always starts at sexual debut (t0) with chlamydia-negative status. Screening (+) = having a positive PCR-test outcome in the screening study; self-reported (+) = a self-reported chlamydia infection; serology (+) = presence of chlamydia IgG antibodies in serum. # serology (+) only applies if no other known chlamydia diagnoses (screening-PCR (+) or self-reported (+)). $ remains classified as symptomatic even after subsequent asymptomatic infection. CT, Chlamydia trachomatis; PCR, polymerase chain reaction; IgG, Immunoglobulin G.
For planned pregnancies, attempts to conceive were reported in calendar months and timing of chlamydia infections in calendar years. For these analyses we fixed chlamydia status based on the status at the end of the observed follow-up period.
Potential confounders were assessed time-varying: gonorrhea diagnosis and intrauterine device (IUD) insertion, or fixed: age, smoking, body mass index (BMI), chlamydia tests, sex partners, condom use, education level and migration background (Table S2). We examined whether the relationship between chlamydia status and outcomes varied across age groups and, if statistically significant (P < 0.05) effect modification was detected, conducted stratified analyses. All covariates were initially assessed one-by-one in a model together with chlamydia. To build the association model, we used forward selection to assess relevant confounders. Starting with the covariate that had the largest impact on the chlamydia regression coefficient, covariates to the multivariable model if they resulted in a ≥10% change in the chlamydia status (or chlamydia ‘group’) regression coefficient.
Analyses were performed using R Statistical Software (v4.1.2; R Core Team 2021).
Sensitivity analyses
We conducted the following analyses:
-
•
Included only women with screening-PCR-positive results (highly sensitive and specific) or self-reported diagnoses, to assess potential recall bias.
-
•
Excluded women with serology+ only results from the chlamydia-positive group to assess the impact of including serological outcomes.
-
•
Restricted analyses for PID to hospitalized cases due to residual uncertainties in PID diagnosis.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 5704 women contributed to 104,612 person-years of exposure time. In total, 2066 (36.2%) women participated in all four rounds, 1307 (22.9%) in three rounds, 1125 (19.7%) in two rounds, and 1206 (21.1%) only in the first round (Figure S2).
Mean age at sexual debut (start exposure) was 16.9 (SD:2.4) years and mean exposure time was 18.3 (SD:4.6) years. For 459 women (8.1% of all women, 22.8% of chlamydia-positive women) multiple imputation was used to impute the year of infection (Table S4).
Study population
Mean age at last data collection was 35.3 (SD:4.5) years. Compared with chlamydia-negative women (n = 3691), chlamydia-positive women (n = 2103) had a higher BMI, younger age at sexual debut, more lifetime sex partners and higher gonorrhea positivity. Overall pregnancy proportions were similar between chlamydia-positive and chlamydia-negative women (64.9% vs. 65.1%), but chlamydia-positive women had less often planned pregnancies and were younger at first pregnancy (Table S3). Women with asymptomatic chlamydia infections (n = 767), as opposed to serology+ only women (n = 449), showed higher rates of having ≥12 lifetime sex partners (68.2% vs. 53.1%) and ≥6 chlamydia tests (31.6% vs. 12.1%) (Table S5). Women in whom the first diagnosed infection was symptomatic (n = 781) as opposed to asymptomatic (n = 783) were more often diagnosed <18 years (9.0% vs. 4.7%) (Table S6).
