Abstract
Background
Conventional medicine (CM) for paroxysmal atrial fibrillation (PAF) have limitations and side effects. Integrative approaches, including traditional herbal medicines like Liriope Tuber, are being explored for potential benefits, although evidence remains limited.
Methods
In April 2023, a literature search was conducted across nine databases, focusing on randomized controlled trials assessing the effects of Liriope Tuber in traditional herbal medicine (LTHM) on PAF. The risk of bias was evaluated using Version 2 of the Cochrane risk-of-bias tool for randomized trials. A random-effects model was employed for the meta-analysis.
Results
A total of 43 studies with 3,743 participants were included. The meta-analysis indicated that adding LTHM to CM reduced PAF frequency (SMD = -0.99, 95 % CI = -1.40 to -0.57, I² = 88 %, N = 16, n = 1266), left atrium diameter (LAD) (MD = -2.39 mm, 95 % CI = -3.09 to -1.68), P-wave dispersion (Pd) (MD = -6.41 ms, 95 % CI = -8.44 to -4.37), high sensitive C-Reactive Protein (hs-CRP) (MD = -1.10 mg/l, 95 % CI = -1.73 to -0.47), and improved left ventricular ejection fraction (LVEF) (MD = 4.71 %, 95 % CI = 3.17 to 6.25). Thirty-four studies raised concerns about bias, with eight showing high risk. Certainty of evidence was rated as "low" for PAF frequency, LAD, Pd, hs-CRP, and LVEF.
Conclusion
LTHM combined with CM may reduce PAF frequency. However, due to the complexity of interventions, with Liriope Tuber being only one component of the regimen, high risk of bias, substantial heterogeneity, and indirectness, interpretations should be cautious.
Study registration
PROSPERO (ID: CRD42023477926).
Keywords: Liriope Tuber, Paroxysmal atrial fibrillation, Systematic review, Meta-analysis, East Asian traditional medicine
1. Introduction
Atrial Fibrillation (AF) is a supraventricular tachycardia, the most common type of arrhythmia in adults. It is characterized by irregular and disorganized electrical activity throughout the atria, causing them to quiver instead of contracting uniformly. The global prevalence of AF is increasing. According to the Global Burden of Disease project, in 2016, approximately 46.3 million AF patients were estimated to have AF worldwide.1 In the Asia, it is projected that by 2050, at least 720 million individuals will be diagnosed with AF.2 AF is also a known risk factor for conditions like stroke and heart failure.3, 4, 5 The increasing prevalence of AF and its associated complications imposes a growing burden on public health. Over the past decade, medical expenses related to AF have increased by more than 5.7 times.6
AF can be categorized by its duration: paroxysmal AF (PAF) ends spontaneously or with treatment within 7 days; persistent AF lasts over 7 days; long-standing Persistent AF persists for 12 months or more; and permanent AF occurs when all attempts to restore normal rhythm have been abandoned.7 If PAF is left untreated, it may eventually progress to persistent or permanent AF, making early treatment of PAF particularly important.8 Currently, AF treatment is primarily based on catheter and cryoballoon ablation. However, approximately half of the patients undergoing Catheter Ablation and half of those undergoing Cryoballoon Ablation experience recurrence within 12 months.9 Drug therapy mainly relies on anti-arrhythmic drugs to control heart rate or maintain a normal rhythm. However, in terms of heart rate control, beta-blockers have shown no significant reduction in overall mortality compared to placebo.10 In the context of rhythm control, the use of anti-arrhythmic increases the probability of maintaining a normal rhythm by approximately two-fold compared to not using them. However, these treatments are not effective in reducing mortality or cardiovascular complications. It is important to exercise caution with long-term use because of the potential for increased hospitalization owing to side effects.11, 12, 13, 14, 15, 16
Efforts are underway to overcome the limitations of conventional AF treatments by using evidence-based, integrated, and multidisciplinary approaches. These approaches have been shown to reduce all-cause mortality and hospitalization rates related to cardiovascular diseases.17,18 In East Asia, East Asian traditional medicine (EATM) interventions have been utilized for the treatment of AF.19 Numerous systematic reviews have been published to gather relevant evidence, making this an emerging focus for integrated treatment approaches for AF.20, 21, 22, 23, 24, 25 Furthermore, while systematic literature reviews have previously focused on prescriptions, there is growing interest in conducting systematic reviews of individual herbal remedies.26, 27, 28 In EATM, several herbal remedies are commonly used to treat AF. These include Liriope Tuber, Salvia Miltiorrhiza Root, and Nardostachyos Radix et Rhizoma.29 The most commonly utilized Liriope Tuber (Maidong, 麦冬) is the root of a perennial herb belonging to the Lily family. It is known for its efficacy in nourishing the yin, moistening the lungs, calming the mind, benefiting the stomach, and generating fluids.30 From a pharmacological perspective, it contains compounds such as saponins, flavonoids, and polysaccharides, which offer cardiovascular protection, anti-inflammatory properties, immune regulation, and potential effects against cancer and diabetes.31, 32, 33 However, there is insufficient evidence regarding the safety and efficacy of Liriope Tuber included traditional herbal medicine (LTHM), in patients with AF.
To the best of our knowledge, this is the first systematic review on the effects of LTHM on PAF. This study aimed to confirm the efficacy and safety of LTHM in PAF, identify the factors influencing these effects through subgroup analysis and meta-regression, evaluate the level of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, and visually represent the network of included herbal remedies to understand the relationship between Liriope Tuber and frequently used herbal ingredients.
2. Methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.34 The study protocol is registered in PROSPERO (ID: CRD42023477926).
2.1. Criteria for inclusion and exclusion
2.1.1. Study types
The study design encompassed only Randomized Controlled Trials (RCTs) where the term "randomization" was mentioned. There were no restrictions on the treatment duration or clinical settings.
2.1.2. Participant types
The participants were diagnosed with paroxysmal fibrillation, without age or sex restrictions. Participants with PAF, even when accompanied by other conditions, such as hypertension or heart failure, were also included.
2.1.3. Intervention types and controls
Only studies that combined the EATM herbal medicine intervention (HM) with conventional medicines intervention (CM) were included. There were no restrictions on the forms of HM, such as decoctions, powders, or pills, and only oral herbal preparations, including Liriope Tubers, were included. The control group included studies that used the same interventions as the experimental group. Three or more comparison groups were included if they met the purpose of the study.
2.1.4. Outcomes measures
Studies that only reported the Total Effective Rate (TER) or the efficacy of the Traditional Chinese Medicine (TCM) symptom score were excluded, and only studies with one or more evaluation indicators related to AF were included. The primary outcome was the frequency of paroxysmal atrial fibrillation (PAF Frequency), defined in continuous units such as episodes per day, episodes per week, or episodes per month, according to the criteria of each study. Secondary outcomes included Left Atrial Diameter (LAD), P-wave Dispersion (Pd), high sensitive C-Reactive Protein (hs-CRP), Left Ventricular Ejection Fraction (LVEF), 6 Min Walking Distance (6 MWD), and Quality of Life (The Minnesota Living with Heart Failure Questionnaire, QoL). Adverse events (AEs) were presented as the number of occurrences of each symptom.
2.2. Literature searches
Electronic databases, including PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Excerpta Medica Database (Embase), Oriental Medicine Advanced Searching Integrated System (OASIS), Korea Citation Index (KCI), Cumulative Index of Nursing and Allied Health Literature (CINAHL), China National Knowledge Infrastructure (CNKI), Citation Information by NII (CiNii), and International Clinical Trials Registry Platform (ICTRP) were searched until April 13, 2023, without restrictions on country or language. In addition, a snowball search was conducted to identify further studies by searching the reference lists of publications that were eligible for a full-text review. The search strategy was developed through consultation with a bibliographic expert and consisted of three categories: ‘Atrial Fibrillation’, ‘East Asian Traditional Medicine’, and ‘Randomized Controlled Trials’. These three categories were combined using the AND Boolean operator with the keywords appropriate for each database. The detailed search terms are provided in Supplementary Table 1.
2.3. Study selection
Studies were selected by two independent researchers (SJK and HBJ). In cases of disagreement, the researchers reached mutual agreement through discussion, and when disagreements persisted, the advice of a third researcher (JTL) was sought to make the final decision. The studies identified through literature searches and reference list checking were imported into EndNote, where duplicates were removed and selected according to pre-established criteria. The titles and abstracts of the studies were initially screened to determine their suitability for further consideration. The full texts of the selected studies were then reviewed in depth to ensure their inclusion in the final selection. For studies identified through other methods, a comprehensive review of the full text was conducted to facilitate the final selection process.
2.4. Data extraction
The data extraction process was conducted by two independent researchers (SJK and HBJ), and discrepancies were solved in the manner mentioned above. The analysis framework utilized in this study was developed using Google spreadsheets and through discussions within the research team. Subsequently, a review of existing studies was conducted, and a pilot extraction was performed using five articles to develop the final analysis framework. This framework was then finally applied to the main extraction. The extracted data included information on primary outcomes, secondary outcomes, first author, publication year, type and number of clinical institutions, number of participants, age, sex, type and dosage of intervention, composition of herbal interventions, treatment duration, outcome variables, country of the first author, diagnosis criteria, comorbidity conditions, funding source, AEs, and number of dropouts. The primary and secondary outcomes were extracted in their original form when reporting change data (mean ± standard deviation) for both the experimental and control groups. If the mean and standard deviation were not reported, we obtained the raw data and presented them as mean and standard deviation. Otherwise, the study was excluded. In instances where units of measurement differed, they were converted to a unified unit through discussion within the study team. In cases where there were multiple measurement time points, only measurements taken immediately after the end of treatment were used. If data were missing, they were excluded from the extraction process. If the units of measurement were unclear, the authors were contacted via email for clarification. If we did not receive any response or were unable to establish contact, we made decisions through internal discussions within the research team. Studies for which a consensus could not be reached were excluded from the analysis.
2.5. Risk of bias assessment
The risk of bias assessment was performed according to Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2)35 and conducted by two independent researchers (SJK and HBJ). In cases of disagreement, solved it the same way as above. Five areas were evaluated for potential bias: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in the measurement of the outcome; and (5) bias in the selection of reported results.
2.6. Data analysis
2.6.1. Statistical analysis
The meta-analysis was conducted using RStudio software (version 2023.06.0; R Foundation for Statistical Computing, Vienna, Austria) with the "meta", "dplyr" and "dmetar" packages. For further details, please refer to Supplementary 1.
