Abstract
Keloids are abnormal skin growths occurring in a significant portion of the global population. Despite their pervasiveness, the underlying pathophysiology of this scarring process is yet to be fully understood. In this review article, we delve into the current literature on the pathophysiological mechanisms of keloids. We take a top-down approach, first looking at host factors such as genetics and endocrine factors and then taking a more granular approach describing specific control factors such as germline keloid predisposition variants, epigenetics and transcriptomics, inflammatory and immune dysregulation, and the role of profibrotic and angiogenic cell signaling pathways. We then discuss current knowledge gaps, propose further research avenues, and explore potential future treatment options considering our increased understanding of keloid pathogenesis.
Keywords: Keloid, Pathophysiology, Scar tissue
Introduction
Keloids are chronic, often debilitating, cutaneous fibroproliferative growths that affect a sizeable global population (Limandjaja et al, 2020; Tan et al, 2019). Although keloids are like hypertrophic scars (HTSs) in progression and appearance, keloids differ in their ability to outgrow the initial wound margin at poorly regulated rates. Both keloids (Bijlard et al, 2017) and HTSs (Butzelaar et al, 2015) can cause pain and pruritus and can negatively impact the patient's QOL beyond the debilitating physical and cosmetic concerns (Bijlard et al, 2017). In terms of natural progression, HTSs harbor a greater capacity to spontaneously resolve over time than keloids, which often require surgical, laser, or steroid injections for improvement. (Butzelaar et al, 2015).
Clinically, keloids are firm nodules or plaques of varying sizes with colors ranging from red to white to brown/black. The collagen fibers in keloids are abnormally thick and densely packed. Unlike in normal scars, where collagen fibers are aligned in a parallel fashion, in keloids, these fibers are often arranged haphazardly (Macarak et al, 2021). This disorganized arrangement contributes to the firm, rubbery texture of keloid scars. Keloids also exhibit an overabundance of extracellular matrix (ECM) components, including types I and III collagen, elastin, fibronectin, and proteoglycans, along with a greater number of blood vessels than normal scars, which reflects their tendency to appear redder or more purple than the surrounding skin. Keloids may also contain inflammatory cells such as lymphocytes and macrophages (Ehrlich et al, 1994).
A myriad of host and environmental factors have been etiologically linked to keloidal formation (Figure 1) (Huang and Ogawa, 2020; Ogawa et al, 2012; Tulandi et al, 2011; Wang et al, 2019; Young et al, 2014). This review article aims to provide a broad overview of the pathophysiological mechanisms underlying keloid development. We take a top-down approach, first looking at biological host factors, including patient genetics and endocrine contributions. We also delineate some recent updates in epigenetics and transcriptomics, inflammatory and immune system regulation, and the role of profibrotic and angiogenic cell signaling pathways. Finally, we discuss current knowledge gaps and propose future opportunities.
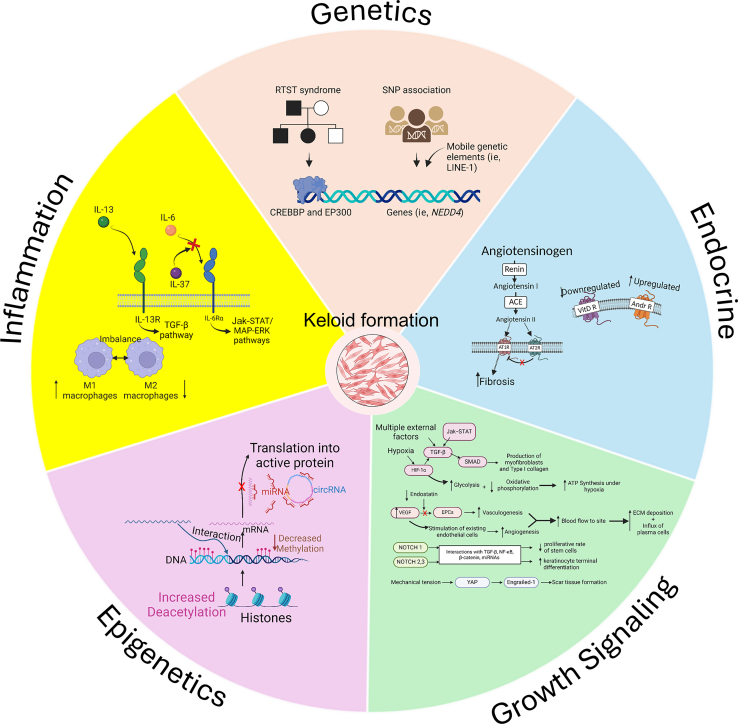
Figure 1.
Pathogenic factors that contribute to keloid formation. The diagram is divided into 5 major sections: genetics, endocrine system, GF signaling, epigenetics, and inflammation. The genetics section illustrates the interaction of genes and mobile genetic elements within a genome. The endocrine system section shows the role of angiotensin, VitD R, and ARs. The growth signaling section summarizes the interaction between the Jak–STAT, TGFβ, and HIF-1α pathways. This section also illustrates the mechanism of VEGF in the production of vasculature and the role of NOTCH1, 2, and 3. The epigenetics section shows the interaction of multiple epigenetics components, including histone modification, DNA methylation, miRNAs, lncRNAs, and circRNAs. The inflammation section illustrates the role of IL-6, IL-37, IL-13, and macrophages M1 and M2. This image was prepared with BioRender. ACE, angiotensin-converting enzyme; AR, androgen receptor; AT1R, angiotensin type 1 receptor; AT2R, angiotensin type 2 receptor; circRNA, circular RNA; ECM, extracellular matrix; EPC, endothelial progenitor cell; lncRNA, long noncoding RNA; miRNA, microRNA; RTST, Rubinstein–Taybi syndrome; STAT, signal transducer and activator of transcription; VitD R, vitamin D receptor.
Genetics of Keloid Predisposition
Familial studies
One of the first comprehensive studies on the inheritance of keloids was conducted by Marneros et al (2001). Ten African American families, along with 1 White, 1 Japanese, and 1 Afro-Caribbean (n = 341 individuals) families with keloid, were analyzed and found to exhibit an autosomal dominant mode of inheritance with incomplete penetrance. The expression of the keloid phenotype was also highly variable, with some individuals having small earlobe keloids and others having much larger keloids involving other parts of the body (Marneros et al, 2001).
Subsequent studies attempted to identify the genes responsible for the observed mode of inheritance. Marneros et al (2001) analyzed genome-wide linkage in 1 Japanese and 1 African American family. The researchers found evidence of linkage to chromosome 2q23 (2-point maximum logarithm of the odds [LOD] score of 3.01) for the Japanese family and 7p11 (2-point maximum LOD score of 3.16) for the African American family. They hypothesized that the gene TNFAIP6, which encodes TNFAIP6, is contained within the locus identified for the Japanese family. Another marker was identified for the African American family, linked to the gene EGFR, which codes for EGFR (Marneros et al, 2004).
Keloids can also occur as a clinical feature in a few congenital disorders, with Rubinstein–Taybi syndrome (RSTS) and Goeminne syndrome being the most prominent (van de Kar et al, 2014). In a clinical study of RSTS, van de Kar et al (2014) described a 24% rate of keloid formation among patients with RSTS with a mean age of onset of 11.9 years. RSTS is primarily associated with germline sequence variants in 2 genes: CREBBP and EP300. These genes encode histone acetyltransferases (HATs), which play crucial roles in transcriptional coactivation by modifying chromatin structure and facilitating gene expression (Milani et al, 2015; Roelfsema and Peters, 2007). Variations in these genes lead to alterations in the epigenetic regulation of gene expression, impacting various developmental pathways and contributing to the phenotype of RSTS. Goeminne syndrome is very rare and is characterized by pronounced keloid development, congenital torticollis, nevi, and varicosities that emerge in early puberty. Other rare syndromes (www.omim.org) associated with keloid development include Warburg-Cinotti syndrome (Online Mendelian Inheritance in Man [OMIM] number 618175; alteration in DDR gene); frontometaphyseal dysplasia 2 (OMIM number 617137; alteration in MAP3K7 gene); lateral meningocele syndrome (OMIM number 130720; alteration in NOTCH3 gene); premature aging syndrome, Penttinen type (OMIM number 601812; alteration in PDGFRB gene); and cardiac valvular dysplasia, X linked (OMIM number 314400; alteration in FLNA gene).
Single nucleotide variation–based association studies
Further genetic studies have identified additional candidate loci. Keloids appear to conform to an oligogenic rather than monogenic inheritance pattern, with multiple genes affecting different groups (Liu et al, 2022). A review by Liu et al (2022) compiled the primary known genes associated with keloids across multiple genetic studies. A GWAS by Nakashima et al (2010) found 4 susceptibility loci in the Japanese population. The strongest associated single nucleotide variation (SNV) was rs873549 on chromosome 1q41. Two other SNVs were identified in chromosome 3q22: rs11511412 (including FOXL2) and rs8032158. The fourth identified loci, rs8032158, was located within NEDD4 on chromosome 15q23.3 (Nakashima et al, 2010).