Pelvic inflammatory disease
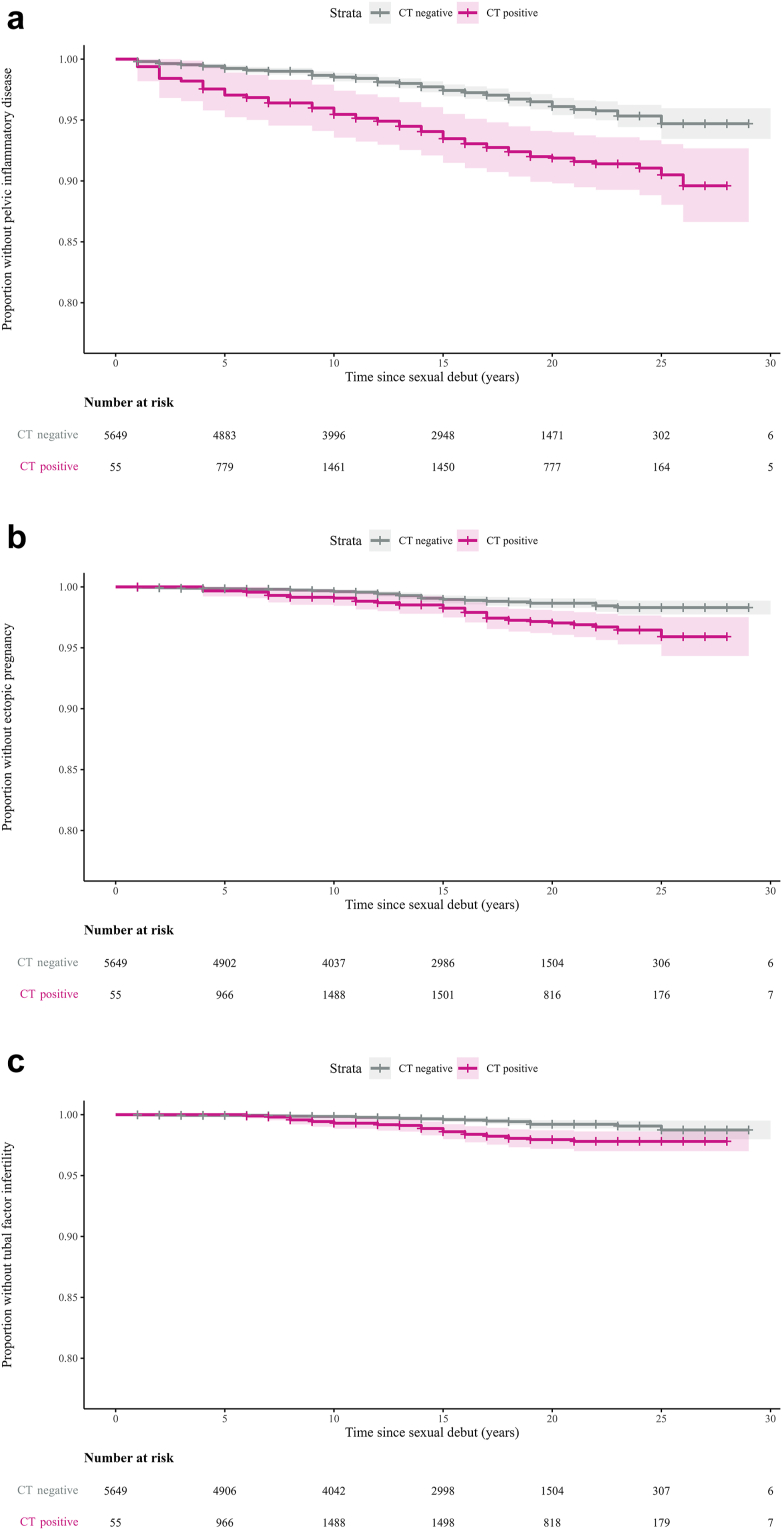
In total 236 women (4.1%) reported at least one PID episode. Among chlamydia-positive women, PID incidence was significantly higher than in chlamydia-negative women: 3.80/1000 py (95% CI 3.08–4.62) vs. 1.80/1000 py (95% CI 1.55–2.14). Symptomatic chlamydia showed the highest incidence at 5.82/1000 py (95% CI 4.39–7.53) (Table 1, Fig. 2a).
Table 1.
Incidence rates of outcomes chlamydia in negative and chlamydia positive women in Netherlands chlamydia cohort study.
| Overall |
Chlamydia-negative |
Chlamydia-positive |
Sub-analysis chlamydia positive | Asymptomatic |
Symptomatic |
Serology+ only |
|
|---|---|---|---|---|---|---|---|
| Per 1000 person-yearsa or 1000 person-monthsb | Per 1000 person-yearsa or 1000 person-monthsb | Per 1000 person-yearsa or 1000 person-monthsb | Per 1000 person-yearsa | Per 1000 person-yearsa | Per 1000 person-yearsa | ||
| Pelvic inflammatory disease | 2.30 (2.02–2.60) | 1.80 (1.55–2.14) | 3.80 (3.08–4.62) | 2.57 (1.66–3.76) | 5.82 (4.39–7.53) | 2.50 (1.41–4.04) | |
| Ectopic pregnancy | 0.87 (0.71–1.07) | 0.63 (0.47–0.83) | 1.63 (1.18–2.18) | 1.42 (0.78–2.34) | 1.90 (1.15–2.91) | 1.41 (0.64–2.62) | |
| Tubal factor infertility | 0.53 (0.40–0.68) | 0.30 (0.21–0.46) | 1.21 (0.81–1.67) | 0.98 (0.47–1.77) | 1.79 (1.07–2.78) | 0.70 (0.22–1.62) | |
| Pregnancies | 46.9 (45.5–48.5) | 41.9 (40.3–43.5) | 67.2 (63.2–71.3) | – | – | – | |
| Planned pregnancies | 99.0 (95.4–102.6) | 106.2 (101.7–110.8) | 84.9 (79.3–90.7) | – | – | – |
Chlamydia-positive was defined as a positive PCR test outcome in the CSI study (screening-PCR), and/or the presence of chlamydia IgG and/or a self-reported chlamydia infection. Chlamydia status was determined at the time of the event. Symptomatic was defined as screening-PCR+ and/or self-reported+ with symptoms. Asymptomatic was defined as screening-PCR+ and/or self-reported + without symptoms. Serology+ only was defined as presence of chlamydia IgG antibodies without any other chlamydia indicators. For these analyses, multiple imputations were used to estimate time of first chlamydia infection in women without a known first year of chlamydia infection.
PCR, polymerase chain reaction; IgG, immunoglobulin G.
Person-years for pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility and pregnancies.
Person-months for planned pregnancies.
Fig. 2.
Kaplan–Meier plots of time (years since sexual debut) to complications by chlamydia status. (a) Pelvic inflammatory disease, (b) Ectopic pregnancy, (c) Tubal factor infertility. Chlamydia-positive was defined as a positive PCR-test outcome in the CSI study (screening-PCR+), and/or a self-reported chlamydia infection (self-reported+) and/or presence of chlamydia IgG antibodies in serum (serology+)∗. Shaded areas represent corresponding 95% confidence intervals. Time was censored at a sample size reduction of less than 15 participants per stratum. ∗Median serology+ cases of 15 multiple imputation. PCR, polymerase chain reaction; IgG, Immunoglobulin G.
