Abstract
Background
Early initiation of antiretroviral therapy improves human immunodeficiency virus (HIV) outcomes. However, achieving earlier treatment initiation is challenging for many reasons including provider awareness and clinic barriers; this study sought to understand perceptions of an early initiation program.
Methods
We interviewed 10 providers from 3 HIV clinics in North Carolina (October-November 2020). We asked providers about overall perceptions of early initiation and the pilot program. We developed narrative summaries to understand individual contexts and conducted thematic analysis using NVivo.
Results
Providers believed earlier initiation would signal an “extra sense of urgency” about the importance of antiretroviral therapy—a message not currently reflected in standard of care. Safety was a consistent concern. Cited implementation barriers included transportation assistance, medication sustainability, and guidance to address increased staff time and appointment availability.
Conclusion
Our qualitative findings highlight the need for training on the safety of early initiation and addressing staffing needs to accommodate quicker appointments.
Keywords: HIV, healthcare providers, ART therapy, rapid treatment initiation, people living with HIV (PLWH)
Plain Language Summary
Doctor and clinic staff perspectives on a program to immediately start HIV treatment among patients newly diagnosed with HIV
Treating human immunodeficiency virus (HIV) is easier than ever. Starting newly diagnosed persons on HIV medication as soon as possible is a now recommended goal. However, starting patients right away can be challenging. This study interviewed doctors and clinic staff to better understand their perspectives prior to implementing a program that would provide newly diagnosed patients with HIV treatment immediately. Results showed that some doctors are worried patients will not return after receiving their medications. Providers want support for linking patients to the clinic and ensuring they will be able to receive their next dose of medication when they come in. Other providers saw the benefits of reducing HIV stigma if the program can more quickly start patients on treatment. Some providers explained that when you go to the doctor and are sick you receive medications immediately, yet for newly diagnosed patients living with HIV, patients can be told to come back a month later to start treatment. Some providers believe shifting this messaging may also help patients take their medications better. Most providers saw the need for clinics to have more same-day appointment availability to meet the needs of the new program. Overall, providers were excited about the opportunity to improve the HIV care by offering HIV medications to newly diagnosed patients immediately.
Introduction
Access to human immunodeficiency virus (HIV) care has drastically increased across the globe with roughly 26 million people living with HIV (PLWH) on antiretroviral therapy (ART) in 2020 compared to just 6.6 million in 2010.1,2 In 2016, the World Health Organization (WHO) encouraged countries to adopt a policy of initiating ART as soon as possible after the HIV diagnosis was confirmed. 3 Earlier initiation of ART safely decreases time to HIV viral suppression, thereby reducing viral transmission potential.4–7 However, despite this evidence, implementation of earlier ART initiation remains a challenge. In the United States (US), roughly 20% of people diagnosed with HIV in 2019 did not visit an HIV healthcare provider (HCP) within one month of their diagnosis 8 despite recommendations to start treatment “as close to the date of HIV diagnosis as possible.” 9 Moreover, states located in the US South (ie, Washington, District of Columbia, North Carolina, South Carolina, etc) account for only one-third of the total US population, yet represent over half of all new HIV cases annually. 10 Barriers distinctly prominent in the rural and semirural US South, such as long distances to health facilities, limited public transportation and health infrastructure, lower rates of health insurance coverage (ie, states not adopting Medicaid expansion), pervasive stigma, and confidentiality concerns, all lead to delays in scheduling first clinic visits, receiving lab results, and accessing ART.11–15 These barriers result in over 40% of persons in the US South waiting 3 or more months before initiating ART following HIV diagnosis. 15 Furthermore, these delays increase loss to follow-up, time to viral suppression, and the potential for new HIV transmission events in the community.14–17
Rapid treatment initiation (RTI) was first proposed to allow ART initiation to occur as soon as possible after HIV diagnosis.18–21 These RTI programs provide ART along with medical, psychological, and social support and have improved access and sustained engagement of care for vulnerable populations.18,19,22 RTI programs outside the US report that proper training materials, strong intradepartment relationships, accountability, and peer counselors are strong facilitators of implementation. 23 Despite limited national or statewide guidelines for the implementation of RTI programs in the US (Table 1), RTI programs have recently been implemented in large, urban centers with success in producing higher rates of viral suppression and increased retention in care compared to standard care models.21,24–31 However, RTI has not been uniformly implemented across HIV care sites.