2.6.2. Heterogeneity
The presence and extent of statistical heterogeneity were identified using RStudio software with the "meta" packages. Heterogeneity was assessed using the chi-square test and Higgins I2 statistic. In the chi-squared test, a significance level of 0.10 was employed. The Higgins I2 statistic was evaluated according to the following criteria: 0 % ≤ I2 ≤ 40 %, "heterogeneity may not be important"; 30 % ≤ I2 ≤ 60 %, "may have moderate heterogeneity"; 50 % ≤ I2 ≤ 90 %, "may be actual heterogeneity"; and 75 % ≤ I2 ≤ 100 %, "significant heterogeneity."35 As I2 was greater than or equal to 50 %, subgroup analyses were conducted based on treatment duration, daily dosage of Liriope Tuber, total dosage of Liriope Tuber, and age to explore factors that may influence treatment effects and heterogeneity. For continuous variables, meta-regression was conducted.
2.6.3. Sensitivity analysis
A leave-one-out Meta-Analysis was conducted to assess the influence of each study on the effect estimates by excluding one study at a time, identifying studies that significantly influenced heterogeneity, and evaluating the robustness of the meta-analysis results when excluding those studies. A cumulative meta-analysis was also performed to evaluate how the average effect estimates and CI changed as studies were added individually over time, assessing the stability of the meta-analysis results over time.
2.7. Publication bias
Publication bias was assessed for outcome variables that included 10 studies in the meta-analysis.36 Publication bias was visually evaluated using funnel plots and statistically assessed using Egger's test, with a significance level of 0.05.37 Contour-enhanced funnel plots were used to assess the relationships between asymmetrical patterns in funnel plots and statistical significance.38 When publication bias was suspected, the trim-and-fill method was used to analyze its impact. In cases where both publication bias was suspected and there was significant heterogeneity (I2 ≥ 50 %), sensitivity analysis was performed by excluding outliers and then applying the trim and fill method. Subsequently, the results of the trim-and-fill method were visually reevaluated using contour-enhanced funnel plots.39
2.8. Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was used to assess the certainty of evidence. For further details, please refer to Supplementary 2.
2.9. Network analysis
The herb network of LTHM was constructed and modularity was analyzed to identify specific patterns or combinations of herbs. The degree, closeness, betweenness, and eigenvector centralities of each node in each module were calculated. For further details, please refer to Supplementary 3.
3. Results
3.1. Study selection
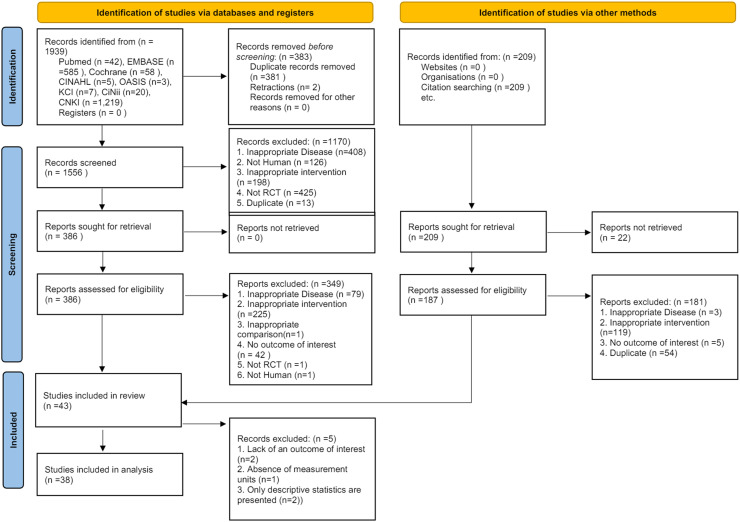
A search of medical research databases initially retrieved 1939 studies. Using the reference management tool ENDNOTE X9, 381 duplicate studies and two withdrawn studies were excluded. The remaining 1556 studies underwent the first round of selection and exclusion based on titles and abstracts, resulting in the exclusion of 1170 studies. The remaining 386 studies underwent a second round of selection and exclusion based on their full texts. This process resulted in the inclusion of 37 studies, with 349 studies excluded. Details of the 349 excluded studies are provided in Supplementary Table 2. In addition to the medical research database search, 209 studies were identified using other methods. After selection and exclusion based on their full texts, six studies met the inclusion criteria via other methods. Among the final 43 studies that employed both methods, five were included in the qualitative analysis only and excluded from the meta-analysis. The study selection process was organized according to the PRISMA flowchart criteria (Fig. 1).
Fig. 1.
PRISMA 2020 flow diagram.
3.2. Study characteristic
The general characteristics of the included studies are summarized in Table 1. All these studies were conducted in China between 2007 and 2022 and were written in Chinese. Studies 1 and 3 were treated as separate studies in the qualitative synthesis and as one study in the quantitative synthesis. This was because they were derived from the same RCTs but published in different papers using different outcomes. The total number of participants were included in the meta-analysis was 3136. In the clinical setting, all studies were conducted at single institutions, except for one multicenter study. One study reported unclear information regarding the clinical settings. PAF frequency was reported in 16 studies, Pd in 10 studies, hs-CRP in 11 studies, LVEF in 7 studies, and LAD in 17 studies. Additionally, 5 studies each reported 6 MWD and QoL. In the case of the three studies initially identified as having incorrect measurement units for hs-CRP,40, 41, 42 we contacted the authors via email for clarification. However, despite our best efforts, we were unable to obtain email addresses. After a thorough internal discussion, the research team ultimately decided to exclude these studies. Additionally, we excluded one study43 that used measurements taken 3 months after treatment for LAD instead of immediately after treatment. Therefore, the meta-analysis included 8 studies for hs-CRP and 16 studies for LAD. The most used prescription in these studies was the Shensong Yangxin Capsule (SSYX), which appeared in 26 studies. The composition and dose of SSYX were obtained from previous studies.44 The names of the prescriptions and details of their compositions and dosages in each study are summarized in Supplementary Table 3. Among the included studies, 20 specified diagnostic criteria and 18 specified comorbidities. Further details can be found in Supplementary Table 4.
Table 1.
Detailed characteristics of included studies.
| Year_ First author |
Clinical setting | Sample size (Initial E:C → Final E:C) |
Mean age (year) |
Sex (M:F) |
Intervention | Treatment period (wk) |
Outcome (Primary, secondary) |
Outcome (Others) |
Funding |
|---|---|---|---|---|---|---|---|---|---|
| 2014_Chen56 | single center (Hospital, IP and OP) |
30 : 32 | Total: 59.3 ± 6.2 | NR | (E) JXPL (6 g*tid) (C) Amiodarone |
12 | None | SRM, NT-proBNP, ALD | Y |
| 2014_He57 | single center (Hospital, IP) |
40 : 40 | (E) 70.5 ± 5.27 (C) 71.61±5.41 |
(E) 20:20 (C) 22:18 |
(E) JXPL (6 g*tid) (C) Amiodarone |
48 | LVEF, LAD | LVIDd, LVST, LVPWT | Y |
| 2014_Chen58 | single center (Hospital, IP and OP) |
30 : 32 | Total: 59.3 ± 6.17 | NR | (E) JXPL (6 g*tid) (C) Amiodarone |
12 | hs-CRP | SRM | Y |
| 2021_Cui59 | single center (Hospital, IP and OP) |
35:35 → 33:32 | (E) 61.91±10.85 (C) 60.38±9.25 |
(E) 18:15 (C) 16:16 |
(E) GZFL (200ml*qd) (C) Warfarin + Metoprolol |
8 | PAF Frequency | IL-6, TNF-α, TCM symptom score, AFEQT score | N |
| 2019_Pan60 | single center (Hospital, IP) |
30 : 30 | (E) 61.1 ± 8.4 (C) 63.0 ± 7.6 |
(E) 17 : 13 (C) 14 : 16 |
(E) SSYX (4C*tid) (C) Amiodarone |
12 | None | Left ventricular systolic GLS, mSRs, mSRe, mSRa, TER | Y |
| 2011_Ma43 | single center (Hospital) |
31 : 30 | (E) 56.83±7.25 (C) 56.42±7.51 |
NR | (E) LTHM (100ml*bid) (C) Amiodarone |
8 | hs-CRP, LAD | SRM | Y |
| 2019_Zhang61 | single center (Hospital) |
23 : 23 → 20 : 20 | (E) 72.55±8.46 (C) 72.85±8.10 |
(E) 9:11 (C) 8:12 |
(E) CGBF (NR) (C) Metoprolol |
8 | PAF Frequency, LAD | DCG, TCM symptom score | N |
| 2022_Wu62 | single center (Hospital, IP) |
22 : 26 | (E) 69.20±9.70 (C) 71.73±8.00 |
(E) 13:9 (C) 13:13 |
(E) CGBF (NR) (C) Metoprolol |
8 | PAF Frequency | DCG, TCM symptom score | Y |
| 2018_Jiao63 | single center (Hospital, IP and OP) |
54 : 54 | (E) 56.9 ± 6.21 (C) 56.7 ± 6.26 |
(E) 26 : 28 (C) 24 : 30 |
(E) JXPL (6 g*tid) (C) Amiodarone |
16 | LAD | MMP-2, TER | NR |
| 2013_Luo64 | single center (Hospital, OP) |
30 : 30 | (E) 62.50±7.19 (C) 63.90±6.59 |
(E) 15 : 15 (C) 17 : 13 |
(E) YXXF (200ml*bid) (C) Usual care |
12 | PAF Frequency, Pd, hs-CRP, LAD | DCG, HRV, Pmax, NT-proBNP, TCM symptom score, TER | N |
| 2021_Ying65 | single center (Hospital) |
63 : 63 | (E) 57.02±5.49 (C) 56.14±5.06 |
(E) 36 : 27 (C) 34 : 29 |