In multivariable analysis, adjusted for age, educational level and lifetime chlamydia tests, chlamydia-positivity remained associated with PID (aHR 1.62, 95% CI 1.20–2.17) (Table 2).
Table 2.
Association between chlamydia positivity and pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility, pregnancy and planned pregnancy in women participating in Netherlands chlamydia cohort study.
| Events |
Time |
Crude hazard ratio (HR) |
Adjusted Hazard Ratio (aHR)f, g, h,i,j |
|||||
|---|---|---|---|---|---|---|---|---|
| Na | Person-yearsb or Person-monthsc | HRd | 95% CI | P value | aHRd | 95% CI | P value | |
| Pelvic inflammatory disease | ||||||||
| Chlamydia negative | 146 | 78,201 | Ref | Ref | ||||
| Chlamydia positive | 90 | 24,484 | 1.95 | 1.47–2.59 | <0.0001 | 1.62 | 1.20–2.17 | 0.001 |
| Ectopic pregnancy | ||||||||
| Chlamydia negative | 52 | 78,897 | Ref | Ref | ||||
| Chlamydia positive | 39 | 25,161 | 2.11 | 1.34–3.31 | 0.002 | 1.84 | 1.14–2.95 | 0.012 |
| Tubal factor infertility | ||||||||
| Chlamydia negative | 25 | 79,002 | Ref | Ref | ||||
| Chlamydia positive | 30 | 25,208 | 2.90 | 1.65–5.09 | 0.0004 | 2.75 | 1.53–4.94 | 0.001 |
|
Stratified analysese | ||||||||
| Pregnancy (years following sexual debut 0–12 years) | ||||||||
| Chlamydia negative | 1233 | 58,966 | Ref | Ref | ||||
| Chlamydia positive | 723 | 17,285 | 1.08 | 1.00–1.17 | 0.04 | 1.10 | 1.02–1.19 | 0.01 |
| Pregnancy (years following sexual debut 13–21 years) | ||||||||
| Chlamydia negative | 1126 | 9001 | Ref | Ref | ||||
| Chlamydia positive | 559 | 4749 | 0.94 | 0.85–1.04 | 0.21 | 1.02 | 0.92–1.13 | 0.77 |
| Pregnancy (years following sexual debut 22–28 years) | ||||||||
| Chlamydia negative | 23 | 390 | Ref | Ref | ||||
| Chlamydia positive | 28 | 246 | 2.00 | 1.14–3.53 | 0.02 | 1.84 | 1.01–3.36 | 0.05 |
| Planned pregnancy (age 16–25 years) | ||||||||
| Chlamydia negative | 214 | 2712 | Ref | Ref | ||||
| Chlamydia positive | 81 | 1825 | 0.68 | 0.51–0.92 | 0.01 | 0.67 | 0.50–0.91 | 0.01 |
| Planned pregnancy (age 26–33 years) | ||||||||
| Chlamydia negative | 1473 | 13,146 | Ref | Ref | ||||
| Chlamydia positive | 567 | 6443 | 0.81 | 0.74–0.90 | <0.0001 | 0.81 | 0.73–0.89 | 0.0002 |
| Planned pregnancy (age 34–42 years) | ||||||||
| Chlamydia negative | 383 | 3640 | Ref | Ref | ||||
| Chlamydia positive | 200 | 1722 | 1.08 | 0.91–1.28 | 0.40 | 1.06 | 0.89–1.27 | 0.48 |
Chlamydia positive was defined as a positive PCR-test outcome in the CSI study (screening-PCR), and/or the presence of chlamydia IgG (serology) and/or a self-reported chlamydia infection (self—reported). For these analyses, multiple imputations were used to estimate time of first chlamydia infection in women without a known first year of chlamydia infection.
HR, Hazard Ratio; CI, Confidence Interval; Ref, Reference category.
Median cases of 15 multiple imputation datasets.
Person-years for pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility and pregnancies.
Person-months for planned pregnancies.
Estimated from 15 multiple imputation datasets.
Analyses for pregnancies were stratified by follow-up time because of violation of the proportional hazard assumption, analyses for planned pregnancy were stratified by age of starting to conceive because of effect modification. Risk estimates for potential confounders and full multivariable models are shown in Tables S11 and S12.
Pelvic inflammatory disease model adjusted for educational level, age (linear) and number of lifetime chlamydia tests.
Ectopic pregnancy model adjusted for age (linear), migration background, educational level, age at sexual debut, number of lifetime sex partners, number of lifetime chlamydia tests and intrauterine device insertion.
Tubal factor infertility model adjusted for age (linear) and number of lifetime chlamydia tests.
Pregnancy model adjusted for: age (linear), number of lifetime sex partners and age of sexual debut.
Planned pregnancy model adjusted for: age (linear).