This study was later replicated in the Chinese Han population by Zhu et al (2023), who also found associations between keloid formation rs1442440 (also on chromosome 1q41) and rs2271289 (also within NEDD4 on 15.21.3). The Chinese Han population has been one of the most thoroughly analyzed, with subsequent studies finding multiple other genes associated with keloid development. A GWAS performed by Teng et al (2015) found an association with SIRT3 (s181924090 on 11p15.5), MYH8 (rs1813178644 on 6p25.3), and HUS1B (rs1813178644 on 6p25.3). An improved multiple ligase detection reaction performed by Liu et al (2021) found an association between leptin receptor gene (LEPR) sequence variants and keloid development at SNVs rs1137101, rs1938496, and rs7555955.
Candidate SNV analyses have also been performed. A PCR-restriction fragment length polymorphism analysis by Han et al (2014) found an association between the gene ADAM33 (rs612709 on 20q13) and keloid scar development. Furthermore, a PCR-sequence–specific primer performed by Lu et al (2008) found associations with specific HLA-DQA1 and HLA-DQB1 haplotypes and certain types of keloid development (Lu et al, 2008).
Studies on other ethnicities have identified different sets of genes associated with keloid development. A study performed by Velez Edwards (2014) on African American patients found an association between myosin genes MYO1E (rs747722 and rs28394564) and MYO7A (rs35641839) and keloids. The researchers also validated the association between the NEDD4 gene (rs138585173) and keloid development in this population (Velez Edwards et al, 2014). Another study performed by Santos-Cortez et al (2017) on a large Nigerian Yoruba family found an association between the acid ceramidase gene ASAH1 on 8q23.3-p21.3 and keloid development.
Studies performed on White patients have also found significant associations between HLA-DRB1∗15 (Brown et al, 2008; Shih and Bayat, 2012) and HLA-DRB5 and keloid development (Shih and Bayat, 2012). Furthermore, another study by Farag et al (2020) found a positive association between the NEDD4 gene (rs8032158 on 15q21.3) and keloid development in Egyptian patients.
The NEDD4 gene appears to play a vital function in keloid development, given its relationship to keloid development across multiple ethnic groups. NEDD4 is a ubiquitin ligase that regulates ubiquitin-mediated protein degradation (Chung et al, 2011; Yang and Kumar, 2010). A study by Chung et al (2011) found that NEDD4 is induced by T-cell factor/β-catenin transcriptional activity, and upregulated production of fibronectin and type 1 collagen leads to excessive ECM deposition (Chung et al, 2011). Of note, β-catenin is more highly expressed in keloids than in normal skin (Roh et al, 2017). Fujita et al (2019) has also found that NEDD4, particularly transcriptional variant 3 (TV3), is crucial for NF-κB activation in vivo and in vitro. NF-κB activation has been shown to play a role in chronic inflammation of keratinocytes, fibroblasts, and endothelial cells. The investigators found that patients with keloid have a higher expression of NEDD4-TV3 than control patients, leading to increased NF-κB activation. They hypothesized that NEDD4-TV3 modulates the activation NF-κB through ubiquitination of an adaptor protein RIP, which is essential for TNF-α–induced phosphorylation of NF-κB (Fujita et al, 2019).
More recent research has looked at the contributions of mobile elements (MEs) in gene diversification and regulation. MEs, also known as jumping genes, are discrete fragments of DNA capable of moving within genomes akin to a cut-and-paste function (Cordaux and Batzer, 2009). Kojima et al (2023) developed a tool that utilizes whole-genome sequencing to genotype ME variants. Using this new tool, they found that insertion of ME L1 (LINE-1) in an intron of NEDD4 (ie, L1-NEDD4) was associated with the development of keloids and fasciitis (Kojima et al, 2023).
Endocrine Systems and Keloid Formation
Renin–angiotensin system
Growing evidence suggests a role for the renin–angiotensin system (RAS) in the fibrotic processes observed across various organ systems. Key elements of the RAS, including angiotensin II (Ang II), angiotensin type 1 (AT1), and angiotensin type 2 (AT2) receptors, along with angiotensin-converting enzyme (ACE), are localized within the dermal tissue, operating independently of systemic RAS activities. AT1 receptors facilitate fibrogenesis and scar formation, whereas AT2 receptors mitigate the fibrotic effects mediated through AT1 receptor activation. The profibrotic influence of Ang II, mainly through AT1 receptor stimulation, is implicated in cutaneous scar development, notably through the upregulation of proinflammatory mediators such as IL-6; angiogenic factors such as VEGF; and fibrogenic factors, including TGF-β1 and connective tissue GF. Concurrently, Ang II appears to downregulate antifibrotic agents, namely the tissue inhibitors of metalloproteinases. Both AT1 and AT2 receptors are differentially expressed depending on the stage of fibrosis. Studies have shown a spike in AT1 receptor expression during the inflammatory phase (with slower concurrent upregulation of AT2). This upregulation is followed by a simultaneous downregulation of both AT1 and AT2 receptors in the proliferative phase. Finally, AT2 receptors are then quickly upregulated in the remodeling phase (Silva et al, 2020). Initial clinical trials have begun to elucidate the therapeutic potential of targeting the ACE/Ang II/AT1 receptor pathway in managing HTSs and keloids (reviewed in ref Hedayatyanfard et al [2020]).
Sex hormones
Noishiki et al (2019) reported that Japanese females are more likely to develop keloids than Japanese males at all ages; however, this was particularly skewed toward younger females (before age 15 years) with a female:male ratio of 2.7:1 (Noishiki et al, 2019). There was no difference in age of onset between the sexes. However, the disease occurred during puberty for both sexes. Ford et al (1983) analyzed 6 keloid samples, the surrounding skin tissue, and 6 nonkeloidal scars and found that keloids exhibited significantly elevated androgen binding levels, ranging from 510 to 1149 femtomoles per milligram of cytosolic protein. Conversely, estrogen and progesterone binding levels were minimal. Adjacent nonkeloidal tissue displayed androgen-binding capacities between 177 and 476 femtomoles per milligram of cytosolic protein, with similarly low estrogen and progesterone activities. Nonkeloidal scar tissue expressed markedly lower androgen-binding levels (37–60 femtomoles per milligram of cytosolic protein), with estrogen and progesterone bindings at nearly undetectable levels. This evidence suggests a potential role for localized hyperandrogenism in the etiology or progression of keloid formation (Ford et al, 1983). Interestingly, finasteride, an androgen blocker, can decrease pruritus and the texture of chest keloids (Yang et al, 2022). Therefore, androgens may serve as a driving force in the development of keloids.
Vitamin D
Vitamin D has also been hypothesized to play a pivotal role in the regulation of cellular proliferation and differentiation, notably attenuating tissue fibrosis progression mediated by keloid fibroblasts and impeding collagen production in dermal fibrosis (Akoh and Orlow, 2020). Mamdouh et al (2022) performed weekly intralesional injections of vitamin D (0.2 ml [200,000 IU] per cm2) in 40 Egyptian patients with keloid scars and found a statistically significant reduction in Vancouver Scar Scale scores after treatment (P ≤ .001).
It is known that vitamin D synthesis can be attenuated by darker skin pigmentation and that keloid formation is more common among individuals possessing darker skin pigmentation, such as African Americans. Beyond its classical function in calcium homeostasis, vitamin D is also integral to cellular proliferation, differentiation, oncogenesis, inflammation, and fibrotic responses. Vitamin D's biological effect is mediated through the vitamin D receptor (VDR), a constituent of the steroid nuclear receptor family, which, upon ligand binding, operates as a transcription factor essential for the ligand-dependent modulation of gene expression. Using immunohistochemistry, Hahn and Supp (2017) assessed the expression and nuclear localization of VDR within keloid lesions (n = 24) and corresponding normal skin biopsies (n = 24). They found diminished VDR protein levels in most keloid lesions and a notably reduced proportion of epidermal cells with nuclear localization of VDR in keloid tissue relative to that in normal skin. Intriguingly, a comparative analysis of VDR-positive nuclei across various normal skin samples indicated a significant decrease in nuclear localization within the epidermis of donors with darker skin tones compared with that in those with lighter skin. These findings propose a potential involvement of VDR in keloid pathogenesis and suggest its contributory role in the heightened keloid scar susceptibility among individuals with darkly pigmented skin (Hahn and Supp, 2017).
Progress in the Epigenetics and Transcriptomics of Keloidogenesis
Methylation and histone modification
DNA methylation and hydroxymethylation are crucial epigenetic mechanisms that preserve the distinct cellular identity and the functional integrity of various tissues. During DNA methylation, DNA methyltransferases catalyze the conversion of cytosine to 5-methylcytosine, which can be further processed into 5-hydroxymethylcytosine (5-hmC) by the action of TET (ten-eleven translocation) enzymes (TET1–3) through the incorporation of a hydroxyl group. The presence of 5-hmC across various normal tissues and cell types underscores its significance as an epigenetic mark that regulates fibroblast plasticity. Extant research has elucidated the role of DNA methylation dynamics in the differentiation processes of myofibroblasts and fibroblasts implicated in keloid and scar formation, indicating its potential influence on pathological fibrosis. Liu et al (2019) recently reported a decreased level of 5-hmC in human scar fibroblasts, indicating a decrease in methylation and an upregulation in gene expression, which could lead to increased fibroblast proliferation.