Table 1.
Current HIV Treatment Guidelines and Definitions for RTI.
| What are the current guidelines for HIV treatment in the US? |
|
| What is happening in North Carolina? |
|
| RTI definitions |
|
| NC RAPID's definition of RTI |
|
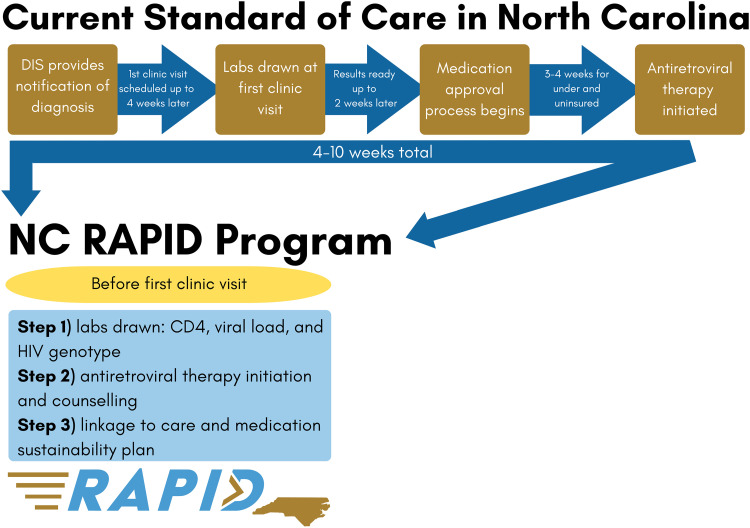
The lack of more widespread RTI program guidelines and implementation in the US is due in part to a gap in research on RTI outside urban settings, a lack of HCP awareness of evidence for RTI benefits, 32 and potential clinic-level facilitators and barriers, especially in rural and semirural settings. In many care settings in the US South, it can take 4 to 10 weeks from HIV diagnosis to the initiation of ART (Figure 1). The complex barriers to RTI implementation in rural and semirural settings are particularly poorly understood.11,14,15,30
Figure 1.
A comparison of the NC RAPID 3-step RTI program with current standard of HIV care in North Carolina.
RTI, rapid treatment initiation.
The North Carolina (NC) Rapid ART Program for Individuals with an HIV Diagnosis (NC RAPID) is an RTI pilot program designed to address common barriers in rural and semirural settings by providing ART initiation in the field (ie, health department, participant's home) before a first HIV care appointment (Figure 1). Currently in NC, the majority of newly diagnosed persons are contacted and interviewed by a NC disease intervention specialist (DIS) who may be the first person the newly diagnosed person talks to about their diagnosis (Table 1). The DIS provides HIV counseling, answers questions, conducts anonymous contact-tracing, and sets up an HIV care appointment at the individual's clinic of choice.
The initial design of the NC RAPID program included having a NC RAPID nurse accompany a DIS on field visits with newly diagnosed individuals to allow for education, nurse assessment, and initiation of a 30-day supply of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) in the field. However, the cessation of in-field notification by DIS due to the COVID-19 pandemic necessitated modifying the program to prompt referral for rapid ART initiation at a local health department prior to the scheduled clinic visit. Under this revised program, a DIS would introduce the program to newly diagnosed individuals and refer interested individuals to the NC RAPID nurse. The NC RAPID nurse would contact interested individuals to describe the program and schedule prompt appointments for them at the health department.
To inform the development and implementation of the NC RAPID program, we sought to answer the following three research questions with HCPs prior to NC RAPID program implementation: (1) Awareness and acceptability of RTI?; (2) Who do HCPs believe are appropriate candidates for RTI?; and (3) What are HCPs thoughts on the NC RAPID program, including clinic facilitators and barriers?
Methods
Study Design and Recruitment
Between October and November 2020, we conducted formative qualitative in-depth interviews with HCPs located in a semirural county where the new RTI pilot program—NC RAPID—would be implemented. We recruited 10 HCPs from a list of HCPs representing HIV care sites located in the county, and purposefully selected a sample that reflected the many different staff (eg, physicians, nurses, patient navigators) within HIV care clinics typically engaged with people newly diagnosed with HIV entering care (Table 2). We also sought to include a diverse sample of HCPs in terms of position, education level, years of experience working with PLWH, and years working at the current clinic. This study was guided by the Consolidated criteria for reporting qualitative research, a 32-item checklist for interviews and focus groups (see supplemental materials). 33
Table 2.