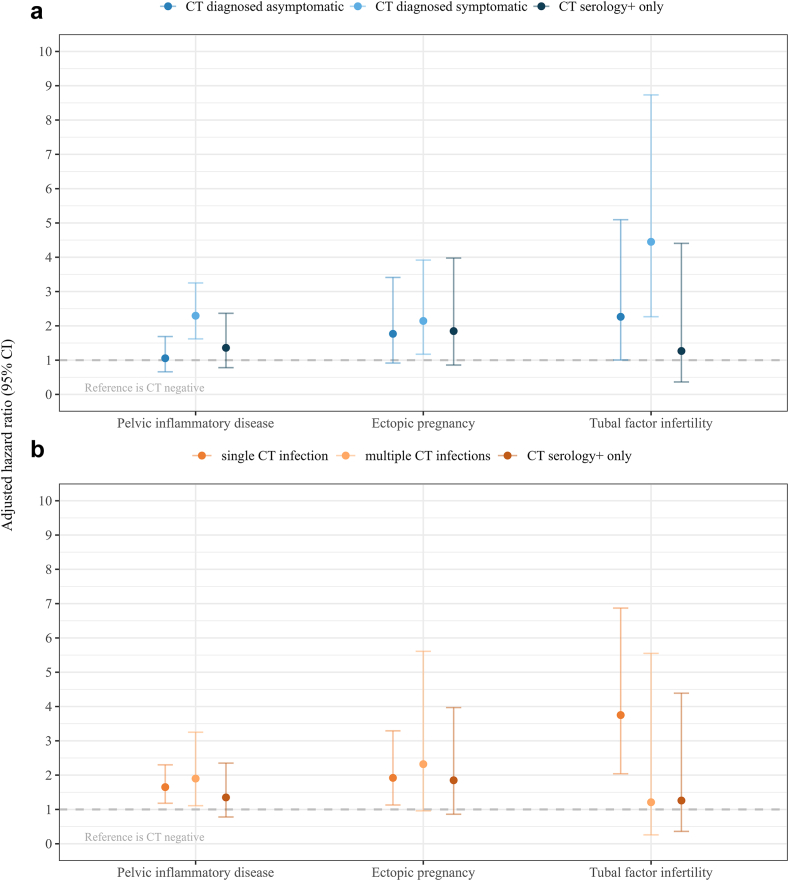
In sub-analyses an association with PID was observed for symptomatic infection compared to chlamydia-negatives (aHR 2.29, 95% CI 1.62–3.25) but not for asymptomatic infection (aHR 1.06, 95% CI 0.66–1.69) or serology+ only (aHR 1.35, 95% CI 0.78–2.35). Furthermore an association between chlamydia-positivity and PID was found for single infection (aHR 1.65, 95% CI 1.18–2.30) and multiple infections (aHR 1.90, 95% CI 1.11–3.25) (Fig. 3, Table S8).
Fig. 3.
Complication risks for chlamydia subgroups, reference is chlamydia-negative. (a) Chlamydia diagnosis: asymptomatic, symptomatic, and serology+ only, (b) Repeat chlamydia infection: single infection (self-reported + or screening-PCR+), multiple infections (≥2 or more) (self-reported+ and/or screening-PCR+), and serology+ only. Error bars represent 95% confidence intervals. Number of events per group and unadjusted hazard ratios can be found in Table S8. CT, Chlamydia trachomatis; PCR, polymerase chain reaction.
Ectopic pregnancy
In total, 91 women (1.6%) reported one or more ectopic pregnancies. Among chlamydia-positive women, ectopic pregnancy incidence was significantly higher than in chlamydia-negative women: 1.63/1000 py (95% CI 1.18–2.18) vs. 0.63/1000 py (95% CI 0.47–0.83). Symptomatic chlamydia showed the highest incidence at 1.90/1000 py (95% CI 1.15–2.91) (Table 1, Fig. 2b).
In multivariable analysis, adjusted for age, migration background, educational level, lifetime sex partners, lifetime chlamydia tests and IUD insertion, chlamydia-positivity remained associated with ectopic pregnancy (aHR 1.84, 95% CI 1.14–2.95) (Table 2).
In sub-analyses, an association between chlamydia-positivity and ectopic pregnancy was found for single infection (aHR 1.92, 95% CI 1.13–3.29) and symptomatic infection (aHR 2.14, 95% CI 1.17–3.92). For the remaining categories no significant association between chlamydia-positivity and ectopic pregnancy was observed (Fig. 3, Table S8).
Tubal factor infertility
In total 55 women (1.0%) reported to be diagnosed with tubal factor infertility. Among chlamydia-positive women, tubal factor infertility incidence was significantly higher than among chlamydia-negatives: 1.21/1000 py (95% CI 0.81–1.67) vs. 0.30/1000 py (95% CI 0.21–0.46). Symptomatic chlamydia showed the highest incidence at 1.79/1000 py (95% CI 1.07–2.78) (Table 1, Fig. 2c).
In multivariable analysis, adjusted for age and lifetime chlamydia tests, chlamydia-positivity remained strongly associated with tubal factor infertility (aHR 2.75, 95% CI 1.53–4.94) (Table 2).
In sub-analyses, an association between tubal factor infertility and chlamydia-positivity was found for single infection (aHR 3.75, 95% CI 2.04–6.87), and symptomatic infection (aHR 4.45, 95% CI 2.27–8.73). For the remaining categories no association between chlamydia-positivity and tubal factor infertility was observed (Fig. 3, Table S8).
Sensitivity analyses are given in the Supplementary Materials (Tables S7, S9, S10), which did not change any of the results significantly.
Pregnancy
In total 3692 women (64.7%) had been pregnant at least once. Among chlamydia-positives, the incidence rate of pregnancies was significantly higher compared with chlamydia-negatives: 67.7 per 1000 py (95% CI 63.2–71.3) and 41.9 per 1000 py (95% CI 40.3–43.5) respectively (Table 1).