Two aberrant methylation patterns have been identified in proliferating and malignant cells: gene-specific hypermethylation and genome-wide hypomethylation. Gene-specific hypermethylation involves the accumulation of methylation at CpG islands within the promoter regions of genes, typically resulting in diminished gene expression. Conversely, genome-wide hypomethylation, predominantly affecting repetitive DNA sequences such as long interspersed nuclear elements and short interspersed nuclear elements (also known as Alu in primates), has been observed across various cancer cell lines and primary tumors. Genome-wide hypomethylation leads to chromosomal instability and an elevated incidence of DNA strand breaks, despite the usual association of DNA methylation with transcriptional repression. Jones et al (2015) analyzed 6 keloids with matching normal skin samples and found that of 685 genes differentially expressed in keloids, 510 genes were hypomethylated, whereas 175 genes were hypermethylated (n = 190 CpGs [28%] in promoter and n = 495 CpGs [72%] in nonpromoter regions). The predominance of hypomethylation in keloids suggests that methylation and transcriptional deregulation could also participate in the formation of keloids. As has been observed in other tumors (Almeida et al, 1993; Rodriguez et al, 2006), increased levels of hypomethylation likely predispose keloidal genomes to higher levels of DNA strand breaks and chromosomal instability. However, studies on this relationship in keloids have yet to be performed.
The rearrangement of genes through chromatin restructuring and histone modification can also contribute to keloid proliferation. Histone deacetyltransferases decrease keloid proliferation by removing the acetyl group, thereby allowing the chromatin to wrap tightly around the histone and decreasing the expression of cells that activate the production of fibroblasts. Conversely, HATs add an acetyl group and have the opposite effect on gene expression, accelerating the development of fibroblasts (Stevenson et al, 2021). Fitzgerald O'Connor et al (2012) discovered that owing to the overproduction of HDAC2s in keloids, CUDC-907, an inhibitor of histone deacetylases, effectively decreased cell proliferation, ECM deposition, and collagen accumulation.
Noncoding RNA
Noncoding RNAs (ncRNAs) have also been found to play a significant role in keloid epigenetics. ncRNAs are not translated into protein but can regulate gene expression at the transcriptional and post-transcriptional levels. About 98% of genomic DNA is transcribed as ncRNAs, including microRNAs (miRNA), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs) (Lv et al, 2020).
miRNAs are the smallest type of ncRNA (around 22 nucleotides in length) and are highly conserved evolutionarily (Lee et al, 2020). miRNAs are the most extensively characterized ncRNA in the context of keloids (Lv et al, 2020). Upregulated keloid miRNAs include miR-152-3p, miR-v21/miR-21-5p, miRNA-31, and miR-181a. On the other hand, downregulated miRNAs include miR-1-3p, miR-4417, miR-152-5p, miR-203, miR-141-3p, miR-29a, miR-214-5p, miR-188-5p, miR-637, miR-1224-5p, miR-205-5p, miR-200b, and miR-196a. The confluence of these miRNAs plays a major role in regulating signaling pathways involved in keloid pathogenesis, such as phosphoinositide 3-kinase/protein kinase B and TGF-β, by interfering with their corresponding RNA products, thereby preventing their translation into biologically active proteins (Lv et al, 2020).
lncRNAs are ncRNA molecules over 200 nucleotides long and regulate gene expression (Su and Han, 2024). Cis-acting lncRNAs localize near their transcriptional origins, impacting the expression and chromatin configurations of proximal genes on the identical allele. In contrast, trans-acting lncRNAs dissociate from their sites of transcription to exert regulatory effects on gene expression, protein functionality, and RNA dynamics at distant sites. lncRNAs mediate regulatory functions across transcriptional, translational, and post-translational stages through interactions with proteins, RNA, and DNA. lncRNAs modulate gene transcription through alterations in histone modifications and chromatin architecture, recruit transcription factors or repression of target gene promoters to modulate gene expression, and function as molecular decoys that sequester transcription-associated proteins, thus inhibiting their interaction with DNA targets. lncRNAs also contribute to post-transcriptional and translational regulation, notably serving as competing endogenous RNAs (ceRNAs) that bind miRNAs to counteract miRNA-induced gene silencing. In addition, lncRNAs act as precursors or templates for the synthesis of certain small RNAs and are involved in the alternative splicing of mRNAs. At the post-translational level, lncRNAs influence protein localization and transport by binding to specific proteins (He et al, 2020). In the context of keloids, mechanistically significant upregulated lncRNAs include lncRNA-H19, lncRNAHOXA11-AS, lncRNA- CAS1, and lncRNA-ATB. These lncRNAs interact with genes involved in keloid cell differentiation and proliferation, cell cycle and apoptosis, deposition of the ECM, and induction of Wnt pathways (Lv et al, 2020).
circRNAs are a newly identified type of RNA composed of closed loops of RNA (Lv et al, 2020). Their lack of 3' and 5' ends renders greater stability than their linear RNA counterparts. circRNAs serve as ceRNAs, akin to sponges that interact with miRNAs and prevent their action (Yu et al, 2022). circRNAs relevant to keloids include the downregulation of cricRNA_0008259 and circCOL3A1-859267. Current research indicates that these circRNAs are involved in regulation of type I collagen gene expression (Lv et al, 2020). However, research in this novel field is still ongoing.
Gene expression profiling of keloids
Using a cDNA microarray of 8400 genes, Chen et al (2003) compared the gene expression profiles (GEPs) of 3 keloid and 3 normal specimens and found that 4.79% (2.98% upregulated and 1.81% downregulated in keloids) exhibited differential expression. Since then, many GEP studies have been performed to elucidate programmatic differences between normal skin and scars or keloids. Using the Affymetrix GeneChip, which contains sequences from 38,500 human genes, Smith et al (2008) profiled cultures from 5 keloid fibroblasts and 5 normal skin fibroblasts. There were 175 and 559 genes that were upregulated and downregulated, respectively, in keloid fibroblasts. The authors noted altered expression in multiple genes within the Wnt pathway (Smith et al, 2008).
Kang et al (2020) profiled 49,372 transcripts in 5 keloid and 5 normal skin fibroblast lines and found 1406 genes (2.85%) that exhibited >2-fold changes in gene expression (673 upregulated and 733 downregulated in keloid cells). Pathway analysis revealed top transcriptional footprints in TGFB1 (z-score = 2.88, P = 9.66E-61) and HIF1A (z-score = 2.02, P = 1.05E-19) pathways. They subsequently showed increased HIF1A protein expression in keloid specimens compared with that in normal skin and a significant correlation between HIF1A and COL1A1 mRNA levels (q = 5.67E-45) across >800 cell lines. Most interestingly, functional studies indicated that keloid cells grown in 1% oxygen demonstrated increases in both intracellular and extracellular COL1A protein compared with cells grown in 20% oxygen. Furthermore, HIF1A inhibitor CAY10585 abrogated this increase (Kang et al, 2020). These results suggest a direct link between ambient hypoxia and neocollagenesis.
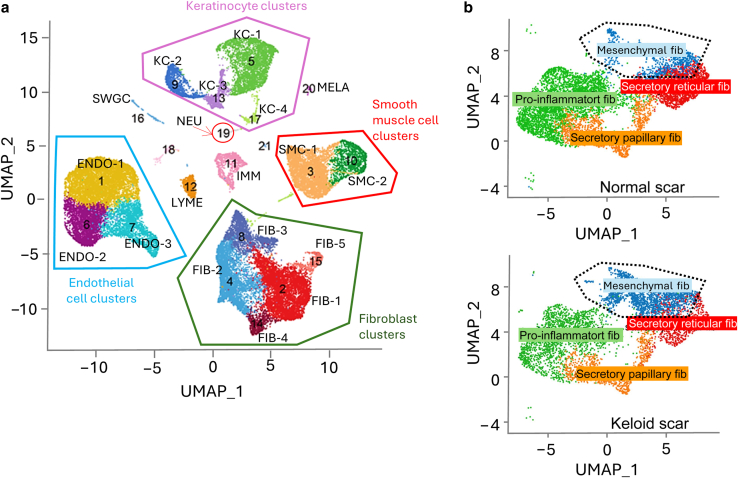
Most recently, Zhu et al (2023) performed bulk RNA sequencing to profile the transcriptome of 60 skin-punch biopsies of lesional keloid skin and nonlesional skin. The authors found that keloidal tissue demonstrated strong upregulation of pathways associated with collagen formation, ECM remodeling, IFN-γ response, immune cells, and neural system development markers (Zhu et al, 2023). Among the most prominent keloid-associated genes was COL2A1, which encodes the pro-alpha1(II) chain for type II collagen. Although COL2A1 is more typically expressed in osseous tissue, aberrant expression of this gene may account for some of the keloidal phenotype. The most precise molecular profiling of keloid tissue was performed by Deng et al (2021) using single-cell RNA sequencing (scRNAseq). A total of 40,655 cells from dermal keloid and normal scar tissue were used to obtain molecular phenotyping of resident cell types. Emerging evidence suggests that there are 4 subpopulations of human dermal fibroblasts: secretory reticular, mesenchymal, proinflammatory, and secretory papillary. scRNAseq analysis of 3 keloid specimens revealed that keloids were enriched for the mesenchymal subpopulation (Figure 2) compared with normal scars, whereas they were diminished in the fraction of proinflammatory, secretory reticular, and secretory papillary subtypes. Mesenchymal fibroblasts appear to express more osteoblast differentiation genes (eg, POSTN, COMP, COL11A1, COL12A1, and COL5A2), suggesting that an imbalance in fibroblast subpopulations could be 1 critical factor in the formation of keloids.