(E) YXDJ(6C*bid) (C) Irbesartan + Amiodarone + Warfarin or Aspirin |
8 | PAF Frequency | SRM, ROS, AOPP, LARV, LACV, LAAEF, Peak-A, TCM symptom score | NR |
| 2018_Pan40 | single center (Hospital, IP) |
36 : 36 | (E) 59.2 ± 7.1 (C) 58.7 ± 6.5 |
(E) 20 : 16 (C) 21 : 15 |
(E) SSYX (4C*tid) (C) Usual care |
12 | hs-CRP, LVEF, LAD | MMP-2, TER | NR |
| 2015_Liu66 | single center (Hospital) |
30 : 30 | Total: 56 | Total: 34 : 26 | (E) LTHM (300ml*qd) (C) Propafenone |
12 | Pd, PAF Frequency | Pmax, TCM symptom score | NR |
| 2017_Chen67 | single center (Hospital) |
30 : 30 | (E) 54.1 ± 7.7 (C) 52.2 ± 6.5 |
(E) 19 : 11 (C) 16 : 14 |
(E) ZGC (NR) (C) Amiodarone |
12 | Pd, hs-CRP | NT-proBNP, ALD, Pmax, TER | Y |
| 2009_Luo68 | single center (Hospital, IP and OP) |
54 : 52 | (E) 58±15 (C) 55±10 |
(E) 41 : 13 (C) 40 : 12 |
(E) LTHM (100ml*tid) (C) Usual care |
12 | PAF Frequency | None | NR |
| 2021_Mu69 | single center (Hospital, IP and OP) |
32 : 32 → 30 : 30 | (E) 45.67±7.28 (C) 46.20±6.47 |
(E) 12 : 18 (C) 16 : 14 |
(E) LTHM (200ml*bid) (C) Usual care |
12 | PAF Frequency, Pd, LAD | HbA1C, TCM symptom score, TER | N |
| 2016_Huang41 | single center (Hospital, IP and OP) |
30 : 30 | (E) 72.46±7.1 (C) 72.96±6.8 |
(E) 16 : 14 (C) 13 : 17 |
(E) LTHM (NR) (C) Usual care |
8 | hs-CRP, LAD, LVEF | IL-6, TNF-α, FS, TCM symptom score, TER | Y |
| 2017_Liu70 | single center (Hospital, IP) |
34 : 34 | (E) 52.37±8.62 (C) 51.24±7.93 |
(E) 19 : 15 (C) 18 : 16 |
(E) LTHM (2c*bid) (C) Metoprolol |
16 | hs-CRP, PAF Frequency, Pd | Pmax, Pmin, TER | NR |
| 2022_Ge71 | single center (Hospital) |
40 : 40 | (E) 55.1 ± 4.0 (C) 55.4 ± 4.2 |
(E) 22 :18 (C) 23 : 17 |
(E) XJHY (3c*tid) (C) Amiodarone |
12 | LAD, LVEF | Recurrence of atrial fibrillation | Y |
| 2015_Fan72 | single center (Hospital, IP) |
56 : 56 | (E) 57.29±4.69 (C) 58.39±5.36 |
(E) 27 : 29 (C) 29 : 27 |
(E) SSYX (3c*tid) (C) Amiodarone |
24 | LAD, LVEF | LVIDd, LVIDs | NR |
| 2012_Jin73 | single center (Hospital) |
24 : 21 : 22 | (E) 68.61±5.12 (C1) 63.94±14.26 (C2) 66.03±14.17 |
(E) 14 : 10 (C1) 12 : 9 (C2) 10 : 12 |
(E) SSYX (3c*tid) (C1) SSYX (C2) Metoprolol |
12 | hs-CRP, Pd, LAD | Pmax | Y |
| 2007_Wu74 | single center (Hospital, IP and OP) |
54 : 58 : 55 | (E) 59±4 (C1) 56±3 (C2) 58±5 |
(E) 30 : 24 (C1) 34 : 24 (C2) 32 : 23 |
(E) SSYX (NR) (C1) SSYX (C2) Metoprolol |
12 | Pd | None | NR |
| 2012_Tang75 | NR | 51 : 51 → 50 : 50 | (E) 75.7 (C) 76.3 |
(E) 24 : 27 (C) 21 : 30 |
(E) SSYX (2∼4c*3∼4/d) (C) Usual care |
16 | PAF Frequency | SRM | NR |
| 2013_Shao76 | single center (Hospital) |
40 : 40 | (E) 67.7 ± 5.2 (C) 68.1 ± 5.7 |
(E) 23 : 17 (C) 22 : 18 |
(E) SSYX (3c*tid) (C) Rosuvastatin |
8 | PAF Frequency | TER | NR |
| 2021_Pan77 | single center (Hospital, IP) |
30 : 30 | NR | NR | (E) SSYX (4c*tid) (C) Amiodarone |
12 | PAF Frequency, LAD, LVEF | LAVmax, LAVmin, LAVIpre, LAVImin | Y |
| 2012_Lu78 | single center (Hospital, IP) |
58 : 56 → 56 : 53 | (E) 57.2 ± 8.4 (C) 56.3 ± 8.3 |
(E) 24 : 34 (C) 26 : 30 |
(E) SSYX (4C*tid) (C) Aspirin + Simvastatin |
24 | LAD, LVEF, Pd | Whole blood high and low shear viscosity, plasma viscosity, hematocrit, Red blood cell aggregation and deformation index, fibrinogen, Pmax, Pmin | NR |
| 2010_Han79 | single center (Hospital) |
50 : 50 → 47 : 48 | (E) 59±12.6 (C) 57±11.3 |
(E) 34 : 13 (C) 36 : 12 |
(E) SSYX (4C*tid) (C) Usual care |
24 | PAF Frequency | None | NR |
| 2020_Liu80 | single center (Hospital) |
38 : 38 | (E) 71.3 ± 5.5 (C) 70.1 ± 5.1 |
(E) 20 : 18 (C) 19 : 19 |
(E) SSYX (4C*tid) (C) Bisoprolol |
24 | LAD | NT-proBNP, TER | Y |
| 2022_Chen81 | single center (Hospital, IP) |
110 : 110 | (E) 62.22±9.17 (C) 61.48±8.04 |
(E) 50 : 60 (C) 62 : 48 |
(E) SSYX (1C*tid) (C) Sacubitril/Valsartan |
24 | hs-CRP, LVEF | BNP, Angiotensin 2, LVIDd, LVIDs | Y |
| 2010_Liu82 | single center (Hospital, IP) |
40 : 40 → 38 : 37 | (E) 63±6 (C) 62±7 |
(E) 23 : 17 (C) 25 : 15 |
(E) SSYX (3c*tid) (C) Amiodarone |
96 | LAD, LVEF | LVIDd, LVIDs, TER | NR |
| 2019_Li83 | single center (Hospital, IP) |
43 : 43 | (E) 61.74±4.17 (C) 60.48±3.97 |
(E) 23 : 20 (C) 21 : 22 |
(E) SSYX (4C*tid) (C) Amiodarone |
8 | LVEF | BUN, SCr, ALT, Mean ventricular rate, TER | NR |
| 2021_Xiao84 | single center (Hospital) | 55 : 55 | (E) 52.56±8.92 (C) 52.84±8.37 |
(E) 29 : 26 (C) 30 : 25 |
(E) SSYX (4C*tid) (C) Usual care |
24 | PAF Frequency, QoL(MLHFQ), 6MWD | TER | Y |
| 2013_Cao85 | single center (Hospital, IP) | 58 : 58 | (E) 62.1 ± 9.3 (C) 61.3 ± 9.7 |
(E) 34 : 24 (C) 35 : 23 |
(E) SSYX (2∼4C*tid) (C) Usual care |
20 | PAF Frequency, Pd, 6MWD | Pmax | NR |
| 2010_Sheng86 | single center (Hospital, IP and OP) |
30 : 30 | (E) 55.7 (C) 56.3 |
(E) 16 : 14 (C) 15 : 15 |
(E) SSYX (4C*tid) (C) Usual care |
24 | PAF Frequency, QoL(MLHFQ), 6MWD | None | NR |
| 2012_Men87 | single center (Hospital) | 42 : 42 | (E) 63.5 ± 7.2 (C) 64.6 ± 6.9 |
(E) 31 : 11 (C) 32 : 10 |
(E) SSYX (4C*tid) (C) Amiodarone |
12 | PAF Frequency, QoL(MLHFQ) | None | NR |
| 2011_Wang88 | multicenter (11) (Hospital, OP) |
107 : 107 : 105 → 106 : 106 : 99 |
(E) 60.1 ± 10.1 (C1) 58±12 (C2) 63±9 |
(E) 54 : 52 (C1) 71 : 35 (C2) 59: 40 |
(E) SSYX (4C*tid) (C1) Propafenone placebo + SSYX (C2) Propafenone + SSYX Placebo |
8 | PAF Frequency, | TCM symptom score, TER | Y |
| 2007_Han89 | single center (Hospital, IP and OP) |
60 : 60 → 54 : 52 | (E) 58±16 (C) 54±11 |
(E) 41 : 13 (C) 40 : 12 |
(E) SSYX (4C*tid) (C) Usual care |
12 | PAF Frequency, QoL(MLHFQ), 6MWD | None | Y |
| 2013_Liu90 | single center (Hospital) | 59 : 52 : 56 | (E) 74.4 ± 16.3 (C1) 75.4 ± 16.1 (C2) 72.4 ± 15.2 |
(E) 30 : 29 (C1) 29 : 23 (C2) 31 : 25 |
(E) SSYX (4C*tid) (C1) SSYX (C2) Amiodarone |
8 | Pd | None | NR |
| 2012_Peng91 | single center (Hospital, IP and OP) |
25 : 25 | (E) 65.8 ± 5.5 (C) 64.8 ± 5.3 |
(E) 13 : 12 (C) 14 : 11 |
(E) SSYX (4C*tid) (C) Indapamide |
12 | Pd | Pmax | NR |
| 2014_Kang92 | single center (Hospital, IP) | 40 : 40 | (E) 52.1 ± 3.3 (C) 50.3 ± 2.5 |
(E) 19 : 21 (C) 24 : 16 |
(E) SSYX (4C*tid) (C) Amiodarone |
24 | LVEF | TER | NR |
| 2016_Li42 | single center (Hospital) | 45 : 45 | (E) 62.3 ± 5.5 (C) 63.4 ± 5.6 |
(E) 24 : 21 (C) 23 : 22 |
(E) SSYX (3C*tid) (C) Amiodarone |
4 | hs-CRP, LAD | MMP-2, TER | NR |
| 2011_Liu93 | single center (Hospital, IP and OP) |
60 : 60 → 55 : 53 | (E) 57±13 (C) 55±10 |
(E) 40 : 15 (C) 39 : 14 |
(E) SSYX (4C*tid) (C) Usual care |
12 | PAF Frequency, QoL(MLHFQ), 6MWD | None | NR |
| 2018_Zhou94 | single center (Hospital, OP) |
59 : 59 | (E) 55.6 ± 3.1 (C) 55.1 ± 3.7 |
(E) 27 :32 (C) 28 : 31 |
(E) SSYX (3C*tid) (C) Valsartan |
4 | LAD, hs-CRP | NO, ET-1, TNF-a, IL-6, SRM, LVIDd, LVIDs, LVMI | NR |
6MWD, 6 Min walking distance; ALD, Aldosterone; AFEQT, Atrial fibrillation effect on qualiTy-of-life; ALT, Alanine aminotransferase; AOPP, Advanced oxidative protein products; BNP, Brain natriuretic peptide; BUN, Blood urea nitrogen; DCG, Dynamic electrocardiogram; ET-1, Human endothelin-1; FS, Fractional shortening; GLS, Global longitudinal strain; HRV, Heart rate variability; IL-6, Interleukin-6; IP, In patients; LAAEF, Left atrial appendage ejection fraction; LACV, Left atrial cavity volume; LAD, Left atrium diameter; LARV, Left atrial reservoir volume; LAV, Left atrial volume; LAVI, Left atrial volume index; LVEF, Left ventricular ejection fraction; LVIDd, Left ventricular internal dimension at end-diastole; LVIDs: : Left ventricular internal dimension at end-systole; LVMI, Left ventricular mass index; LVPWT, Left ventricular posterior wall thickness; LVST, Left ventricular septal thickness; mmp-2, Matrix metalloproteinase-2; mSRa, mean late diastolic peak strain rate; mSRe, mean early diastolic peak strain rate; mSRs, mean systolic peak strain rate; NO, Nitric oxide; NR, Not reported; OP, Out patients; PAF, Paroxysmal atrial fibrillation; Pd, P-wave dispersion; Pmax, The maximum P-wave duration; Pmin, The minimum P-wave duration; QoL(MLHFQ), Quality of life(The Minnesota Living with Heart Failure Questionnaire); ROS, Reactive oxygen species SCr, Serum creatinine; SRM, Sinus rhythm maintenance; TCM, Traditional chinese medicine; TER, Total effective rate; TNF-a, Tumor necrosis factor-a.