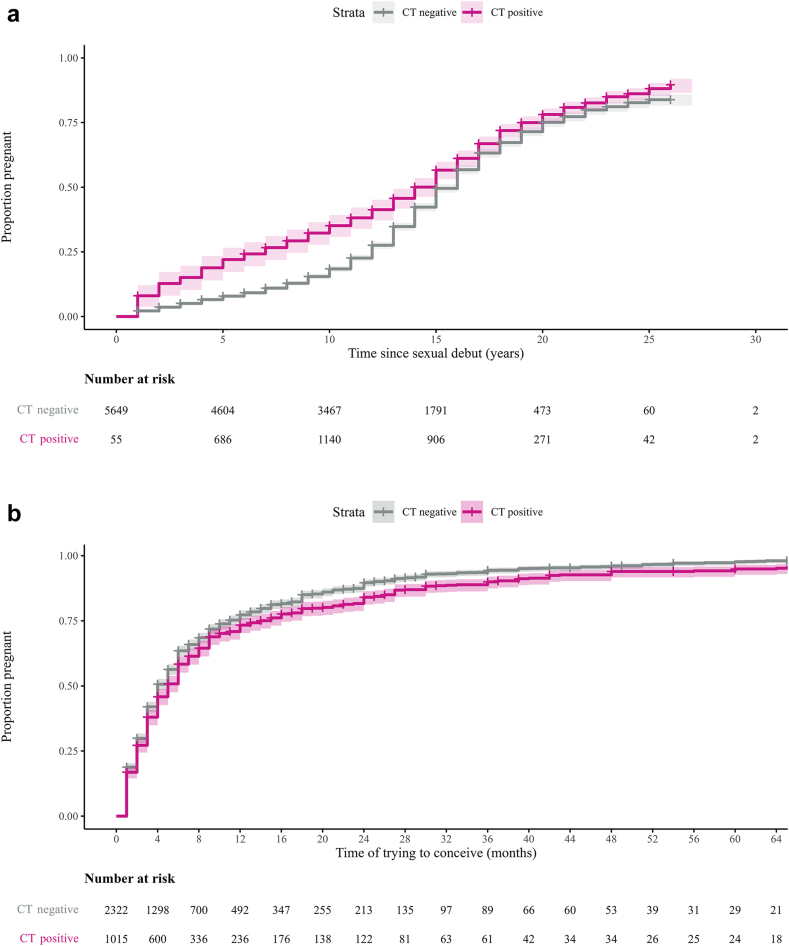
Multivariable analysis, stratified by follow-up time due to a proportional hazard assumption violation (Fig. 4a), showed higher pregnancy chances in chlamydia-positives (aHR 1.10, 95% CI 1.02–1.19) during the first exposure interval (0–12 years after sexual debut). No significant differences were observed in subsequent exposure intervals (Table 2).
Fig. 4.
Kaplan–Meier plots of time (years or months) to pregnancy by chlamydia status. (a) Pregnancy, (b) Planned pregnancies. Chlamydia-positive was defined as a positive PCR-test outcome in the CSI study (screening-PCR+), and/or a self-reported chlamydia infection (self-reported+) and/or presence of chlamydia IgG antibodies in serum (serology+)∗. Shaded areas represent corresponding 95% confidence intervals. Time was censored at a sample size reduction of less than 15 participants per stratum.∗Median serology+ cases of 15 multiple imputation. PCR, polymerase chain reaction; IgG, immunoglobulin G.
Planned pregnancy
A total of 3236 women (56.7%) had either a planned pregnancy or tried but failed to become pregnant (Table S3). After removing records with missing time values (n = 27), 3209 women were included with a total of 29,496 months of person-time follow-up, during which 91.0% (n = 2918) became pregnant. 77.2% of women became pregnant within 12 months, 73.4% (95% CI: 70.3–76.1) in chlamydia-positives and 78.5% (95% CI: 76.6–80.1) in chlamydia-negatives (Fig. 4b).
Multivariable analysis, stratified by age of starting to conceive because of effect modification, showed lower chances of planned pregnancy in chlamydia-positives vs. chlamydia-negatives in age categories 16–25 years (aHR: 0.67, 95% CI: 0.50–0.91) and 26–33 years (aHR: 0.81, 95% CI: 0.73–0.89). No significant difference was found in the 34–42 age category (Table 2).
Discussion
In this prospective cohort of 5700 Dutch women who were followed up for up to 14 years, a previous chlamydia infection increased the risk of complications by 1.6 times for PID, 1.8 times for ectopic pregnancy and 2.8 times for tubal factor infertility. Complication risks were low, with women having a prior chlamydia infection experiencing rates between 1.2 and 3.8 per 1000 py, and those without a prior chlamydia infection rates between 0.3 and 1.8 per 1000 py. Symptomatic, but not asymptomatic chlamydia infections were associated with increased PID risk. The same findings were observed for the other adverse reproductive outcomes. Overall pregnancy chances were not reduced following a chlamydia infection; but duration of trying to conceive was increased.
This is the first large-scale, long-term cohort study to collect prospective data on complications and potential confounders from a setting with widespread chlamydia control activities. While the majority of studies used hospital records only,10,12, 13, 14, 15 this study included diagnoses from all clinical settings, which enhanced the accuracy of estimates, especially for PID.22 Our cohort provides an unique opportunity, as serology testing allowed to compare the risk of complications in never-diagnosed women to women with diagnosed infections.