Figure 2.
Cellular composition of keloids and normal scars.(a) Deconvolution of cell types by scRNAseq (adapted from Figure 1 in Deng et al [2021]). (b) scRNAseq of normal scars versus keloid scars show amplification of mesenchymal fibroblast subpopulation among all fibroblasts (adapted from Figure 2 in Deng et al [2021]). ENDO, endothelial cell, FIB, fibroblast; IMM, immune cell; KC, keratinocyte; LYME, lymphatic endothelial cell; MELA, melanocyte; NEU, neural cell; scRNAseq, single-cell RNA sequencing; SMC, smooth muscle cell; SWGC, sweat gland cell; UMAP, Uniform Manifold Approximation and Projection.
Inflammatory Cytokines and Keloid Formation
Systemic cytokines
Systemic inflammation can also modulate keloid development. The inflammatory circuitry includes cytokines, chemokines, and the complement system. Of these, ILs, IFNs, and TGF play critical roles in the pathogenesis of keloid (Huang and Ogawa, 2020).
IL-6 (also known as IFN2) is a proinflammatory cytokine that plays a key role in keloid development (Huang and Ogawa, 2020). In areas of active inflammation, IL-6 is generated, leading to transcription of downstream inflammatory elements through IL-6RA (Abdu Allah et al, 2019; McLoughlin et al, 2004). McCauley et al (1992) noticed increased blood levels of IL-6 in 12 patients with keloids and 8 healthy controls, all of whom were African American. A study by Allah et al (2019) found significantly elevated serum levels of IL-6 among 60 Egyptian patients with keloid compared with those in 30 age- and sex-matched normal controls. Moreover, patients homozygous for a specific IL6 gene sequence variant exhibited a 4-fold increased risk of keloid formation. This upregulation of IL-6 has also been observed in Japanese patients (Quong et al, 2017). It is theorized that the persistent inflammation seen in keloids may be due to an autocrine loop of IL-6 signaling that leads to downstream activation of pathways such as Jak/signal transducer and activator of transcription (STAT)3 or MAPK/extracellular signal regulated kinase, resulting in fibroblastic cell proliferation and matrix synthesis (Ghazizadeh et al, 2007).
IL-37 is a cytokine first characterized in 2000 that belongs to the IL-1 family and acts as an inhibitor of inflammatory responses (Nold et al, 2010). A study by Nold et al (2010) found that inhibition of IL-37 with small interfering RNA (siRNA) correlated with a dose-dependent increase in IL-6 (Nold et al, 2010). Furthermore, a study by Khattab and Samir (2020) in patients with keloid found a negative association between levels of plasma IL-37 and keloid severity in a cohort of 32 patients from Egypt. This association was independent of keloid and age, sex, duration of lesions, and family history (Khattab and Samir, 2020). Unlike IL-6, studies examining differences in IL37 gene sequence variants between patients with keloid and controls have yet to be carried out. Therefore, we can only speculate that genetic susceptibility to decreased systemic IL-37 levels may play a role in greater keloid susceptibility.
Tissue cytokines
Tissue cytokines implicated in keloid formation include IL-22 and IFN-α, -β, and -γ. IL-22 has been implicated in scarring and keloid formation (Zaharie et al, 2022). A study found higher in situ mRNA levels of IL22 in keloid tissues than in regular scars (da Cunha Colombo Tiveron et al, 2018). IFNs partially suppress the proliferation of fibroblasts and decrease the production of the ECM (Huang and Ogawa, 2020). Among patients with keloid patients, McCauley et al (1992) found higher levels of INF-β production by PBMCs and lower levels of IFN-α and -γ production.
T helper 2 cytokines may also play a crucial role in the development of scars and keloids, particularly through the action of IL-4 and IL-13 as profibrotic mediators. A study found that IL-13 is a potent stimulator and activator of TGFβ1 in vivo, suggesting its significant role in mediating tissue fibrosis. This fibrogenic effect of IL-13 is mediated through a mechanism that involves plasmin/serine protease– and MMP9-dependent pathways but is independent of CD44. The study further demonstrated that the fibrogenic effects of IL-13 are primarily mediated by this TGFβ pathway, highlighting IL-13's central role in fibrosis (Lee et al, 2001). IL-13 has also been shown to upregulate collagen production in both normal and keloid fibroblasts, with effects equivalent to those induced by similar concentrations of IL-4 and TGFβ1 (Oriente et al, 2000). Finally, dupilumab, a human mAb that blocks the signaling of IL-4 and IL-13 cytokines, was used both systemically and intralesionally to successfully treat several patients with large keloids (Min et al, 2023; Wong and Song, 2021).
Macrophages are also known to play an important role in scar formation. During tissue repair, macrophages can polarize into 2 major phenotypes: M1 and M2. M1 macrophages are primarily induced by microbial products or proinflammatory cytokines (particularly IFN-γ), resulting in a proinflammatory cascade that includes nitric oxide formation and the release of large quantities of cytokines, including TNF-α, IL-1β, IL-12, and IL-23 (Chen et al, 2023). On the other hand, M2 macrophages are induced by cytokines such as IL-4 and IL-13 and are primarily involved in wound healing, angiogenesis, and immune suppression (Chen et al, 2023). A complex confluence of biological factors orchestrates the balance of M1/M2 macrophage polarity. An imbalance in M1/M2 macrophage polarization has been shown to play a role in pathologic keloid formation (Xu et al, 2020). First, a higher baseline number of M2 cells has been associated with subsequent HTS formation (Butzelaar et al, 2016). Second, decreased expression of M1 in the early stages of wound healing, followed by delayed expression of M2 macrophages, has been associated with pathological scarring (Xu et al, 2020). In addition, M2 macrophages appear upregulated around the margin and superficial region of keloids, correlating with their invasive nature (Xu et al, 2020). A putative explanation for this pathologic unbalancing of M1/M2 macrophage polarization in patients with keloid is poorly understood. However, recent studies suggest that inflammasomes (multiprotein complexes with important proinflammatory functions) could play a role in this observed phenomenon (Huang and Ogawa, 2022).
GF Signaling and Keloid Pathogenesis
Angiogenic factors
The formation of new blood vessels (neovasculogenesis) is a tightly regulated process in wound healing. Important factors in this process include VEGF, endothelial progenitor cells (EPCs), and endostatin. VEGF plays an important role in neovasculogenesis by inducing EPCs, resulting in the production of new blood vessels (Li et al, 2017). VEGF also plays a prominent role in angiogenesis (sprouting of existing blood vessels) by inducing the proliferation of endothelial cells to form capillary-like tubules, preventing endothelial cell apoptosis, and increasing vascular permeability. The increased vascular permeability results in an influx of plasma proteins (Carmeliet, 2005) and the deposition of the ECM (Huang and Ogawa, 2020).
Dysregulation in the balance of proangiogenic and antiangiogenic factors has been posited to play a key role in the abnormal blood vessel development observed in pathological scar formation (Mogili et al, 2012). Several studies have found that patients with keloid have significantly higher serum levels of VEGF, ranging from 1.3 to 2 times higher than normal controls (Mogili et al, 2012; Zhang et al, 2016). A study by Zhang et al (2016) found that patients with keloid also have increased circulating EPC levels (1.6 times higher) compared with control patients. This result was confirmed by Tanaka et al (2019), who reported >2-fold increase in circulating EPCs among patients with keloid. Zhang et al (2016) also found that keloid duration correlated inversely with the number of circulating EPCs. However, EPC levels did not correlate with VEGF levels, and both factors were independent of patient age, sex, or keloid etiology (Zhang et al, 2016). The lack of correlation between VEGF and EPC numbers could be partly explained by the variability in quantity of VEGF receptors in keloid tissues. For example, a study by Sudha et al (2018), found that 57.5% of studies keloid samples contained the receptor for VEGF.
Endostatin is a C-terminal fragment of type XVIII collagen and a potent inhibitor of angiogenesis through the inhibition of VEGF (Mogili et al, 2012). A study by Mogili et al (2012) found that patients with keloid had lower levels of endostatin both in serum and in tissue than controls. A characteristically high VEGF/endostatin ratio in both serum and tissue was observed in patients with keloid, likely resulting in uncontrolled vascular tissue proliferation (Mogili et al, 2012).
The use of VEGF receptor inhibitors in treating keloids has been explored, focusing on the role of VEGF in keloid pathogenesis and the potential therapeutic effects of inhibiting VEGF expression and its signaling pathways. Zhang et al (2008) reported that short hairpin RNA against VEGF significantly depleted VEGF expression in keloid fibroblasts (Zhang et al, 2008). Wu et al (2006) found that dexamethasone effectively suppresses VEGF expression in keloid fibroblasts and induces keloid regression, suggesting that modulation of VEGF production could be a valuable treatment approach for keloids.
HIF-1α and hypoxia
Another pathway is HIF-1α, the essential sensor and mediator of hypoxia in the cell. HIF-1α has been implicated in multiple aspects of keloid pathogenesis (Qiu et al, 2023). A study by Kang et al (2020) found that keloids expressed higher levels of HIF-1α than normal skin tissue. Targeting HIF-1α with specific inhibitors such as CAY10585 significantly reduced collagen levels, suggesting a novel treatment avenue for keloids by modulating the hypoxic microenvironment and HIF-1α activity (Kang et al, 2020). Furthermore, HIF-1α expression during hypoxia significantly increases proliferation and inhibits the apoptosis of keloid fibroblasts but not normal skin fibroblasts (Kang et al, 2020; Lei et al, 2019). This effect is likely a result of HIF-1α activating the TGF-β1/SMAD pathway, resulting in increased collagen deposition (Qiu et al, 2023).