HIV Provider Types and Corresponding HIV Care Role in North Carolina for Clinics not Providing Rapid Treatment Initiation.
| Position title | Required credentials | Role played in providing HIV care to newly diagnosed persons |
|---|---|---|
| Clinician (n = 3) | Doctor of medicine (MD), physician assistant (PA), or nurse practitioner (NP) | Provides counseling on HIV infection and natural history, HIV treatment, and adherence; answers questions; reviews lab results and next steps for starting HIV medications. |
| Clinical pharmacist (n = 2) | Doctor of pharmacy (PharmD) | May see people newly diagnosed with HIV at diagnosis and can complete all duties as described above under clinician. May have more time available to see people than physicians. |
| Nurse (n = 1) | Registered nurse (RN) | Provides support to people newly diagnosed with HIV by taking vitals and answering any questions people have. Some serve as the contact liaison between a person newly diagnosed with HIV and their provider. |
| Patient navigator (n = 3) | None | Performs outreach to facilitate connections to care and provide resources, including transportation assistance. Once person newly diagnosed with HIV is in the clinic, works with the clinical team to complete required paperwork for medication sustainability (ie, HIV Medication Assistance Program [HMAP]). Reviews any questions people have and may serve as the main point of contact between clinic and the person newly diagnosed with HIV. |
| Bridge counselor (n = 1) | None | Works closely with NC Department of Health and Human Services to perform outreach to people who have been out of care for longer than 12 months and attempt to link them to care. |
Data Collection
Using a basic interpretive qualitative description design, 34 we developed an in-depth interview guide based on the three research questions (ie, RTI awareness/perceptions, appropriate candidates, and thoughts on the NC RAPID program prior to implementation) (see supplemental materials). To elicit responses about RTI in general, participants were asked about their concerns and perceived benefits of RTI and to identify and describe an ideal RTI candidate. To answer research questions specific to the NC RAPID pilot program, we described each step of the program and asked participants for their perceptions including potential clinic barriers and facilitators to implementation of each program step (see “Program Description” below). All interviews were conducted in English, over the phone, audio recorded, and lasted 30–45 min. This study was approved by The University of North Carolina in Chapel Hill (IRB study number: 19-1482). Participants were emailed the consent form prior to the interview. At the beginning of the call, interviewers read and reviewed the consent and documented verbal consent. Verbal consent, approved by the IRB, was used due to the minimal risk involved and the circumstances of conducting interviews during the COVID-19 pandemic. All participants were compensated for their time. We continued interviewing until data saturation was reached.
Program Description Provided to HCPs
We described the 3-step NC RAPID program to each HCP as follows (see Figure 1). In Step 1, the NC RAPID nurse or another advanced practitioner performs a standard sexually transmitted infection assessment; collects blood for CD4 cell count, HIV viral load, and HIV genotype testing; reviews a checklist of potential comorbidities; and screens for symptoms of opportunistic infections. Assuming no contraindications, in Step 2, the program nurse provides the participant with a 30-day supply of BIC/FTC/TAF and counsels the participant on the importance of daily adherence and possible side effects. Participants may opt to take the first dose with the nurse or take it later that day. The nurse's emergency contact number is provided to participants for questions and concerns. Step 3 involves scheduling a clinic appointment (or confirming appointment already made) within 1–14 days. The program nurse also provides a list of all items to bring to the first clinic appointment to facilitate submission of applications for ongoing ART access if needed. After the visit, the program nurse ensures laboratory results are sent to the clinic of choice.
Data Management and Analysis
All interviews were audio recorded and transcribed verbatim. Once transcripts were received, each transcript underwent a quality assurance/quality control (QA/QC) step whereby a study team member listened to the audio and reviewed the transcript to ensure the audio reflected the transcript. Each study participant was given a unique study identifier. All personal identifiers (ie, references to staff names or clinic names) were removed during the transcription phase and during the QA/QC phase. Audio and transcripts were saved in a secure, IRB-approved, password-protected study portal only accessible to IRB-approved study personnel.