This study is not without limitations. Lifetime chlamydia status may still be misclassified. Chlamydia serology can be used in epidemiological studies to capture additional infections when PCR testing intervals are not frequent enough to detect infections before they resolve.23,24 However, not all women will generate detectable or persistent antibodies after a chlamydia infection23; approximately 30% of women with prior infections may go unnoticed by the serology test we used.20 Combining chlamydia exposure measures partially addressed this challenge. We expect the under-detection of antibodies to be differential, as prior studies found that women with recurrent chlamydia infections25 or complications26 are more likely to test and remain seropositive. Thus, the estimated risk of complications associated with chlamydia serology positivity may be skewed toward highly exposed women, potentially overestimating the actual risk.
Outcomes were self-reported which could introduce bias. We reduced this by several measures. Diagnostic bias toward chlamydia-positive women could take place due to chlamydia history being part of the diagnostic criteria for PID.16 This could lead to an overestimation of effect size. However, restricting analyses for PID to hospitalized cases yielded similar results. To improve validity of self-reported fertility outcomes,21 we incorporated risk estimates for (planned) pregnancies and verified medical records for self-reported tubal factor infertility.
The NECCST cohort is a subset of a population-based chlamydia screening study, and participants could enroll when they had already experienced an outcome. Selection bias could increase the association between chlamydia and complications, but is likely least pronounced for ectopic pregnancy which is less known to be linked to chlamydia. Risks at study inclusion were published elsewhere and were slightly higher for PID and tubal factor infertility, but lower for ectopic pregnancy, compared to the completed cohort study.27 Besides selection bias, differences could be explained by the association between chlamydia and complications altering with reproductive lifetime, captured by longer follow-up time. Some factors related to loss-to-follow-up in our cohort (e.g., non-Western migration background and practical education level) were associated with both chlamydia positivity and complication risks. While we adjusted the risk estimates for these factors, unmeasured residual confounding related to retention cannot be excluded. For example, migration background likely reflects unmeasured factors such as variation in host genetic factors, as well as structural drivers such as socio-economic status. As a result, our incidences and overall estimates might not be representative of high chlamydia disease burden groups.
Despite the differences in cohort structure, definition of chlamydia status, confounders and outcome determination, the risk for PID after chlamydia infection (aHR 1.6) in our study was very similar to estimates from four large population-based cohorts (aHR 1.3–2.4).11, 12, 13, 14 For ectopic pregnancies (aHR 1.8) results are also consistent with prior studies (aHR 1.3–1.9).10,11,13, 14, 15 Our estimated risk (aHR 2.8) for tubal factor infertility was notably higher than two similar studies that reported risks of 1.3–1.5.10,13 In these studies the comparison group consisted of women who tested negatively by PCR, thereby excluding low-risk never-tested women. On contrary, in our study low-risk women were tested during the initial screening and included in the reference group. The absolute incidences of tubal factor infertility and ectopic pregnancy were low and in line with estimates from a previous statistical inferences modeling study.7 This limited the statistical power in sub-group analyses, likely leading to non-significant risk observed for repeat diagnoses, while large retrospective studies did find a dose–response relationship10,11,13
We observed no reduction in overall pregnancy rates, in line with population-based cohort studies on birth rates.15,28 We calculated more unplanned pregnancies (15%) in chlamydia-positives as compared to chlamydia-negatives, likely due to more unprotected sexual contact. The large proportion of (younger) chlamydia-positive women who already had an unintended pregnancy could lead to an overestimation of the effect of chlamydia on fertility in the remaining women with intended pregnancies. This hypothesis aligns with our observation that there was no difference in chances of planned pregnancy among older women (≥34 years).
Stratified analyses showed that women with a symptomatic chlamydia infection have an over twofold increased risk of PID and tubal factor infertility compared to women with an asymptomatic infection. This may be explained by symptomatic infections being caused by a more virulent bacterium, and/or higher bacterial load, and/or it could be indicative of a more severe host response.7,8 Furthermore, chlamydia symptoms could indicate undiagnosed PID, potentially leading to higher complication rates in symptomatic women compared to asymptomatic women.
The majority of chlamydia infections in our cohort were PCR confirmed or self-reported diagnoses and therefore we may assume that women received antibiotic treatment according to clinical guidelines. During the study period single-dose oral azithromycin was the regular treatment which is highly accessible in the Netherlands and known for a very high patient adherence.29 On the opposite, women who were only positive by serology or chlamydia negative were unlikely to be treated, although we cannot rule out incidental (i.e., prescribed for another infection) chlamydia-effective antibiotic use. However, accidental effective azithromycin or doxycycline treatment without chlamydia diagnosis is expected to be minimal given the Netherlands' very low background antibiotic consumption.30 While our study was not designed to directly asses the effect of treatment on complications, we observed that risk for PID after asymptomatic chlamydia infections (presumably treated) and women only positive by serology (presumably untreated) were comparable and not significantly different from women without a chlamydia infection, in contrast to symptomatic infections which had significantly increased risk for PID. These findings may be taken into account when reconsidering the chlamydia treatment policy, which may result in prioritizing symptomatic individuals in test-and-treatment efforts.