3.3. Risk of bias of included studies
Most studies have raised concerns regarding the risk of bias. Of the five areas of risk of bias, only four studies explicitly mentioned allocation concealment, which contributed to the low risk of bias in the random sequence generation domain. The remaining studies were assessed for concerns or high risk of bias. None of the studies provided protocols for the selection of the reported result domain, making it impossible to assess whether they followed a prespecified analysis plan. Consequently, all studies were rated as having concerns or a high risk of bias in this domain. Overall, the assessment of bias risk revealed that 34 studies had some concerns, while 8 were at a high risk of bias. (Supplementary Fig. 1)
3.4. Intervention effects
3.4.1. Frequency of paroxysmal atrial fibrillation
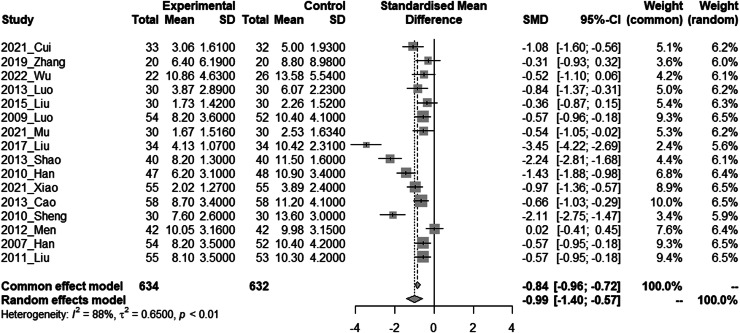
PAF Frequency was considered clinically effective when it decreased. As each study used different evaluation metrics, such as events per day, events per week, or events per month, we conducted a meta-analysis using the SMD. In a meta-analysis of 16 studies that measured PAF Frequency, the combination of HM and CM showed a significant decrease in PAF frequency compared to CM alone. (SMD = −0.99, 95 % CI = −1.40 to −0.57, I² = 88 %, REM, N = 16, n = 1266) (Fig. 2)
Fig. 2.
Forest plot of paroxysmal atrial fibrillation frequency
(Experimental: Liriope Tuber included Traditional Herbal Medicine + Conventional Medicine; Control: Conventional Medicine only). The meta-analysis was conducted using RStudio software. The results were presented as standardized mean difference with 95 % confidence intervals. A random-effects model was employed for the meta-analysis with a significance level of 0.05. Additionally, the results from a common effect model were provided for sensitivity analysis. Heterogeneity was assessed using the chi-square test and Higgins I2 statistic. In the chi-squared test, a significance level of 0.10 was employed.
SD; Standard Deviation; SMD; Standardized Mean Difference.
3.4.2. Left atrium diameter
LAD (unit: mm) was considered clinically effective when it decreased, in the meta-analysis of 16 studies that measured LAD, the combination of HM and CM showed a significant decrease compared to CM alone. (MD= −2.39 mm, 95 % CI= −3.09 to −1.68, I²=75 %, REM, N = 16, n = 1246) (Supplementary Fig. 2 (a))
3.4.3. P-wave dispersion
Pd (unit: ms) was considered clinically effective when it decreased, in the meta-analysis of 10 studies that measured Pd, the combination of HM and CM showed a significant decrease compared to CM alone. (MD= −6.41 ms, 95 % CI= −8.44 to −4.37, I²=90 %, REM, N = 10, n = 807) (Supplementary Fig. 2 (b))
3.4.4. high sensitive C-Reactive protein
hs-CRP (unit: mg/l) was considered clinically effective when it decreased, and the MCID was estimated to be 0.5 mg/L.45 In a meta-analysis of 8 studies that measured hs-CRP, the combination of HM and CM showed a significant decrease in hs-CRP compared to CM alone, with effect estimates larger than the MCID. (MD= −1.10 mg/l, 95 % CI= −1.73 to −0.47, I²=94 %, REM, N = 8, n = 693) (Supplementary Fig. 2 (c))
3.4.5. Left ventricular ejection fraction
LVEF (unit:%) was considered clinically effective when it increased, in the meta-analysis of 7 studies that measured LVEF, the combination of HM and CM showed a significant increase compared to CM alone. (MD=4.71%, 95 % CI=3.17 to 6.25, I²=77 %, REM, N = 7, n = 713) (Supplementary Fig. 2 (d))
3.4.6. 6 min walking distance
6 MWD (unit: m) was considered clinically effective when it increased, and the MCID is estimated to be 30m.46 In a meta-analysis of five studies that measured 6 MWD, the combination of HM and CM showed a significant increase compared to CM alone, with effect estimates larger than the MCID. (MD = 75.07 m, 95 % CI=47.52 to 102.62, I² = 78 %, REM, N = 5, n = 500) (Supplementary Fig. 2 (e))
3.4.7. Quality of life (The Minnesota living with heart failure questionnaire)
QoL (points) was considered clinically effective when it increased, and the MCID was estimated to be 8.2.47 In a meta-analysis of five studies that measured QoL, the combination of HM and CM showed a significant increase in QoL compared with CM alone, with effect estimates larger than those of MCID. (MD=18.17 points, 95 % CI=8.72 to 27.63, I² = 98 %, REM, N = 5, n = 468) (Supplementary Fig. 2 (f))
3.5. Subgroup analyses
Subgroup analyses were conducted when possible, based on treatment duration, daily dosage of Liriope Tuber, total dosage of Liriope Tuber, and patient age for each outcome variable. In most subgroup analyses, no significant differences were observed. However, subgroup analysis based on the daily dosage of Liriope Tuber for LAD and hs-CRP and the total dosage of Liriope Tuber for Pd and hs-CRP revealed that a higher dosage of Liriope Tuber was associated with a reduced effect. (Supplementary Fig. 3–1) A meta-regression analysis using the total dose of Liriope Tubers of Pd as a predictor variable also showed a significant difference, and the results were consistent with those of the subgroup analysis. (Supplementary Fig. 3–2)
3.6. Adverse events
A total of 43 studies were reviewed, of which 19 reported AEs. However, a quantitative synthesis of the incidence of AEs through meta-analysis is challenging due to the limited number of studies reporting the number of cases of each AE in both the intervention and control groups. Therefore, we aimed to provide a descriptive presentation of the number of cases of each AE symptom. The most common AEs were gastrointestinal symptoms, and other details can be found in Supplementary 4.
3.7. Sensitivity analysis
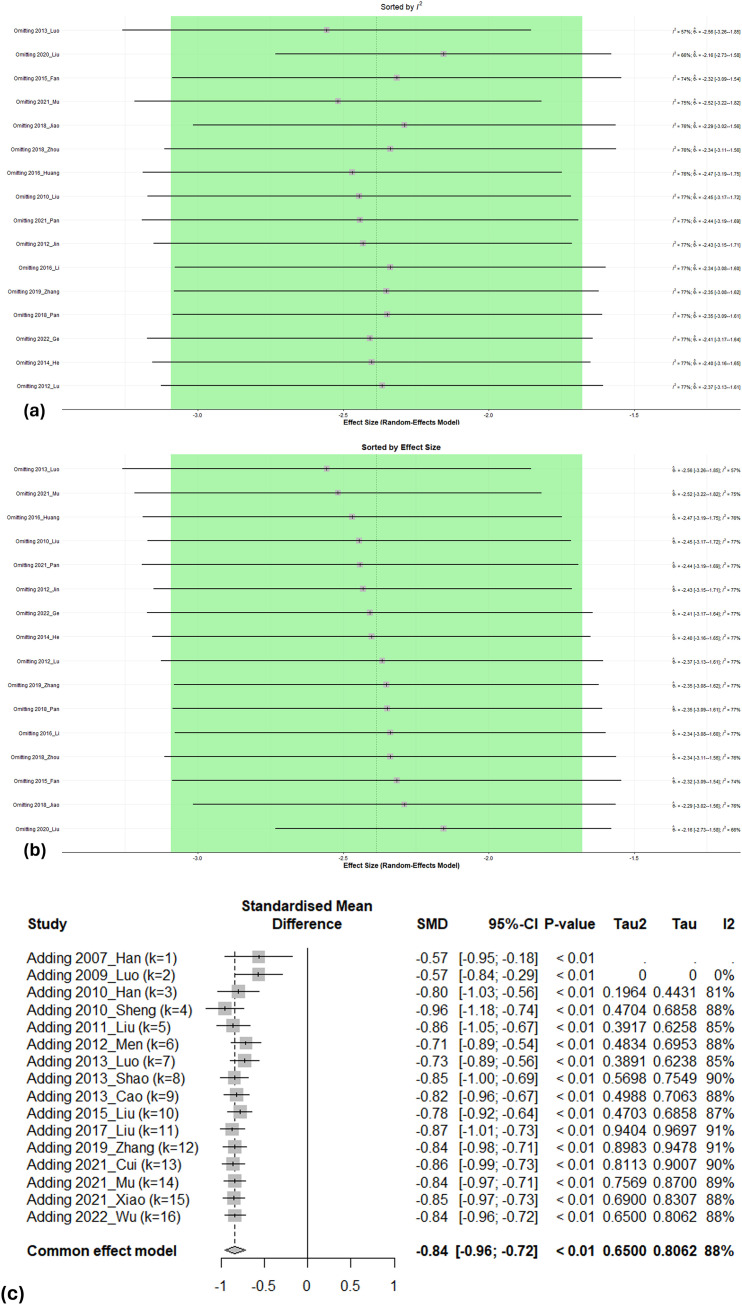
When comparing the results of the meta-analysis between the fixed- and random-effects models, there was no difference in the direction of the effect estimates. For sensitivity analysis, we also conducted a Leave-One-Out Meta-Analysis of the PAF frequency and outcomes considered important in the GRADE evaluation, including Pd, hs-CRP, and LAD. When performing a Leave-One-Out analysis for LAD, it was observed that the studies "2020_Liu" and "2013_Luo" had the most influence on the overall effect estimate but when they were excluded, the effect estimates remained within the 95 % CI of the overall effect estimate. (Fig. 3 (a∼b)) When individual studies were excluded from the meta-analysis for PAF Frequency, Pd, and hs-CRP, it was found that the effect sizes were not significantly affected, and the results remained robust. Heterogeneity also showed minimal changes when individual studies were excluded. (Supplementary Figure. 4) We also conducted a cumulative meta-analysis for the sensitivity analysis. Cumulative meta-analyses were conducted for the PAF Frequency and outcomes considered important in the GRADE assessment, including Pd, hs-CRP, and LAD. Regarding the frequency of PAF, there were 16 studies conducted between 2007 and 2022. As more studies were added to the analysis, the CI of the effect estimates gradually narrowed, and the point estimate steadily converged towards a singular value. The stable cumulative meta-analysis results indicated that subsequent study findings were likely to have a minimal impact on the meta-analysis of PAF Frequency (Fig. 3(c)). The cumulative meta-analysis of Pd, hs-CRP, and LAD showed the same results. (Supplementary Figure. 5)
Fig. 3.