Using finalized transcripts, narrative summaries were developed to understand the individual context for each study participant. Narrative summaries covered the following areas with integrated quotes: (1) professional background; (2) provider awareness of RTI; (3) provider's thoughts on “ideal candidate” for RTI; (4) perspectives of the program and clinic readiness; and (5) program scalability.
A codebook was developed that included question-based codes and emerging domain codes revealed in the narrative summaries. The team reviewed the codebook prior to coding to confirm definitions and review example codes. All transcripts were coded in NVivo Release 1.6 by 2 independent data analysts. Approximately one-third of the transcripts were selected to assess interrater reliability. Any discrepancies in the application of codes (with Kappa scores of 0.7 or less) were identified, discussed, and resolved by recoding transcripts accordingly to ensure consistency in code application. Code outputs were generated for all domains and reviewed by the team for salient themes. Code summaries were developed by 3 data analysts to review themes across all providers; matrices were used to understand relationships by prescribing (n = 5) or nonprescribing provider (n = 5) types within each key domain.
Results
Sample Characteristics
Most HCP study participants identified as non-Hispanic or Latino (80%), white (80%), and female (60%) (Table 3). Median age was 41.5 years. Half (n = 5) of the providers were able to prescribe and had terminal medical degrees. Providers had experience working in HIV care settings (median 9.5 years) and working at their current clinic (median 6.5 years). Clinic size varied with smaller clinics reporting 500 patients and larger clinics reporting 5000 (median load was 2100). Providers saw anywhere from 4 to 12 people per day (median per day was 6 people).
Table 3.
Demographics of All Participants Interviewed Prior to NC RAPID Implementation.
| Characteristic | n (%) |
|---|---|
| Gender | |
| Female | 6 (60) |
| Male | 4 (40) |
| Age | |
| Median (IQR) | 42 (33-52) |
| Race | |
| White | 8 (80) |
| African American or Black | 1 (10) |
| Other† | 1 (10) |
| American Indian or Alaska Native | N/A |
| Asian Pacific Islander | N/A |
| Ethnicity | |
| Non-Hispanic or Latino | 8 (80) |
| Hispanic or Latino | 2 (20) |
| Provider type | |
| Physician‡ | 3 (30) |
| Patient navigator§ | 3 (30) |
| Clinical pharmacist‡ | 2 (20) |
| Registered nurse§ | 1 (10) |
| NC HIV state bridge counselor§ | 1 (10) |
| Years at current HIV clinic | |
| Median (IQR) | 7 (0.25-21) |
| Total years providing HIV care | |
| Median (IQR) | 10 (1-24) |
| ≤ 1 year | 2 (20) |
| >1 year and ≤ 10 years | 3 (30) |
| > 10 years | 5 (50) |
| No of patients per day‡ | |
| Median (IQR) | 6(4-12) |
| Total No of patients at clinic | |
| Median (IRQ) | 2100 (100-5000) |
† Includes self-reported Indigenous.
‡ Prescribers—able to prescribe medication.
§ Nonprescribers—not able to prescribe medication.
Overview of Qualitative Findings
Qualitative findings were grouped into 4 broad themes: (1) awareness of RTI; (2) acceptability of RTI as an approach to treat newly diagnosed PLWH; (3) perceptions of the appropriate candidate for RTI; and (4) clinic implementation of the NC RAPID program. Below we present each theme in turn and, where appropriate, provide a figure and/or point out differences between prescribing and nonprescribing providers.
Awareness of RTI
Most providers, regardless of medical training level, were aware of RTI as a recommended approach. All prescribing providers were aware of RTI and identified first hearing about RTI either in meetings, discussions with colleagues, or within the scientific literature. Two prescribing providers specifically mentioned the success of an RTI program in California:
It [RTI] has always made sense. I think there was, like, some work from California in particular that showed some benefit with that. (Prescribing provider)
Acceptability of RTI
Providers shared their perceptions of concerns and benefits of RTI both for people newly diagnosed with HIV and for themselves as HCPs.