However, it's important to note that the findings of observational studies are specific to the context of their respective study settings. Current chlamydia (opportunistic) screening policies vary widely in Europe, reflecting ongoing uncertainty about the extent to which severe complications, such as infertility, can be prevented through screening.4,31,32 These policies are partly informed by evidence from observational studies, which provide data on the incidence and preventable fraction of complications. Our study suggests that the impact of chlamydia on infertility may be limited at a population level. However, to extrapolate to other settings and inform chlamydia screening policies, it's crucial to account for variations in social factors, health literacy, access to medical care and medications for which our study could not account, as well as reproductive characteristics across different populations.
Primary prevention and timely testing and treatment of symptomatic chlamydia infections, especially in young women, remains important. Monitoring and surveillance for complications is needed to evaluate the public health impact of chlamydia.
Contributors
BHBvB, SAM, CJPAH, NHTMD-M, MABvdS, HMG, HJCdV and BMH contributed to conceptualization of this study, design of the methodology, and funding acquisition. ZWA and BMH collected the data for NECCST and performed among others the laboratory analyses. SAM coordinated the laboratory analyses. Data curation and formal analysis was performed by ZWA with supervision of BMH and BHBvB. All authors had full access to all the data in the study and vouch for the accuracy and completeness of the data. ZWA and BMH directly accessed and verified the underlying data reported in the manuscript. The data interpretation was done by ZWA, BMH, BHBvB, SAM and CJPAH discussed with NHTMD-M, MABvdS, HMG, HJCdV and JEdH. All authors contributed to revision of the paper, approved the final manuscript and accept responsibility to submit for publication.
Data sharing statement
On request (anonymised) data and available biological material can be provided for research related to STI, after approval by an advisory committee. Data available at doi: https://doi.org/10.35086/07c7cff8-3a03-4141-a72d-7ec4694e563f.
Declaration of interests
None declared.
Acknowledgements
We gratefully acknowledge the participants of the NECCST study. Second, we thank the representatives of Freya, Dutch patient association for people experiencing fertility problems, for their valuable feedback on the questionnaires.
Ethics Approval: This study was initially approved by the Medical Ethical Committee Noord-Holland, Alkmaar the Netherlands. In 2018 this committee ceased to exist, after which the approval of this study was taken over by the Medical Ethical Committee VU medical Center, Amsterdam the Netherland (NL 51553.094.14/2015.903(A2019.336)). Date of approval 10/13/2015. Dutch Trial Register NTR-5597.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101027.
Appendix A. Supplementary data
References
- 1.WHO . GenevaWorld Health Organization; Geneva: 2023. Fact sheet: sexually transmitted infections (STIs) world health organization.https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) Available from: [Google Scholar]
- 2.Low N., Redmond S., Uuskula A., et al. Screening for genital chlamydia infection. Cochrane Database Syst Rev. 2016;9 doi: 10.1002/14651858.CD010866.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oakeshott P., Kerry S., Aghaizu A., et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340:c1642. doi: 10.1136/bmj.c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor A., Dana T., Griffin J.C., et al. Screening for chlamydial and gonococcal infections: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;326(10):957–966. doi: 10.1001/jama.2021.10577. [DOI] [PubMed] [Google Scholar]
- 5.Hocking J.S., Temple-Smith M., Guy R., et al. Population effectiveness of opportunistic chlamydia testing in primary care in Australia: a cluster-randomised controlled trial. Lancet. 2018;392(10156):1413–1422. doi: 10.1016/S0140-6736(18)31816-6. [DOI] [PubMed] [Google Scholar]
- 6.van den Broek I.V., van Bergen J.E., Brouwers E.E., et al. Effectiveness of yearly, register based screening for chlamydia in the Netherlands: controlled trial with randomised stepped wedge implementation. BMJ. 2012;345 doi: 10.1136/bmj.e4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price M.J., Ades A.E., Soldan K., et al. The natural history of Chlamydia trachomatis infection in women: a multi-parameter evidence synthesis. Health Technol Assess. 2016;20(22):1–250. doi: 10.3310/hta20220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisler W.M. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis. 2010;201(Suppl 2):S104–S113. doi: 10.1086/652402. [DOI] [PubMed] [Google Scholar]
- 9.Herzog S.A., Althaus C.L., Heijne J.C., et al. Timing of progression from Chlamydia trachomatis infection to pelvic inflammatory disease: a mathematical modelling study. BMC Infect Dis. 2012;12:187. doi: 10.1186/1471-2334-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reekie J., Donovan B., Guy R., et al. Risk of ectopic pregnancy and tubal infertility following gonorrhea and Chlamydia infections. Clin Infect Dis. 2019;69(9):1621–1623. doi: 10.1093/cid/ciz145. [DOI] [PubMed] [Google Scholar]
- 11.den Heijer C.D.J., Hoebe C., Driessen J.H.M., et al. Chlamydia trachomatis and the risk of pelvic inflammatory disease, ectopic pregnancy, and female infertility: a retrospective cohort study among primary care patients. Clin Infect Dis. 2019;69(9):1517–1525. doi: 10.1093/cid/ciz429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reekie J., Donovan B., Guy R., et al. Risk of pelvic inflammatory disease in relation to Chlamydia and gonorrhea testing, repeat testing, and positivity: a population-based cohort study. Clin Infect Dis. 2017;66(3):437–443. doi: 10.1093/cid/cix769. [DOI] [PubMed] [Google Scholar]