Sensitivity analysis
(a) Leave-one-out meta-analysis of left atrium diameter (Sorted by I²)
(b) Leave-one-out meta-analysis of left atrium diameter (Sorted by effect size)
(c) Cumulative meta-analysis of paroxysmal atrial fibrillation frequency (Sorted by year)
The sensitivity analysis was conducted using R Studio software. A leave-one-out meta-analysis was conducted to assess the influence of each study on the effect estimates by excluding one study at a time, identifying studies that significantly influenced heterogeneity. A cumulative meta-analysis was also performed to evaluate how the average effect estimates, and CI changed as studies were added individually over time. The results in (a) and (b) are presented as mean differences with 95 % confidence intervals and results in (c) are presented as standardized mean differences. A random-effects model with a significance level of 0.05 was employed for the sensitivity analysis in (a) and (b), whereas a common effect model was utilized in (c).
SMD; Standardized Mean Difference.
3.8. Publication bias
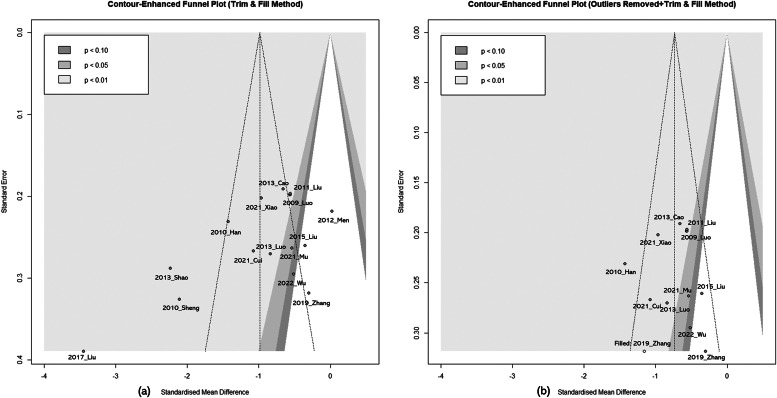
LAD and Pd had low probabilities of publication bias (Supplementary Figure 6). However, for PAF, frequency asymmetry was observed in the funnel plot, and Egger's test indicated a high probability of publication bias, with P-values of 0.02. Two methods were employed to analyze the impact of publication bias on the effect estimate of PAF frequency. First, the trim-and-fill method was applied and resulted in the inclusion of no additional studies. Second, due to substantial heterogeneity (I² = 88 %) in PAF frequency, potential outliers were identified using the "find.outliers" command in RStudio "meta" package, revealing four studies: "2017_Liu," "2013_shao," "2010_sheng," and "2012_men." When the trim and fill method was applied with these four studies excluded, one additional study, "2019_zhang," was included. The results of the meta-analysis (SMD=−0.73, 95 % CI=−0.90, −0.56, I² = 44 %) remained statistically significant (P < 0.01). (Fig. 4)
Fig. 4.
Contour-enhanced funnel plot
(a) Paroxysmal atrial fibrillation frequency (Trim & Fill Method)
(b) Paroxysmal atrial fibrillation frequency (Outliers Removed + Trim & Fill Method)
(a) No studies added. (b) The "find.outliers" command in the RStudio "meta" package revealed four studies: "2017_Liu," "2013_shao," "2010_sheng," and "2012_men." The trim and fill method added '2019_zhang'.
3.9. Certainty of evidence
Certainty of evidence was rated as "low" for PAF frequency, LAD, Pd, hs-CRP, and LVEF. The downgrade in the certainty of evidence for PAF frequency was due to variations in measurement units among studies, with no clear explanation. All outcome variables that assessed the certainty of the evidence were downgraded because of risk of bias, inconsistency, or indirectness. (Table 2)
Table 2.
GRADE recommendation.
| Certainty assessment |
№ of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Liriope Tuber included traditional herbal medicine | Conventional Medicine | Relative (95 % CI) |
Absolute (95 % CI) |
||
| PAF Frequency | ||||||||||||
| 16 | randomised trials | seriousa | seriousb,c | seriousd | not serious | none | 634 | 632 | – | SMD 0.99 SD fewer (1.4 fewer to 0.57 fewer) |
⨁⨁◯◯ Low |
CRITICAL |
| LAD | ||||||||||||
| 16 | randomised trials | seriousa | seriousc | seriousd | not serious | none | 406 | 401 | – | MD 2.39 lower (3.09 lower to 1.68 lower) |
⨁⨁◯◯ Low |
CRITICAL |
| hs-CRP | ||||||||||||
| 8 | randomised trials | seriousa | seriousc | seriousd | not serious | none | 348 | 345 | – | MD 1.10 lower (1.73 lower to 0.47 lower) |
⨁⨁◯◯ Low |
IMPORTANT |
| Pd | ||||||||||||
| 10 | randomised trials | seriousa | seriousc | seriousd | not serious | none | 357 | 356 | – | MD 6.41 lower (8.44 lower to 4.37 lower) |
⨁⨁◯◯ Low |
IMPORTANT |
| LVEF | ||||||||||||
| 7 | randomised trials | seriousa | seriousc | seriousd | not serious | none | 626 | 620 | – | MD 4.71 more (3.17 more to 6.25 more) |
⨁⨁◯◯ Low |
IMPORTANT |
CI, confidence interval; MD, mean difference; SMD, standardised mean difference, PAF, paroxysmal atrial fibrillation, LAD, Left atrium diameter, Pd, P-wave dispersion, LVEF, Left ventricular ejection fraction.
Explanations.
. All studies were assessed for some concerns or high risk of bias.
. The units of measurement are different, and the explanation is not clear.
. Substantial or considerable heterogeneity.
. The interventions were complex, with Liriope Tuber being only one component of the regimen.
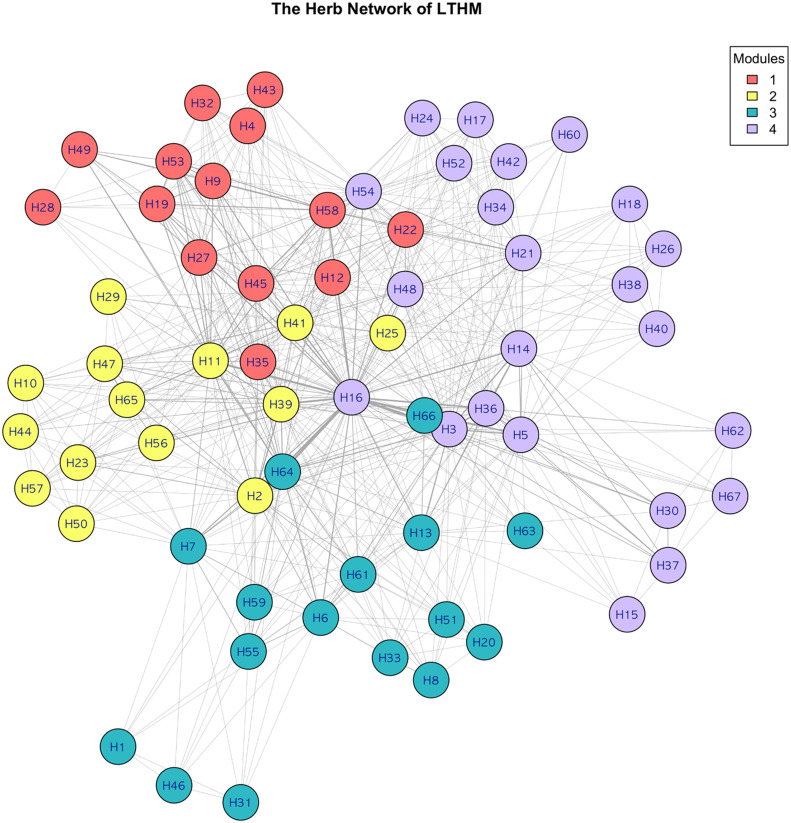
3.10. Network analysis
The herb network of LTHM was constructed and is represented in Supplementary Table 5. Four modules were identified in this study. (Fig. 5) For each module, the top five herbs with the highest centrality values are represented Supplementary Table. 6. The rankings of herbs for each centrality metric were generally consistent, and herbs frequently used in LTHM tended to exhibit higher centrality values.
Fig. 5.
The herb network of traditional herbal medicine including Liriope Tubers.