Oxidative metabolism within keloidal cells is another significant pathway likely affected by HIF-1α. When compared with normal skin tissue, the local hypoxic keloidal tissue has been shown to induce HIF-1α, resulting in the upregulation of adenosine triphosphate (ATP) synthesis through glycolysis and downregulation of mitochondrial ATP synthesis (Vincent et al, 2008; Wang et al, 2021). This points to a likely intrinsic molecular reprogramming in keloids, resulting in upregulation of the glycolytic pathways and downregulation of the oxygen-dependent oxidative phosphorylation pathway. A similar effect is observed in malignant neoplasms, called the Warburg effect (Qiu et al, 2023).
Resveratrol, known for its antiproliferative effects, was studied for its ability to inhibit proliferation and promote apoptosis of keloid fibroblasts under hypoxic conditions by targeting HIF-1α. Si et al (2020) demonstrated that resveratrol could reverse the effects of hypoxia on keloids through the downregulation of HIF-1α, also affecting collagen synthesis in keloid fibroblasts. These findings support the potential of resveratrol in treating keloids by inhibiting cell proliferation and promoting apoptosis through the modulation of HIF-1α (Si et al, 2020).
TGFβ
The TGFβ/SMAD signaling pathway is one of the best known orchestrators of scar formation. TGFβ is a cytokine ubiquitously expressed during wound healing and can be produced by damaged tissues, fibroblasts, and M2 macrophages (Zhang et al, 2020). Upon binding to its cognate receptor, the intracellular signaling cascade activates SMAD proteins, which in turn induces endothelial–mesenchymal transition, facilitating the production of myofibroblasts and subsequent type I collagen (Ghosh et al, 2000; Macarak et al, 2021).
TGFβ signaling has been shown to be a key modulator of myofibroblast induction arising from keratinocyte–fibroblast interactions (Shephard et al, 2004). Aberrant signaling of the TGFβ/SMAD pathway can result in collagen overproduction and subsequent uncontrolled scar tissue deposition (Zhang et al, 2020).
TGFβ protein expression (particularly TGFβ1 and β2) is elevated in keloid tissue relative to that in normal skin (Lee et al, 1999). For this reason, TGFβ has sparked great interest as a potential therapeutic target for keloid disease (Zhang et al, 2020). TGFβ1 antisense oligonucleotide treatment significantly downregulated MMP9 secretion in keloid-derived fibroblasts, suggesting a potential therapeutic opportunity (Sadick et al, 2008). Moreover, treatment with SB-431542, a specific inhibitor of TGFβ1 receptor kinase activity, efficiently suppressed TGFβ-induced contraction of collagen gels by keloid fibroblasts, highlighting its therapeutic potential for excessive skin contraction observed in keloids (Hasegawa et al, 2005). Finally, the use of thalidomide has also been shown to inhibit fibronectin production in TGFβ1-treated normal and keloid-derived fibroblasts through the inhibition of the p38/SMAD3 pathway, suggesting another possible therapeutic approach for keloids (Liang et al, 2013). However, it is essential to note the historical association of thalidomide and severe teratogenicity (Franks et al, 2004) when considering this agent.
Studies have not found significant differences in systemic serum TGFβ1 levels between patients with keloid and controls (Bayat et al, 2003). Furthermore, no differences have been observed in the frequency of common TGFB1 gene sequence variants between patients with keloid and controls (Bayat et al, 2003; Tu et al, 2017). It stands to reason that other external pathways may interact with keloidal tissue locally by inducing the TGFβ/SMAD pathway, resulting in uncontrolled tissue proliferation.
NOTCH signaling
The NOTCH signaling pathway has also been implicated in scar tissue formation. NOTCH is a highly conserved cell–cell communication pathway involving a receptor–ligand interaction (Condorelli et al, 2021). This pathway plays a vital role in maintaining skin homeostasis by regulating keratinocyte proliferation and differentiation (Condorelli et al, 2021). Differences between specific NOTCH ligand–receptor structures have also been observed. NOTCH1 has been found to reduce the proliferative rate of stem cells, whereas NOTCH2 and NOTCH3 induce keratinocyte terminal differentiation (Negri et al, 2019).
The NOTCH pathway has been shown to activate profibrotic processes within multiple organs, including lung, kidney, liver, skin, and heart, through direct gene activation and/or through indirect interaction with other signaling pathways such as TGFβ, NF-κB, β-catenin, and various regulatory miRNAs (Hu and Phan, 2016). Although the specific role of NOTCH in keloid development is not fully understood, a study by Syed and Bayat (2012) found significantly elevated NOTCH receptors in keloid tissue compared with that in normal skin. NOTCH activation also appears to correlate positively with the degree of inflammation (Syed and Bayat, 2012). Furthermore, NOTCH signaling blockade combined with a γ-secretase inhibitor and JAG1 depletion with siRNA impaired cell spreading, attachment, and proliferation of keloid fibroblasts (Syed and Bayat, 2012). NOTCH hyperactivation has also been observed in HTSs and is known to play a role in scar inflammation and hyperplasia (Condorelli et al, 2021).
Jak/STAT pathway
The JAK–STAT is another pathway that has been studied in relation to keloid pathogenesis. The Jak–STAT pathway is an evolutionarily conserved cell signaling pathway that mediates cell cycle and homeostatic processes across various tissues (Harrison, 2012). Briefly, binding of a ligand to the Jak–STAT receptor induces dimerization of intracellular Jak proteins, leading to dissociation of STAT proteins, which then translocate to the cell nucleus and induce gene expression (Zeidler et al, 2000).
In the context of keloids, most studies indicate enhanced expression of STAT3 in keloid tissue and keloid fibroblasts (Yin et al, 2023). Upregulation of Jak1 (Ghazizadeh et al, 2007) and Jak2 (Lim et al, 2006) has also been observed in keloid tissue. This pathway has been shown to induce TGFβ gene expression (Lee et al, 2019) leading to fibrosis (Liu et al, 2014; Tang et al, 2017). Upstream activators of Jak–STAT in keloids have also been studied. IL-6, a cytokine known to be elevated in keloid tissue, has been poised as a likely inducer of STAT3 (Yin et al, 2023). STAT inhibitors have shown the suppression of cell proliferation and ECM production in keloidal fibroblasts in vitro (Hong et al, 2022), shedding light on the potential of Jak/STAT inhibitors as a therapeutic option for keloids (Yin et al, 2023).
Conclusion
As demonstrated by the wide breadth of studies performed during recent years, keloids have a complex pathophysiology encompassing a broad range of elements, including genetic, endocrine, epigenetic, immune, and cell-signaling dysregulation. Keloids represent a complex biological ecosystem characterized by unconstrained growth, alterations in its immunological milieu, and changes in cellular signaling and metabolism. Contrary to cancer, keloids have not been shown to have the capacity to metastasize or to pose a direct threat to patient survival. Nevertheless, the disfiguration and debilitating symptoms associated with keloids can be very detrimental to a patient's QOL.
Although still an area of active research, genetics likely plays an important role in determining the likelihood of keloid formation across ethnic groups. However, the precise genetic landscape modulating keloid susceptibility among various populations is unknown. Future research avenues should include comprehensive population studies that include a wider variety of ethnic groups. In addition, we have only recently developed techniques to effectively profile and better understand associated epigenetic elements such as ncRNA and transposons. Together, these approaches will ideally allow us to better characterize the molecular underpinnings of keloid predisposition; progression; and, possibly, pharmacological response. Finally, it is critical to contextualize keloids vis à vis other fibrotic processes, such as HTSs.
Regarding therapies, there are currently no systemic treatments that can eradicate large-volume keloids. However, a broad array of options is currently available for localized disease control. Treatment options are typically based on disease severity, with initial approaches including a combination of silicone gel sheets, compression therapies, topical and/or intralesional steroids, cryotherapy, and laser. More aggressive treatments include radiation, intralesional chemotherapeutics (fluorouracil [5-FU]), and surgical approaches (Ogawa, 2022).
These treatment approaches prevent further proliferation of scar tissue cells through 5 primary mechanisms: cutting the blood supply to scar tissue (compression therapies), reducing inflammation (corticosteroids), DNA damage of highly proliferative cells (chemotherapy, radiation), direct destruction (cryotherapy), or removal (surgery). Often, monotherapy is insufficient, with patients typically necessitating a combination approach to achieve adequate disease control and mitigation.
Our increasing understanding of keloid pathophysiology allows us to conceptualize potential new therapies that could be more effective than the ones currently available. Promising treatment targets include the RAS, androgens, vitamin D, IL-13, HIF-1α, TGFβ1, and Jak–STAT. These pathways are already targeted for other disease processes through commercially available medications. Additional experimental targets include different cytokine inhibitors, VEGF inhibitors, and NOTCH1 inhibitors. Anticollagenic agents have also been proposed as a potential treatment approach (Rajadurai and Tsao, 2023).