- 13.Davies B., Turner K.M., Frolund M., et al. Risk of reproductive complications following chlamydia testing: a population-based retrospective cohort study in Denmark. Lancet Infect Dis. 2016;16(9):1057–1064. doi: 10.1016/S1473-3099(16)30092-5. [DOI] [PubMed] [Google Scholar]
- 14.Low N., Egger M., Sterne J.A., et al. Incidence of severe reproductive tract complications associated with diagnosed genital chlamydial infection: the Uppsala Women's Cohort Study. Sex Transm Infect. 2006;82(3):212–218. doi: 10.1136/sti.2005.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakken I.J., Skjeldestad F.E., Lydersen S., Nordbo S.A. Births and ectopic pregnancies in a large cohort of women tested for Chlamydia trachomatis. Sex Transm Dis. 2007;34(10):739–743. doi: 10.1097/01.olq.0000261326.65503.f6. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf H., Trent M. Management of pelvic inflammatory disease in clinical practice. Ther Clin Risk Manag. 2023;19:183–192. doi: 10.2147/TCRM.S350750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haggerty C.L., Gottlieb S.L., Taylor B.D., Low N., Xu F., Ness R.B. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(Suppl 2):S134–S155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 18.Hoenderboom B.M., van Ess E.F., van den Broek I.V.F., et al. Chlamydia trachomatis antibody detection in home-collected blood samples for use in epidemiological studies. J Microbiol Methods. 2018;144:164–167. doi: 10.1016/j.mimet.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Hoenderboom B.M., van Oeffelen A.A., van Benthem B.H., et al. The Netherlands Chlamydia cohort study (NECCST) protocol to assess the risk of late complications following Chlamydia trachomatis infection in women. BMC Infect Dis. 2017;17(1):264. doi: 10.1186/s12879-017-2376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman K.S., Darville T., Wiesenfeld H.C., Hillier S.L., Kaltenboeck B. Mixed Chlamydia trachomatis peptide antigens provide a specific and sensitive single-well colorimetric enzyme-linked immunosorbent assay for detection of human anti-C. trachomatis antibodies. mSphere. 2018;3(6) doi: 10.1128/mSphere.00484-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer E.J., den Tonkelaar I., Burger C.W., van Leeuwen F.E., Group O.P. Validity of self-reported causes of subfertility. Am J Epidemiol. 2005;161(10):978–986. doi: 10.1093/aje/kwi120. [DOI] [PubMed] [Google Scholar]
- 22.Reekie J., Donovan B., Guy R., et al. Hospitalisations for pelvic inflammatory disease temporally related to a diagnosis of Chlamydia or gonorrhoea: a retrospective cohort study. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0094361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waters M.B., Hybiske K., Ikeda R., et al. Chlamydia trachomatis seroassays used in epidemiologic research: a narrative review and practical considerations. J Infect Dis. 2024;230:250. doi: 10.1093/infdis/jiae199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta K., Harrison S.A., Davis N.A., et al. Prevalence of Chlamydia trachomatis infection in young women and associated predictors. Sex Transm Dis. 2021;48(8):529–535. doi: 10.1097/OLQ.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohman H., Rantsi T., Joki-Korpela P., Tiitinen A., Surcel H.M. Prevalence and persistence of Chlamydia trachomatis-specific antibodies after occasional and recurrent infections. Sex Transm Infect. 2020;96(4):277–282. doi: 10.1136/sextrans-2018-053915. [DOI] [PubMed] [Google Scholar]
- 26.Zuo Y., Jiang T.T., Teng Y., Han Y., Yin Y.P., Chen X.S. Associations of Chlamydia trachomatis serology with fertility-related and pregnancy adverse outcomes in women: a systematic review and meta-analysis of observational studies. eBioMedicine. 2023;94 doi: 10.1016/j.ebiom.2023.104696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoenderboom B.M., van Benthem B.H.B., van Bergen J., et al. Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for chlamydia in a chlamydia screening trial. Sex Transm Infect. 2019;95(4):300–306. doi: 10.1136/sextrans-2018-053778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen B., Ostergaard L., Puho E., Skriver M.V., Schonheyder H.C. Ectopic pregnancies and reproductive capacity after Chlamydia trachomatis positive and negative test results: a historical follow-up study. Sex Transm Dis. 2005;32(6):377–381. doi: 10.1097/01.olq.0000154512.86651.07. [DOI] [PubMed] [Google Scholar]
- 29.WHO . GenevaWorld Health Organization; 2016. Recommendations for treatment of chlamydial infections. WHO guidelines for the treatment of Chlamydia trachomatis. WHO guidelines approved by the guidelines review committee. [Google Scholar]
- 30.ECDC . Stockholm: European Centre for Disease Prevention and Control; European Centre for Disease Prevention and Control; Stockholm: 2023. Antimicrobial consumption in the EU/EEA (ESAC-Net) - annual epidemiological report 2022.https://www.ecdc.europa.eu/sites/default/files/documents/AER-antimicrobial-consumption.pdf Available from: [Google Scholar]
- 31.Kenyon C., Herrmann B., Hughes G., de Vries H.J.C. Management of asymptomatic sexually transmitted infections in Europe: towards a differentiated, evidence-based approach. Lancet Reg Health Eur. 2023;34 doi: 10.1016/j.lanepe.2023.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Broek I.V., Sfetcu O., van der Sande M.A., et al. Changes in chlamydia control activities in Europe between 2007 and 2012: a cross-national survey. Eur J Publ Health. 2016;26(3):382–388. doi: 10.1093/eurpub/ckv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.