The nodes of the network indicate all the herbs used in traditional herbal medicine including Liriope Tuber. A linkage was defined between two herbal nodes if there was a common appearance between the two herbs in a prescription. Visualization was performed using the Fruchterman–Reingold layout.
4. Discussion
4.1. Summary of evidence
We conducted a systematic review and meta-analysis on the effects and safety of LTHM in patients with PAF. In total, 43 studies were analyzed qualitatively, and 38 studies were analyzed quantitatively. The meta-analysis demonstrated that the combination therapy of HM and CM may have a notable impact on PAF frequency, Pd, hs-CRP, LAD, LVEF, 6 MWD, and QoL, when compared with CM monotherapy. Sensitivity analysis revealed that the effect estimates of the meta-analysis were robust. However, the overall risk of bias was high, and the likelihood of publication bias and the certainty of evidence in the primary outcome were rated as "low." Additionally, considerable heterogeneity was noted among the studies included in this meta-analysis, indicating that the results should be interpreted with caution.
4.2. Agreements and disagreements with other reviews
A systematic review and meta-analysis of SSYX for PAF found that it significantly improved Pd (MD= −13.92, 95 % CI= −15.53 to −12.30, I² = 0 %, REM, N = 3, n = 196) and QoL (MD=25.98, 95 % CI=23.30 to 28.65, I²=57 %, REM, N = 3, n = 266). These effects were more favorable than the results of the meta-analysis presented in this study. This aligns with the findings of the subgroup analysis in this study, which indicated that the effect estimates of SSYX were higher than those of the other herbal medicines. Subgroup analyses revealed that SSYX, with a daily dosage of less than 10 g, had superior effects compared to other decoctions with a daily dosage of 10 g or more, possibly due to its convenient pill form, which promotes better compliance. The longer treatment duration of approximately 18 weeks for SSYX, compared to about 13 weeks for other decoctions, may have also contributed to its more favorable outcomes. However, this study did not determine which herbal medicine is most effective for specific outcome variables, leaving room for future research such as network meta-analyses to explore this issue further.
4.3. Possible mechanism of LTHM
Liriope Tuber has demonstrated pharmacological effects by promoting SERCA2a activity and maintaining intracellular calcium homeostasis, thus protecting myocardial cells.48 Additionally, one of the saponin monomers of Liriope Tuber, 13 (DT-1), has cardioprotective effects.49 In the perspective of EATM, PAF is categorized under various terms such as palpitate (心悸), fearful throbbing (怔忡) and dizziness (眩暈). It is believed to result from a combination of various factors that ignite heart fire (心火) and disrupt the heart orifice (心竅). These factors may include phlegm and blood stasis among others, leading to the manifestation of this condition.50 In EATM, Liriope Tuber is known for its efficacy in nourishing yin, moistening the lungs, calming the mind, benefiting the stomach, and generating fluid. Therefore, it is effective for AF caused by Deficiency of the Heart and Kidneys, phlegm, and conditions arising from the transform into fire (化火).
4.4. Diagnostic challenges
The clinical trials included in this study have some limitations regarding the diagnosis of PAF. Among the 43 studies, 23 did not specify the diagnostic criteria. Even among the studies that specified the criteria, the criteria varied. The diagnosis of AF is recommended to involve electrocardiographic measurements for at least 30 s. Based on its duration, AF is classified into PAF, persistent atrial fibrillation, long-standing persistent atrial fibrillation, and permanent atrial fibrillation. In cases where both paroxysmal and persistent atrial fibrillation coexist, which makes it difficult to determine a clear treatment approach based solely on this classification, recent practices have divided AF into categories based on treatment and prognosis.51 Therefore, in future clinical trials of herbal fibrillation, it is essential to design studies that incorporate the latest diagnostic criteria for AF to ensure accurate diagnosis and treatment.
4.5. Strengths and limitations of review
One of the strengths of this study was that it conducted a comprehensive search without restrictions on country or language, resulting in a sufficient number of included studies for the meta-analysis (38 studies). This allowed for robust effect estimates in sensitivity analyses, such as leave-one-out and cumulative meta-analyses. Additionally, this enabled the assessment of publication bias for the three outcome variables. Moreover, in the fields of EATM and AF, systematic reviews often encounter issues related to insufficient sample sizes, leading to frequent downgrades owing to imprecision in the GRADE methodology. However, these issues were not addressed in this study. Second, this study adhered to PRISMA guidelines. Third, this study selected outcome variables that aligned with the consensus recommendations for AF clinical trials. In the selection of outcome variables in RCTs of AF, expert consensus was obtained in seven areas: mortality, stroke, symptoms and quality of life, rhythm, left atrial function, cost, and emerging surrogate outcome parameters.52
However, this study had several limitations. First, 24 of the 43 studies did not report any AEs. Additionally, there were insufficient studies reporting the number of participants experiencing AEs to conduct a quantitative synthesis. Therefore, a descriptive presentation was provided instead of a quantitative synthesis; however, future research should focus on the safety of LTHM in AF treatment. Additionally, it is important to note that all the studies included in this research were conducted in China, and all participants were of Chinese ethnicity. Therefore, the generalizability of the results is uncertain. Third, PAF frequency was evaluated for possible publication bias. The trim and fill method analysis revealed that publication bias had a de minimis influence on the effect estimate of PAF frequency. Although we conducted a sensitivity analysis when interpreting the results of this meta-analysis, it is important to consider the possibility of publication bias. Fourth, there were no long-term outcomes reported, which is an important benefit of herbal medicines. Finally, interpretation must proceed with caution since Liriope Tubers were only one component of the regimen in this study. This may make it challenging to apply these results to the clinical setting; nevertheless, this study will provide a useful resource for future studies.
4.6. Implications for further research
This study requires cautious interpretation owing to the methodological limitations of the included studies. In terms of future research design, to enhance the quality of studies, adherence to reporting standards such as Consolidated Standards of Reporting Trials (CONSORT)53 and guidelines specific to traditional Chinese medicine and herbal interventions54,55 is recommended. Moreover, it is essential to employ appropriate methods of blinding and allocation concealment to minimize the risk of bias. To improve the transparency of reporting, pre-registration, and publicly available protocols are encouraged. Furthermore, clinical trials should be conducted to determine the long-term effectiveness and safety of herbal medicines. This study was a systematic review and meta-analysis of LTHM, with all included studies involving herbal medicines that combined Liriope Tuber with various other herbs. Therefore, the meta-analysis results do not specifically highlight the isolated effects of Liriope Tubers. Conducting research to assess the effects of Liriope Tuber alone can be challenging because of the clinical practice trend in East Asia, where multiple herbs are combined in herbal decoctions. Exploratory analyses such as subgroup analyses based on daily and total dosages, as employed in this study, offer a more feasible approach. For further studies investigating the sole effects of a single herbal medicine, pharmacoepidemiological study designs that consider the dosage of the herbal medicine are required.
4.7. Conclusion
In conclusion, the combination of LTHM and CM may be effective in reducing the frequency of PAF. However, due to the complexity of interventions, with Liriope Tuber being only one component of the regimen, the overall risk of bias was high, and the likelihood of publication bias and the certainty of evidence in the primary outcome were rated as "low." Therefore, these results should be interpreted with caution. The secondary outcomes of Pd, hs-CRP, LAD, LVEF, 6 MWD, and QoL also showed significant improvements; however, there was considerable heterogeneity among the studies included in this meta-analysis. Further studies are needed to confirm the robustness of the results. Moreover, the meta-analysis results do not specifically highlight the isolated effects of Liriope Tubers. For further studies investigating the sole effects of a single herbal medicine, pharmacoepidemiological study designs that consider the dosage of the herbal medicine are required. In the future, clinical trials of herbal medicines for PAF should be conducted to determine their long-term effectiveness and safety. These trials should adhere to strict methodological standards, including the CONSORT guidelines, and should be transparent through pre-registration and publishing of protocols.
CRediT authorship contribution statement
Hanbit Jin: Software, Validation, Formal analysis, Investigation, Writing – original draft, Visualization. Sukjong Kang: Formal analysis, Investigation, Writing – original draft. Dasol Park: Software, Writing – original draft, Visualization. Yeun-Ja Mun: Conceptualization, Data curation, Writing – review & editing, Project administration. Jungtae Leem: Conceptualization, Methodology, Resources, Data curation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Funding
This study was supported by Wonkwang University in 2024.
Acknowledgement
This study is based on the Ph.D. dissertation written in Korean by Sukjong Kang, a co-first author of this research. It has been modified, supplemented, condensed, and reorganized for this publication.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Protocol registration: The study protocol is registered in PROSPERO (ID: CRD42023477926).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2024.101069.