Ethics Statement
No human or mice studies were involved.
ORCIDs
David I. Latoni: http://orcid.org/0000-0001-6104-7601
Danica C. McDaniel: http://orcid.org/0009-0001-8330-0425
Hensin Tsao: http://orcid.org/0000-0002-2204-2071
Sandy S. Tsao: http://orcid.org/0009-0000-2154-7265
Conflict of Interest
The authors state no conflict of interest
Acknowledgments
H.T. was funded by Richard Allen Johnson, MD, Endowed Chair in Dermatology and the generous donors at Massachusetts General Hospital (HT)
Author Contributions
Conceptualization: DIL, HT, ST; Resources: HT, SST; Supervision: HT, SST; Writing – Original Draft Preparation: DIL, DCM, HT, SST; Writing - Review and Editing: DIL, DCM, HT, SST
Declaration of Generative Artificial Intelligence (AI) or Large Language Models (LLMs)
The author(s) did not use AI/LLM in any part of the research process and/or manuscript preparation.
corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2024.100299
References
- Abdu Allah A.M.K., Mohammed K.I., Farag A.G.A., Hagag M.M., Essam M., Tayel N.R. Interleukin-6 serum level and gene polymorphism in keloid patients. Cell Mol Biol (Noisy-le-grand) 2019;65:43–48. [PubMed] [Google Scholar]
- Akoh C.C., Orlow S.J. A review of vitamin D and scarring: the potential for new therapeutics. J Drugs Dermatol. 2020;19:742–745. doi: 10.36849/JDD.2020.4986. [DOI] [PubMed] [Google Scholar]
- Almeida A., Kokalj-Vokac N., Lefrancois D., Viegas-Pequignot E., Jeanpierre M., Dutrillaux B., et al. Hypomethylation of classical satellite DNA and chromosome instability in lymphoblastoid cell lines. Hum Genet. 1993;91:538–546. doi: 10.1007/BF00205077. [DOI] [PubMed] [Google Scholar]
- Bayat A., Bock O., Mrowietz U., Ollier W.E., Ferguson M.W. Genetic susceptibility to keloid disease and hypertrophic scarring: transforming growth factor beta1 common polymorphisms and plasma levels. Plast Reconstr Surg. 2003;111:535–544. doi: 10.1097/01.PRS.0000041536.02524.A3. [DOI] [PubMed] [Google Scholar]
- Bijlard E., Kouwenberg C.A., Timman R., Hovius S.E., Busschbach J.J., Mureau M.A. Burden of keloid disease: a cross-sectional health-related quality of life assessment. Acta Derm Venereol. 2017;97:225–229. doi: 10.2340/00015555-2498. [DOI] [PubMed] [Google Scholar]
- Brown J.J., Ollier W.E., Thomson W., Bayat A. Positive association of HLA-DRB1∗15 with keloid disease in Caucasians. Int J Immunogenet. 2008;35:303–307. doi: 10.1111/j.1744-313X.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- Butzelaar L., Schooneman D.P., Soykan E.A., Talhout W., Ulrich M.M., van den Broek L.J., et al. Inhibited early immunologic response is associated with hypertrophic scarring. Exp Dermatol. 2016;25:797–804. doi: 10.1111/exd.13100. [DOI] [PubMed] [Google Scholar]
- Butzelaar L., Soykan E.A., Galindo Garre F., Beelen R.H., Ulrich M.M., Niessen F.B., et al. Going into surgery: risk factors for hypertrophic scarring. Wound Repair Regen. 2015;23:531–537. doi: 10.1111/wrr.12302. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Chen S., Saeed A.F.U.H., Liu Q., Jiang Q., Xu H., Xiao G.G., et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Fu X., Sun X., Sun T., Zhao Z., Sheng Z. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003;113:208–216. doi: 10.1016/s0022-4804(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Chung S., Nakashima M., Zembutsu H., Nakamura Y. Possible involvement of NEDD4 in keloid formation; its critical role in fibroblast proliferation and collagen production. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:563–573. doi: 10.2183/pjab.87.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli A.G., El Hachem M., Zambruno G., Nystrom A., Candi E., Castiglia D. Notch-ing up knowledge on molecular mechanisms of skin fibrosis: focus on the multifaceted Notch signalling pathway. J Biomed Sci. 2021;28:36. doi: 10.1186/s12929-021-00732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R., Batzer M.A. The impact of retrotransposons on human genome evolution. Nat Rev Genet. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha Colombo Tiveron L.R., da Silva I.R., da Silva M.V., Peixoto A.B., Rodrigues D.B.R., Rodrigues V., Jr. High in situ mRNA levels of IL-22, TFG-β, and ARG-1 in keloid scars. Immunobiology. 2018;223:812–817. doi: 10.1016/j.imbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Deng C.C., Hu Y.F., Zhu D.H., Cheng Q., Gu J.J., Feng Q.L., et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat Commun. 2021;12:3709. doi: 10.1038/s41467-021-24110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H.P., Desmoulière A., Diegelmann R.F., Cohen I.K., Compton C.C., Garner W.L., et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–113. [PMC free article] [PubMed] [Google Scholar]
- Farag A.G.A., Khaled H.N., Hammam M.A., Elshaib M.E., Tayel N.R., Hommos S.E.I., et al. Neuronal precursor cell expressed developmentally down regulated 4 (NEDD4) gene polymorphism contributes to keloid development in Egyptian population. Clin Cosmet Investig Dermatol. 2020;13:649–656. doi: 10.2147/CCID.S253603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald O’Connor E.J., Badshah I.I., Addae L.Y., Kundasamy P., Thanabalasingam S., Abioye D., et al. Histone deacetylase 2 is upregulated in normal and keloid scars. J Invest Dermatol. 2012;132:1293–1296. doi: 10.1038/jid.2011.432. [DOI] [PubMed] [Google Scholar]
- Ford L.C., King D.F., Lagasse L.D., Newcomer V. Increased androgen binding in keloids: a preliminary communication. J Dermatol Surg Oncol. 1983;9:545–547. doi: 10.1111/j.1524-4725.1983.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Franks M.E., Macpherson G.R., Figg W.D. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- Fujita M., Yamamoto Y., Jiang J.J., Atsumi T., Tanaka Y., Ohki T., et al. NEDD4 is involved in inflammation development during keloid formation. J Invest Dermatol. 2019;139:333–341. doi: 10.1016/j.jid.2018.07.044. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh M., Tosa M., Shimizu H., Hyakusoku H., Kawanami O. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105. doi: 10.1038/sj.jid.5700564. [DOI] [PubMed] [Google Scholar]
- Ghosh A.K., Yuan W., Mori Y., Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- Hahn J.M., Supp D.M. Abnormal expression of the vitamin D receptor in keloid scars. Burns. 2017;43:1506–1515. doi: 10.1016/j.burns.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Han J., Han J., Yu D., Xiao J., Shang Y., Hao L. Association of ADAM33 gene polymorphisms with keloid scars in a Northeastern Chinese population. Cell Physiol Biochem. 2014;34:981–987. doi: 10.1159/000366314. [DOI] [PubMed] [Google Scholar]
- Harrison D.A. The Jak/STAT pathway. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T., Nakao A., Sumiyoshi K., Tsuchihashi H., Ogawa H. SB-431542 inhibits TGF-beta-induced contraction of collagen gel by normal and keloid fibroblasts. J Dermatol Sci. 2005;39:33–38. doi: 10.1016/j.jdermsci.2005.01.013. [DOI] [PubMed] [Google Scholar]
- He J., Huang B., Zhang K., Liu M., Xu T. Long non-coding RNA in cervical cancer: from biology to therapeutic opportunity. Biomed Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110209. [DOI] [PubMed] [Google Scholar]
- Hedayatyanfard K., Haddadi N.S., Ziai S.A., Karim H., Niazi F., Steckelings U.M., et al. The renin-angiotensin system in cutaneous hypertrophic scar and keloid formation. Exp Dermatol. 2020;29:902–909. doi: 10.1111/exd.14154. [DOI] [PubMed] [Google Scholar]
- Hong Y.K., Wu C.H., Lin Y.C., Huang Y.L., Hung K.S., Pai T.P., et al. ASC-J9 blocks cell proliferation and extracellular matrix production of keloid fibroblasts through inhibiting STAT3 signaling. Int J Mol Sci. 2022;23:5549. doi: 10.3390/ijms23105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Phan S.H. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res. 2016;108:57–64. doi: 10.1016/j.phrs.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Ogawa R. Systemic factors that shape cutaneous pathological scarring. FASEB J. 2020;34:13171–13184. doi: 10.1096/fj.202001157R. [DOI] [PubMed] [Google Scholar]
- Huang C., Ogawa R. Role of Inflammasomes in keloids and hypertrophic scars-lessons learned from chronic diabetic wounds and skin fibrosis. Int J Mol Sci. 2022;23:6820. doi: 10.3390/ijms23126820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.R., Young W., Divine G., Datta I., Chen K.M., Ozog D., et al. Genome-wide scan for methylation profiles in keloids. Dis Markers. 2015;2015 doi: 10.1155/2015/943176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Roh M.R., Rajadurai S., Rajadurai A., Kumar R., Njauw C.-N., et al. Hypoxia and HIF-1α regulate collagen production in keloids. J Invest Dermatol. 2020;140:2157–2165. doi: 10.1016/j.jid.2020.01.036. [DOI] [PubMed] [Google Scholar]