Appendix. Supplementary materials
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart Disease and Stroke Statistics-2022 Update: a Report From the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C.E., Wang K.L., Lip G.Y.H. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S., Hart C.L., Hole D.J., McMurray J.J.V. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 4.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 5.Krahn A.D., Manfreda J., Tate R.B., Mathewson F.A., Cuddy T.E. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98(5):476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim D., Yang P.S., Jang E., et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018;104(24):2010–2017. doi: 10.1136/heartjnl-2017-312930. [DOI] [PubMed] [Google Scholar]
- 7.January C.T., Wann L.S., Alpert J.S., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 8.Kato T., Yamashita T., Sagara K., Iinuma H., Fu L.T. Progressive nature of paroxysmal atrial fibrillation. Published online 2004. [DOI] [PubMed]
- 9.Andrade J.G., Champagne J., Dubuc M., et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140(22):1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 10.Kotecha D., Holmes J., Krum H., et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235–2243. doi: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 11.Roy D., Talajic M., Nattel S., et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 12.Wyse D.G., Waldo A.L., DiMarco J.P., et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 13.Van Gelder I.C., Hagens V.E., Bosker H.A., et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 14.Opolski G., Torbicki A., Kosior D.A., et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126(2):476–486. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khatib S.M., Allen LaPointe N.M., Chatterjee R., et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160(11):760–773. doi: 10.7326/M13-1467. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S., Sardar P., Lichstein E., Mukherjee D., Aikat S. Pharmacologic rate versus rhythm-control strategies in atrial fibrillation: an updated comprehensive review and meta-analysis. Pacing Clin Electrophysiol. 2013;36(1):122–133. doi: 10.1111/j.1540-8159.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhat A., Khanna S., Chen H.H.L., et al. Integrated care in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2021;14(3) doi: 10.1161/CIRCOUTCOMES.120.007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher C., Elliott A.D., Wong C.X., et al. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart. 2017;103(24):1947–1953. doi: 10.1136/heartjnl-2016-310952. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y., Liao J., Yao K., Jiang W., Wang J. Application of traditional Chinese medicine in treatment of atrial fibrillation. Evid Based Complement Alternat Med. 2017 doi: 10.1155/2017/1381732. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Nie S., Gao H., et al. The effects of wenxin keli on p-wave dispersion and maintenance of sinus rhythm in patients with paroxysmal atrial fibrillation: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/245958. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei Y., Tang Y., Huang L., He P. Systematic review and meta-analysis on efficacy of traditional Chinese medicine for atrial fibrillation through cluster analysis. Ann Palliat Med. 2021;10(8):8982–8990. doi: 10.21037/apm-21-1785. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z., Tang Z., Peng X., et al. Network meta-analysis: aspirin plus traditional Chinese medicine for stroke prevention in patients with atrial fibrillation. J Herb Med. 2020;22 doi: 10.1016/j.hermed.2020.100355. [DOI] [Google Scholar]
- 23.Jiang X., Luo Y., Wang X., et al. Investigating the efficiency and tolerability of traditional Chinese formulas combined with antiarrhythmic agents for paroxysmal atrial fibrillation: a systematic review and Bayesian network meta-analysis. Phytomedicine. 2022;94 doi: 10.1016/j.phymed.2021.153832. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Tang Z., Zhu W., Ge L., Ge J. Efficacy and safety of traditional Chinese medicine on thromboembolic events in patients with atrial fibrillation: a systematic review and meta-analysis. Complement Ther Med. 2017;32:1–10. doi: 10.1016/j.ctim.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Chen G., Wei B., Wang J., et al. Shensongyangxin capsules for paroxysmal atrial fibrillation: a systematic review of randomized clinical trials. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0151880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon S., Jin C., Cho S.Y., et al. Paeoniae Radix-containing herbal medicine for patients with restless legs syndrome: a systematic review and meta-analysis. Complement Ther Clin Pract. 2019;35:329–341. doi: 10.1016/j.ctcp.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Di Y.M., Sun L., Lu C., et al. Benefits of herbal formulae containing Poria cocos (Fuling) for type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2022;17(12) doi: 10.1371/journal.pone.0278536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astragalus-containing Chinese herbal medicine combined with chemotherapy for cervical cancer: a systematic review and meta-analysis - PubMed. Accessed July 5, 2023. https://pubmed.ncbi.nlm.nih.gov/34393766/ [DOI] [PMC free article] [PubMed]
- 29.Jaeyun An. Wonkwang University; 2022. The Effect of Herbal Medicine On Arrhythmia: Systematic Review and Meta-Analysis of Randomized Control Clinical Trial.http://www.riss.kr/link?id=T16377364 [Google Scholar]
- 30.Chungyeol Lee. Effects of ginseng radix and Ophiopogonis tuber on field potentials in rat hippocampal and cardiac muscle slices. J Physiol Pathol Korean Med. 2003;17(6):1463–1467. [Google Scholar]
- 31.Li N., Zhang J.Y., Zeng K.W., Zhang L., Che Y.Y., Tu P.F. Anti-inflammatory homoisoflavonoids from the tuberous roots of Ophiopogon japonicus. Fitoterapia. 2012;83(6):1042–1045. doi: 10.1016/j.fitote.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Zheng X.L., Xing F.W. Ethnobotanical study on medicinal plants around Mt.Yinggeling, Hainan Island, China. J Ethnopharmacol. 2009;124(2):197–210. doi: 10.1016/j.jep.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Chen M.H., Chen X.J., Wang M., Lin L.G., Wang Y.T. Ophiopogon japonicus–A phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol. 2016;181:193–213. doi: 10.1016/j.jep.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cochrane handbook for systematic reviews of interventions. Accessed July 3, 2023. https://training.cochrane.org/handbook/current
- 36.Sterne J.A.C., Sutton A.J., Ioannidis J.P.A., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 37.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26(25):4544–4562. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 40.Pan Q. The effect of Shen Song Yangxin capsule combined with amiodarone in the treatment of atrial fibrillation and Detection and analysis of related indexes (应用参松养心胶囊联合胺碘酮方案对阵发性房颤的治疗作用及相关指标检测分析) Contemp Med. 2018;24(32):134–136. [Google Scholar]
- 41.Huang Y., Zhang Z., Chen R., Ding Y., Chen M., Zhu Y. Effects of Dingji formula on inflammatory factors and myocardial fibrosis in paroxysmal atrial fibrillation(定悸方对阵发性房颤炎症因子及心肌纤维化影响). Inaugural Meeting of the Chinese Society of Traditional Chinese Medicine Branch of Family Medicine and 2016 Academic Annual Meeting.:8.
- 42.Li F., Kuang P., Chen Y., Liu K. Effect of Shensongyangxin capsules combined with amiodarone on MMP-2 and hs-CRP of patients with paroxysmal atrial fibrillation(参松养心胶囊联合胺碘酮治疗对阵发性房颤患者血清MMP-2,hs-CRP水平的影响) Internal Med. 2016;11(05):694–697. [Google Scholar]
- 43.Ma M., Hao W. Clinical research on effects of filling weakness and fasting Ben, Expelling Phlegm and removing blood stasis on c-reactive protein in patients with paroxysmal atrial fibrillation (补虚固本,祛痰化瘀法对阵发性房纤颤患者血浆C-反应蛋白影响的临床研究) West J Tradit Chin Med. 2011;24(09):1–3. [Google Scholar]
- 44.Shi T., Yao Z., Qin Z., Ding B., Dai Y., Yao X. Identification of absorbed constituents and metabolites in rat plasma after oral administration of Shen-Song-Yang-Xin using ultra-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. Biomed Chromatogr. 2015;29(9):1440–1452. doi: 10.1002/bmc.3443. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg J.Z., Day A., Brinkworth G.D., et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan M.S., Siddiqi T.J., Butler J., et al. Functional outcomes with Carillon device over 1 year in patients with functional mitral regurgitation of Grades 2+ to 4+: results from the REDUCE-FMR trial. ESC Heart Fail. 2021;8(2):872–878. doi: 10.1002/ehf2.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Saenz de Tejada M., Bilbao A., Ansola L., et al. Responsiveness and minimal clinically important difference of the Minnesota living with heart failure questionnaire. Health Qual Life Outcomes. 2019;17(1):36. doi: 10.1186/s12955-019-1104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., You W., Wang N., et al. Ophiopogonin D increases SERCA2a interaction with Phospholamban by promoting CYP2J3 upregulation. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/8857906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao J., Wang H., Zhou H., Li S. The saponin monomer of dwarf lilyturf tuber, DT-13, reduces L-type calcium currents during hypoxia in adult rat ventricular myocytes. Life Sci. 2005;77(24):3021–3030. doi: 10.1016/j.lfs.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 50.Kim Jung-chul, Oh Sung-won, Song Chang-Hoon, Lee Seul-hee, Jeong Jong-jin, Sun Seung-ho. case report of Chengsim Yeunja-Tang(CYT) for atrial fibrillation with cerebral-infarction. J Korean Orient Internal Med. 2006;27(3):752–762. [Google Scholar]
- 51.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 52.Kirchhof P., Auricchio A., Bax J., et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German atrial fibrillation competence NETwork and the European Heart Rhythm Association. Europace. 2007;9(11):1006–1023. doi: 10.1093/europace/eum191. [DOI] [PubMed] [Google Scholar]
- 53.Schulz K.F., Altman D.G., Moher D., Group CONSORT. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bian Z., Liu B., Moher D., et al. Consolidated standards of reporting trials (CONSORT) for traditional Chinese medicine: current situation and future development. Front Med. 2011;5(2):171–177. doi: 10.1007/s11684-011-0132-z. [DOI] [PubMed] [Google Scholar]
- 55.Gagnier J.J., Boon H., Rochon P., et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144(5):364–367. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y., He X., Yan Z., Zhang D. Influence of Jianxin PinglüPill on BNP and ALD levels in patients with paroxysmal atrial fibrillation(健心平律丸对阵发性房颤患者BNP,Ald的影响) Chin J Integr Med Cardio Cerebrovasc Dis. 2014;12(07):782–784. [Google Scholar]
- 57.He X., Yan Z., Zhang D., Bi Y., Chen Y. Effect of Jianxin Pingrong pill on cardiac remodeling in patients with paroxysmal atrial fibrillation(健心平律丸对阵发性心房颤动患者心脏重构的影响) J N Chin Med. 2014;46(07):30–31. [Google Scholar]
- 58.Chen Y., He X., Yan Z., Zhang D. Effect of Jianxin PinglüPill on HS-CRP in paroxysmal atrial fibrillation(健心平律丸治疗阵发性房颤的临床疗效及对hs-CRP的影响) Chin J Integr Med Cardio Cerebrovasc Dis. 2014;12(03):271–272. [Google Scholar]
- 59.Cui F. Clinical effect of Guizhi Fuling pill on paroxysmal atrial fibrillation of kidney deficiency and blood stasis type(桂枝茯苓丸加减治疗肾虚血瘀型阵发性房颤的临床疗效研究). Thesis. 2021.
- 60.Pan J., Qiao Y., Yang J. Clinical resaerch of evaluating the influences of Shensong Yangxin capsule on the left atrial function of patients with paroxysmal atrial fibrillation based on the technology of two-dimensional speckle tracking imaging(基于二维斑点追踪成像技术探讨参松养心胶囊对阵发房颤患者左心房功能影响的临床研究) Tianjin J Tradit Chin Med. 2019;36(10):959–962. [Google Scholar]
- 61.Zhang B. Clinical study of compound Guanbaifu in the treatment on paroxysmal atrial fibrillation(复方关白附治疗阵发性心房颤动的临床研究). Thesis. 2019.
- 62.Wu W., Liu C. Clinical observation on the treatment of paroxysmal atrial fibrillation with Qi and Yin deficiency type by combining compound Guanbaifu Decoxtion with metoprolol succinate extended-release tablets(复方关白附汤联合琥珀酸美托洛尔缓释片治疗气阴两虚型阵发性房颤的临床观察) Chin Naturopath. 2022;30(07):83–86. [Google Scholar]
- 63.Jiao Q., He X., Yan Z. Efficacy of amiodarone combined with Jianxin Pingrong pill in the treatment of paroxysmal atrial fibrillation and its effect on serum MMP-2 and LAD(胺碘酮联合健心平律丸治疗阵发性房颤的疗效及对血清MMP-2和LAD的影响) Guid J Tradit Chin Med Pharma. 2018;24(24):118–119. [Google Scholar]
- 64.Luo X. Clinical study on treating paroxysmal atrial fibrillation with broth of Yangxinxifeng(养心熄风汤治疗阵发性房颤的临床研究). Thesis. 2013.
- 65.Ying L., Liu H., Zhu Y. Clinical study on the treatment of hypertension combined with paroxysmal atrial fibrillation by Yangxin Dingji capsule combined with irbesartan(养心定悸胶囊联合厄贝沙坦治疗高血压合并阵发性心房颤动的临床研究) Chin J Integr Med Cardio Cerebrovasc Dis. 2021;19(13):2222–2225. [Google Scholar]
- 66.Liu S., Zhao Y. Clinical observation on supplementing Qi and nourishing Yin therapy for treatment of paroxysmal atrial fibrillation with deficiency syndrome of Qi and Yin(益气养阴法治疗阵发性房颤气阴亏虚证的疗效观察) Chin J Integr Med Cardio Cerebrovasc Dis. 2015;13(18):2047–2048. [Google Scholar]
- 67.Chen T., Chang Z., Fan X., Du B. Effect of Zhigancao decoction combined with amiodarone on paroxysmal atrial fibrillation and atrial remodeling (炙甘草汤联合胺碘酮治疗阵发性心房颤动及对心房重构的影响) China Modern Doctor. 2017;55(15):118–121. [Google Scholar]
- 68.Luo Q. Efficacy observation of 54 cases of paroxysmal atrial fibrillation treated with Chuchan decoction(自拟除颤汤治疗阵发性心房颤动54例疗效观察) Yunnan J Tradit Chin Med Mater Med. 2009;30(06):34–35. [Google Scholar]
- 69.Mou A. Clinical observation of Ningxinanshen decoction in the treatment of paroxysmal atrial fibrillation(Qi and Yin Deficiency) with Type 2 diabetes Mellitus(宁心安神方治疗阵发性房颤(气阴两虚型)合并2型糖尿病患者的临床疗效观察). Thesis. 2021.
- 70.Liu J., Liao Y., Tang Y. Effect of Dingxin capsule combined with metoprolol on P-wave dispersion and serum inflammatory factors in patients with paroxysmal atrial fibrillation(定心颗粒联合美托洛尔对阵发性心房颤动患者P波离散度及血清炎症因子的影响) Modern J Integr Tradit Chin West Med. 2017;26(23):2564–2566. [Google Scholar]
- 71.Ge R., Li J., Xu J., Li M. Study on prevention of early recurrence of Xiaju Huayu capsule after radiofrequency ablation of paroxysmal atrial fibrillation(夏橘化瘀胶囊对预防阵发性房颤射频消融术后早期复发的研究) Syst Med. 2022;7(19):1–5. [Google Scholar]
- 72.Fan X. Study on the efficacy of losartan combined with amiodarone and Shensong Yangxin capsule in the treatment of hypertension with paroxysmal atrial fibrillation(氯沙坦联合胺碘酮与参松养心胶囊治疗高血压伴阵发性心房颤动的疗效研究) For all Health. 2015;9(12):167. [Google Scholar]
- 73.Jin Y. Effects of metoprolol and Shensongyangxin capsule on P wave dispersion and high sensitivity C-reactive protein in patients with paroxysmal atrial fibrillation(美托洛尔联合参松养心胶囊对阵发性心房颤动患者P波离散度和血浆高敏C-反应蛋白的影响) Chin Circ J. 2012;27(5):353–356. [Google Scholar]
- 74.Wu P., Yu H., Guo D., et al. Therapeutic effects of Betaloc combined with Shensong Yangxin capsule on P wave dispersion in patients with paroxysmal atrial fibrillation(倍他乐克联合参松养心胶囊对阵发性房颤患者P波离散度的影响) Chin J Difficult Complic Cases. 2007;(05):270–272. [Google Scholar]
- 75.Tang Y., Liu M., Li J. Observations on the effect of usual care combined with Shensong Yangxin capsule in treating atrial fibrillation in the elderly(常规加参松养心胶囊治疗老年房颤效果观察) People's Milit Surg. 2012;55(01):41–42. [Google Scholar]
- 76.Shao J. Clinical efficacy of rosuvastatin combined with Shensong Yangxin capsule in the treatment of paroxysmal atrial fibrillation in elderly coronary heart disease(瑞舒伐他汀联合参松养心胶囊治疗老年冠心病阵发性心房纤颤的临床疗效观察) Guide Chin Med. 2013;11(24):141–142. [Google Scholar]
- 77.Pan J., Qiao Y., Yang J. Clinical observation on the influence of Shensong Yangxin capsules on left atrial structural remodeling in patients with paroxysmal atrial fibrillation(参松养心胶囊对阵发房颤患者左房结构重构的影响) Guangming J Chin Med. 2021;36(03):389–392. [Google Scholar]
- 78.Lu J. Clinical observation on the effect of Shensong Yangxin capsule on P-wave dispersion in patients with paroxysmal atrial fibrillation(参松养心胶囊对阵发性房颤患者P波离散度影响的临床观察) Prevent Treatment Cardio Cerebr Vascular Dis. 2012;12(02):134–136. [Google Scholar]
- 79.Han Y., Xue R., Zhang X. Effect of Shensong Yangxin capsule on the frequency of paroxysmal atrial fibrillation episodes(参松养心胶囊对阵发性心房颤动发作频率的影响) Chin J Misdiagnos. 2010;10(28):6827–6828. [Google Scholar]
- 80.Liu Y., Miao L., Sun Q. Clinical study of Shensong Yangxin Capsule combined bisoprolol on elderly paroxysmal atrial fibrillation(参松养心胶囊联合比索洛尔治疗老年阵发性心房颤动) J Changchun Univ Chin Med. 2020;36(04):688–691. [Google Scholar]
- 81.Chen S., You C., Huang M., Cheng X., Zheng J., Huang Q. Clinical study on the treatment of paroxysmal atrial fibrillation combined with cardiac insufficiency by Shensong Yangxin capsule combined with sacubitril valsartan(参松养心胶囊联合沙库巴曲缬沙坦治疗阵发性心房颤动合并心功能不全的临床研究) Chin J Integr Medic Cardio Cerebrovasc Dis. 2022;20(01):131–134. [Google Scholar]
- 82.Liu Y., Liu Z., Zhang X., Ban Y., Sun Z. Efficacy of Shensong Yangxin capsule combined with amiodarone in the treatment of paroxysmal atrial fibrillation(参松养心胶囊联合胺碘酮治疗阵发性心房纤颤的疗效观察) Hebei Med J. 2010;32(19):2716–2717. [Google Scholar]
- 83.Li S., Chang Y., Wu Z. Effect of Shensong Yangxin capsule in the adjuvant treatment of heart failure combined with paroxysmal atrial fibrillation in the elderly and its effect on patients’ cardiac function(参松养心胶囊辅助治疗老年心力衰竭合并阵发性房颤的效果及对患者心功能的影响) Clin Res. 2019;27(08):137–138. [Google Scholar]
- 84.Xiao J., Fu J., Li X., Ji D., Chen J. Analysis of the safety and clinical effects of Shensong Yangxin capsules in treating hypertension complicated with paroxysmal atrial fibrillation(参松养心胶囊治疗高血压并发阵发性房颤的临床疗效及安全性分析) West J Tradit Chin Med. 2021;34(08):110–112. [Google Scholar]
- 85.Cao D., Yang X., Shi Y. Clinical observation on 58 cases of chronic heart failure combined with paroxysmal atrial fibrillation treated with Shensong Yangxin capsule(参松养心胶囊治疗慢性心力衰竭合并阵发性心房纤颤58例临床观察) Chin J Clin Rational Drug Use. 2013;6(17):49–50. [Google Scholar]
- 86.Sheng H., Xu M. The clinical observation on ShenSong YangXin capsule in treating paroxysmal atrial firbrillation(参松养心胶囊治疗阵发性心房颤动30例) Gansu J Tradit Chin Med. 2010;23(12):24–25. [Google Scholar]
- 87.Men R. Treatment of paroxysmal atrial fibrillation with Shensong Yangxin capsule in 42 cases(参松养心胶囊治疗阵发性心房颤动42例) China Pharmaceuticals. 2012;21(17):69. [Google Scholar]
- 88.Wang A., Pu J., Yong Q., et al. A multicenter clinical study on the treatment of paroxysmal atrial fibrillation with Shensong Yangxin Capsule(参松养心胶囊治疗阵发性心房颤动的多中心临床研究) National Med J Chin. 2011;(24):1677–1681. [Google Scholar]
- 89.Han H., Zhou Y., Wang Y., Pan Y., Xu C. Clinical study on the therapeutic effect of Shensong Yangxin capsule on paroxysmal atrial fibrillation(参松养心胶囊治疗阵发性心房颤动的临床研究) Chin J Difficult Complicat Cases. 2007;(07):389–391. [Google Scholar]
- 90.Liu Y., Li S. The effect of Shensong Yangxin capsule and amiodarone on p-wave dispersion in patients with paroxysmal atrial fibrillation with coronary artery disease(参松养心胶囊及乙胺碘肤酮对冠心病阵发性房颤病人P波离散度的影响) Heilongjiang Medicine Journal. 2013;26(03):408–409. [Google Scholar]
- 91.Peng G., Li S., Liu B., Zhang W. Effect of Shensong Yangxin Capsuleon P-wave dispersion in patients with hypertension and paroxysmal atrial fibrillation(参松养心胶囊对高血压并阵发性心房颤动患者P波离散度的影响) Chin J Integr Med Cardio Cerebrovasc Dis. 2012;10(01):29–31. [Google Scholar]
- 92.Kang X., Huang X., Zheng Z. Combined with western medicine treatment of paroxysmal atrial fibrillation randomized parallel group study Shensongyangxin Capsules(参松养心胶囊联合西药治疗阵发性心房纤颤随机平行对照研究) J Pract Tradit Chin Internal Med. 2014;28(12):72–74. [Google Scholar]
- 93.Liu H., Zhang J. Treatment of paroxysmal atrial fibrillation with Shensong Yangxin capsule in 60 cases(参松养心胶囊治疗阵发性心房颤动60例) Shaanxi J Tradit Chin Med. 2011;32(11):1509–1510. [Google Scholar]
- 94.Zhou Q. Effect of valsartan combined with Shen Song Yangxin capsule in the treatment of hypertensive patients with atrial fibrillation(缬沙坦联合参松养心胶囊治疗高血压合并心房颤动的效果) Chin J Thrombos Hemostas. 2018;24(02):257–260. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.