- Khattab F.M., Samir M.A. Correlation between serum IL 37 levels with keloid severity. J Cosmet Dermatol. 2020;19:2428–2431. doi: 10.1111/jocd.13290. [DOI] [PubMed] [Google Scholar]
- Kojima S., Koyama S., Ka M., Saito Y., Parrish E.H., Endo M., et al. Mobile element variation contributes to population-specific genome diversification, gene regulation and disease risk. Nat Genet. 2023;55:939–951. doi: 10.1038/s41588-023-01390-2. [DOI] [PubMed] [Google Scholar]
- Lee C.G., Homer R.J., Zhu Z., Lanone S., Wang X., Koteliansky V., et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.J., Yuan X., Kerr K., Yoo J.Y., Kim D.H., Kaur B., et al. Strategies to modulate microRNA functions for the treatment of cancer or organ injury. Pharmacol Rev. 2020;72:639–667. doi: 10.1124/pr.119.019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.Y., Chin G.S., Kim W.J., Chau D., Gittes G.K., Longaker M.T. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–184. [PubMed] [Google Scholar]
- Lee Y.S., Liang Y.C., Wu P., Kulber D.A., Tanabe K., Chuong C.M., et al. STAT3 signalling pathway is implicated in keloid pathogenesis by preliminary transcriptome and open chromatin analyses. Exp Dermatol. 2019;28:480–484. doi: 10.1111/exd.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei R., Li J., Liu F., Li W., Zhang S., Wang Y., et al. HIF-1α promotes the keloid development through the activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell Cycle. 2019;18:3239–3250. doi: 10.1080/15384101.2019.1670508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu H., Xu C., Deng M., Song M., Yu X., et al. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res Ther. 2017;8:237. doi: 10.1186/s13287-017-0684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.J., Yen Y.H., Hung L.Y., Wang S.H., Pu C.M., Chien H.F., et al. Thalidomide inhibits fibronectin production in TGF-β1-treated normal and keloid fibroblasts via inhibition of the p38/Smad3 pathway. Biochem Pharmacol. 2013;85:1594–1602. doi: 10.1016/j.bcp.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Lim C.P., Phan T.T., Lim I.J., Cao X. Stat3 contributes to keloid pathogenesis via promoting collagen production, cell proliferation and migration. Oncogene. 2006;25:5416–5425. doi: 10.1038/sj.onc.1209531. [DOI] [PubMed] [Google Scholar]
- Limandjaja G.C., Niessen F.B., Scheper R.J., Gibbs S. The keloid disorder: heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. 2020;8:360. doi: 10.3389/fcell.2020.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cai L., Zhang Z., Ma Y., Wang Y. Association of leptin receptor gene polymorphisms with keloids in the Chinese Han population. Med Sci Monit. 2021;27 doi: 10.12659/MSM.928503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.Y., Zeng Y., Lei Z., Wang L., Yang H., Liu Z., et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44:1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- Liu S., Yang H., Song J., Zhang Y., Abualhssain A.T.H., Yang B. Keloid: genetic susceptibility and contributions of genetics and epigenetics to its pathogenesis. Exp Dermatol. 2022;31:1665–1675. doi: 10.1111/exd.14671. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xu S., Zu T., Li F., Sang S., Liu C., et al. Reversal of TET-mediated 5-hmC loss in hypoxic fibroblasts by ascorbic acid. Lab Invest. 2019;99:1193–1202. doi: 10.1038/s41374-019-0235-8. [DOI] [PubMed] [Google Scholar]
- Lu W.S., Wang J.F., Yang S., Xiao F.L., Quan C., Cheng H., et al. Association of HLA-DQA1 and DQB1 alleles with keloids in Chinese Hans. J Dermatol Sci. 2008;52:108–117. doi: 10.1016/j.jdermsci.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Lv W., Ren Y., Hou K., Hu W., Yi Y., Xiong M., et al. Epigenetic modification mechanisms involved in keloid: current status and prospect. Clin Epigenetics. 2020;12:183. doi: 10.1186/s13148-020-00981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarak E.J., Wermuth P.J., Rosenbloom J., Uitto J. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol. 2021;30:132–145. doi: 10.1111/exd.14243. [DOI] [PubMed] [Google Scholar]
- Mamdouh M., Omar G.A., Hafiz H.S.A., Ali S.M. Role of vitamin D in treatment of keloid. J Cosmet Dermatol. 2022;21:331–336. doi: 10.1111/jocd.14070. [DOI] [PubMed] [Google Scholar]
- Marneros A.G., Norris J.E., Olsen B.R., Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol. 2001;137:1429–1434. doi: 10.1001/archderm.137.11.1429. [DOI] [PubMed] [Google Scholar]
- Marneros A.G., Norris J.E., Watanabe S., Reichenberger E., Olsen B.R. Genome scans provide evidence for keloid susceptibility loci on chromosomes 2q23 and 7p11. J Invest Dermatol. 2004;122:1126–1132. doi: 10.1111/j.0022-202X.2004.22327.x. [DOI] [PubMed] [Google Scholar]
- McCauley R.L., Chopra V., Li Y.Y., Herndon D.N., Robson M.C. Altered cytokine production in black patients with keloids. J Clin Immunol. 1992;12:300–308. doi: 10.1007/BF00918154. [DOI] [PubMed] [Google Scholar]
- McLoughlin R.M., Hurst S.M., Nowell M.A., Harris D.A., Horiuchi S., Morgan L.W., et al. Differential regulation of neutrophil-activating chemokines by IL-6 and its soluble receptor isoforms. J Immunol. 2004;172:5676–5683. doi: 10.4049/jimmunol.172.9.5676. [DOI] [PubMed] [Google Scholar]
- Milani D., Manzoni F.M., Pezzani L., Ajmone P., Gervasini C., Menni F., et al. Rubinstein-Taybi syndrome: clinical features, genetic basis, diagnosis, and management. Ital J Pediatr. 2015;41:4. doi: 10.1186/s13052-015-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min M.S., Mazori D.R., Lee M.S., Merola J.F., Vleugels R.A., Cobos G., et al. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J Drugs Dermatol. 2023;22:1220–1222. doi: 10.36849/JDD.6385. [DOI] [PubMed] [Google Scholar]
- Mogili N.S., Krishnaswamy V.R., Jayaraman M., Rajaram R., Venkatraman A., Korrapati P.S. Altered angiogenic balance in keloids: a key to therapeutic intervention. Transl Res. 2012;159:182–189. doi: 10.1016/j.trsl.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Nakashima M., Chung S., Takahashi A., Kamatani N., Kawaguchi T., Tsunoda T., et al. A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genet. 2010;42:768–771. doi: 10.1038/ng.645. [DOI] [PubMed] [Google Scholar]
- Negri V.A., Logtenberg M.E.W., Renz L.M., Oules B., Walko G., Watt F.M. Delta-like 1-mediated cis-inhibition of Jagged1/2 signalling inhibits differentiation of human epidermal cells in culture. Sci Rep. 2019;9 doi: 10.1038/s41598-019-47232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noishiki C., Hayasaka Y., Ogawa R. Sex differences in Keloidogenesis: an analysis of 1659 keloid patients in Japan. Dermatol Ther (Heidelb) 2019;9:747–754. doi: 10.1007/s13555-019-00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold M.F., Nold-Petry C.A., Zepp J.A., Palmer B.E., Bufler P., Dinarello C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: A 2020 update of the algorithms published 10 years ago. Plast Reconstr Surg. 2022;149:79e–94e. doi: 10.1097/PRS.0000000000008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R., Okai K., Tokumura F., Mori K., Ohmori Y., Huang C., et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157. doi: 10.1111/j.1524-475X.2012.00766.x. [DOI] [PubMed] [Google Scholar]
- Oriente A., Fedarko N.S., Pacocha S.E., Huang S.K., Lichtenstein L.M., Essayan D.M. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292:988–994. [PubMed] [Google Scholar]
- Qiu Z.K., Zhang M.Z., Zhang W.C., Li Z.J., Si L.B., Long X., et al. Role of HIF-1α in pathogenic mechanisms of keloids. J Cosmet Dermatol. 2023;22:1436–1448. doi: 10.1111/jocd.15601. [DOI] [PubMed] [Google Scholar]
- Quong W.L., Kozai Y., Ogawa R. A case of keloids complicated by Castleman’s disease: interleukin-6 as a keloid risk factor. Plast Reconstr Surg Glob Open. 2017;5 doi: 10.1097/GOX.0000000000001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadurai A., Tsao H. Identification of collagen-suppressive agents in keloidal fibroblasts using a high-content, phenotype-based drug screen. JID Innov. 2023;4 doi: 10.1016/j.xjidi.2023.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J., Frigola J., Vendrell E., Risques R.A., Fraga M.F., Morales C., et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res. 2006;66:8462–9468. doi: 10.1158/0008-5472.CAN-06-0293. [DOI] [PubMed] [Google Scholar]
- Roelfsema J.H., Peters D.J. Rubinstein-Taybi syndrome: clinical and molecular overview. Expert Rev Mol Med. 2007;9:1–16. doi: 10.1017/S1462399407000415. [DOI] [PubMed] [Google Scholar]
- Roh M.R., Kumar R., Rajadurai A., Njauw C., Ryoo U.H., Chung K.Y., et al. Beta-catenin causes fibrotic changes in the extracellular matrix via upregulation of collagen I transcription. Br J Dermatol. 2017;177:312–315. doi: 10.1111/bjd.15079. [DOI] [PubMed] [Google Scholar]
- Sadick H., Herberger A., Riedel K., Bran G., Goessler U., Hoermann K., et al. TGF-beta1 antisense therapy modulates expression of matrix metalloproteinases in keloid-derived fibroblasts. Int J Mol Med. 2008;22:55–60. doi: 10.3892/ijmm.22.1.55. [DOI] [PubMed] [Google Scholar]
- Santos-Cortez R.L.P., Hu Y., Sun F., Benahmed-Miniuk F., Tao J., Kanaujiya J.K., et al. Identification of ASAH1 as a susceptibility gene for familial keloids. Eur J Hum Genet. 2017;25:1155–1161. doi: 10.1038/ejhg.2017.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard P., Martin G., Smola-Hess S., Brunner G., Krieg T., Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih B., Bayat A. Comparative genomic hybridisation analysis of keloid tissue in Caucasians suggests possible involvement of HLA-DRB5 in disease pathogenesis. Arch Dermatol Res. 2012;304:241–249. doi: 10.1007/s00403-011-1182-4. [DOI] [PubMed] [Google Scholar]
- Si L., Zhang M., Guan E., Han Q., Liu Y., Long X., et al. Resveratrol inhibits proliferation and promotes apoptosis of keloid fibroblasts by targeting HIF-1α. J Plast Surg Hand Surg. 2020;54:290–296. doi: 10.1080/2000656X.2020.1771719. [DOI] [PubMed] [Google Scholar]
- Silva I.M.S., Assersen K.B., Willadsen N.N., Jepsen J., Artuc M., Steckelings U.M. The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp Dermatol. 2020;29:891–901. doi: 10.1111/exd.14159. [DOI] [PubMed] [Google Scholar]
- Smith J.C., Boone B.E., Opalenik S.R., Williams S.M., Russell S.B. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol. 2008;128:1298–1310. doi: 10.1038/sj.jid.5701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson A.W., Deng Z., Allahham A., Prêle C.M., Wood F.M., Fear M.W. The epigenetics of keloids. Exp Dermatol. 2021;30:1099–1114. doi: 10.1111/exd.14414. [DOI] [PubMed] [Google Scholar]
- Su L., Han J. Non-coding RNAs in hypertrophic scars and keloids: current research and clinical relevance: a review. Int J Biol Macromol. 2024;256 doi: 10.1016/j.ijbiomac.2023.128334. [DOI] [PubMed] [Google Scholar]
- Sudha D.S., Srinivaasan M., Kalesh S., Krishna G. A study on molecular biology and pathology of keloid. J Med Sci Clin Res. 2018;6 [Google Scholar]
- Syed F., Bayat A. Notch signaling pathway in keloid disease: enhanced fibroblast activity in a Jagged-1 peptide-dependent manner in lesional vs. extralesional fibroblasts. Wound Repair Regen. 2012;20:688–706. doi: 10.1111/j.1524-475X.2012.00823.x. [DOI] [PubMed] [Google Scholar]
- Tan S., Khumalo N., Bayat A. Understanding keloid pathobiology from a quasi-neoplastic perspective: less of a scar and more of a chronic inflammatory disease with cancer-like tendencies. Front Immunol. 2019;10:1810. doi: 10.3389/fimmu.2019.01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Umeyama Y., Hagiwara H., Ito-Hirano R., Fujimura S., Mizuno H., et al. Keloid patients have higher peripheral blood endothelial progenitor cell counts and CD34+ cells with normal vasculogenic and angiogenic function that overexpress vascular endothelial growth factor and interleukin-8. Int J Dermatol. 2019;58:1398–1405. doi: 10.1111/ijd.14575. [DOI] [PubMed] [Google Scholar]
- Tang L.Y., Heller M., Meng Z., Yu L.R., Tang Y., Zhou M., et al. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J Biol Chem. 2017;292:4302–4312. doi: 10.1074/jbc.M116.773085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng G., Liu C., Chen M., Ma K., Liang L., Yan T. Differential susceptible loci expression in keloid and hypertrophic scars in the Chinese Han population. Ann Plast Surg. 2015;74:26–29. doi: 10.1097/SAP.0000000000000364. [DOI] [PubMed] [Google Scholar]
- Tu Y., Lineaweaver W.C., Zhang F. TGF-β1 -509C/T polymorphism and susceptibility to keloid disease: a systematic review and meta-analysis. Scars Burn Heal. 2017;3 doi: 10.1177/2059513117709943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulandi T., Al-Sannan B., Akbar G., Ziegler C., Miner L. Prospective study of intraabdominal adhesions among women of different races with or without keloids. Am J Obstet Gynecol. 2011;204:132.e1–132.e4. doi: 10.1016/j.ajog.2010.09.005. [DOI] [PubMed] [Google Scholar]
- van de Kar A.L., Houge G., Shaw A.C., de Jong D., van Belzen M.J., Peters D.J., et al. Keloids in Rubinstein-Taybi syndrome: a clinical study. Br J Dermatol. 2014;171:615–621. doi: 10.1111/bjd.13124. [DOI] [PubMed] [Google Scholar]
- Velez Edwards D.R., Tsosie K.S., Williams S.M., Edwards T.L., Russell S.B. Admixture mapping identifies a locus at 15q21.2-22.3 associated with keloid formation in African Americans. Hum Genet. 2014;133:1513–1523. doi: 10.1007/s00439-014-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A.S., Phan T.T., Mukhopadhyay A., Lim H.Y., Halliwell B., Wong K.P. Human skin keloid fibroblasts display bioenergetics of cancer cells. J Invest Dermatol. 2008;128:702–709. doi: 10.1038/sj.jid.5701107. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Dudzinski J., Nguyen D., Armbrecht E., Maher I.A. Association of smoking and other factors with the outcome of mohs reconstruction using flaps or grafts. JAMA Facial Plast Surg. 2019;21:407–413. doi: 10.1001/jamafacial.2019.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang P., Qin Z., Yang X., Pan B., Nie F., et al. Altered glucose metabolism and cell function in keloid fibroblasts under hypoxia. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.J.S., Song E.J. Dupilumab as an adjuvant treatment for keloid-associated symptoms. JAAD Case Rep. 2021;13:73–74. doi: 10.1016/j.jdcr.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.S., Wang F.S., Yang K.D., Huang C.C., Kuo Y.R. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. 2006;126:1264–1271. doi: 10.1038/sj.jid.5700274. [DOI] [PubMed] [Google Scholar]
- Xu X., Gu S., Huang X., Ren J., Gu Y., Wei C., et al. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010;17:68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Chen Z., Wu X., Liu W., Gao Z. Androgen-related disorders and hormone therapy for patients with keloids. Chin J Plast Reconstr Surg. 2022;4:44–48. [Google Scholar]
- Yin Q., Wolkerstorfer A., Lapid O., Niessen F.B., Van Zuijlen P.P.M., Gibbs S. The JAK-STAT pathway in keloid pathogenesis: a systematic review with qualitative synthesis. Exp Dermatol. 2023;32:588–598. doi: 10.1111/exd.14747. [DOI] [PubMed] [Google Scholar]
- Young W.G., Worsham M.J., Joseph C.L., Divine G.W., Jones L.R. Incidence of keloid and risk factors following head and neck surgery. JAMA Facial Plast Surgχ. 2014;16:379–380. doi: 10.1001/jamafacial.2014.113. [DOI] [PubMed] [Google Scholar]
- Yu X., Zhu X., Li L., Gao G. Circular RNAs: emerging players in the pathogenesis of keloid. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.1008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharie R.D., Popa C., Schlanger D., Vălean D., Zaharie F. The role of IL-22 in wound healing. Potential implications in clinical practice. Int J Mol Sci. 2022;23:3693. doi: 10.3390/ijms23073693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler M.P., Bach E.A., Perrimon N. The roles of the Drosophila JAK/STAT pathway. Oncogene. 2000;19:2598–2606. doi: 10.1038/sj.onc.1203482. [DOI] [PubMed] [Google Scholar]
- Zhang G.Y., Wu L.C., Liao T., Chen G., Chen Y.H., Meng X.C., et al. Altered circulating endothelial progenitor cells in patients with keloid. Clin Exp Dermatol. 2016;41:152–155. doi: 10.1111/ced.12695. [DOI] [PubMed] [Google Scholar]
- Zhang G.Y., Yi C.G., Li X., Zheng Y., Niu Z.G., Xia W., et al. Inhibition of vascular endothelial growth factor expression in keloid fibroblasts by vector-mediated vascular endothelial growth factor shRNA: a therapeutic potential strategy for keloid. Arch Dermatol Res. 2008;300:177–184. doi: 10.1007/s00403-007-0825-y. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wang X.F., Wang Z.C., Lou D., Fang Q.Q., Hu Y.Y., et al. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- Zhu Y.O., MacDonnell S., Kaplan T., Liu C., Ali Y., Rangel S.M., et al. Defining a unique gene expression profile in mature and developing keloids. JID Innov. 2023;3 doi: 10.1016/j.xjidi.2023.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]