Perceived RTI Concerns for People Newly Diagnosed With HIV
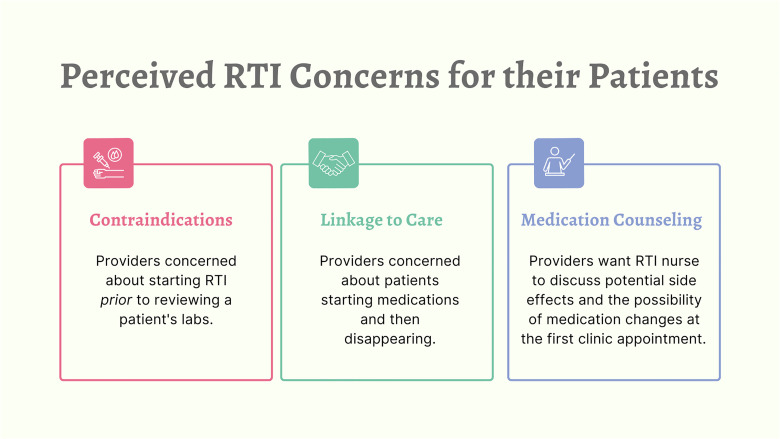
Providers reported 3 main areas of concern when thinking about RTI and people newly diagnosed with HIV (Figure 2). First, several providers described concerns about starting these patients on HIV medication prior to seeing laboratory results and the potential of contraindications. Providers worried about complications of starting people who have limited kidney and liver function, unknown underlying resistance, or other comorbidities:
My concern and this is really not the majority of patients, this is really kind of around the edges, but how do we make sure someone with cryptococcal meningitis …. starting them on therapy immediately could actually be harmful.” (Prescribing provider)
However, linking people to care quickly would help negate some of these concerns:
Figure 2.
Perceived RTI concerns for people newly diagnosed with HIV.
RTI, rapid treatment initiation.
There's a theoretical potential for harm if we don’t know somebody's kidney or liver function … However, having them come to an appointment within one to two weeks after starting it, I think, would greatly minimize that. So I wouldn’t hold that as a risk that's high enough to be a contraindication. (Prescribing provider)
Given the importance of linkage to care quickly, this highlighted several providers’ second concern:
They come in; they get their 30 days; and then for whatever reason, they never come back. And it becomes that loss to follow-up, and inconsistent and interrupted antiretroviral therapy, which is not optimal. (Prescribing provider)
The final area of concern involved step 2 of the NC RAPID program: medication counseling. Several providers highlighted 2 topics they would want covered during this step. The first topic was the importance of the RTI nurse educating newly diagnosed individuals about the potential of regimen changes at the first HIV care clinic visit:
They come in, and they're on Biktarvy, and they think everything is fine because that is what we prescribed to them at the time of the rapid initiation occurred, and then we're suddenly telling them that this isn't the best therapy for them. I think that could result in some confusion on the part of the patient, and even some mistrust that this therapy might not have been the best thing for them. We just have to be transparent and making sure the patient understands why it's important to do rapid initiation of ART, and the benefits of starting immediately after diagnosis compared to waiting. And letting the patient know upfront that this therapy is going to provide these short-term benefits for you, but after going through a more formal and full evaluation … it could be that the provider you see when you come to clinic could change your therapy. (Prescribing provider)
The second topic was discussing potential side effects prior to starting HIV treatment. For example, one provider worried that if potential side effects are not discussed and people has side effects, they could say, “‘I’m never gonna take a medicine ever again. Medicines are the devil.’ You know? They hyperbolize, they catastrophize” and they disappear. (Prescribing provider)
These concerns about the potential variability in medication counseling highlight one provider's concern and personal discomfort with a loss of control:
I will admit it's taking a bit of a leap, essentially what we’re doing is trusting that the beginning of treatment gets handled elsewhere. I will just be straightforward. That makes me extremely nervous. (Prescribing provider)
Yet another physician saw their future role in the program differently, saying they would just be “picking up the ball that's been tossed” following RTI initiation (Prescribing provider).
Perceived Benefits for People Newly Diagnosed With HIV
All providers, regardless of role or years of experience, identified benefits of RTI for people newly diagnosed with HIV. For some, RTI would demonstrate to people that they can take immediate action and that they are not alone in their HIV diagnosis. As one provider stated: “It gives a sense to the patient that they have an immediate support team, and that's something immediately been done.” (Nonprescribing provider)
Most providers also spoke to the benefit of an RTI program in providing an additional visit at which people newly diagnosed with HIV could be educated and to ask questions—potentially allowing providers more time at the first clinic appointment to dispel common HIV misconceptions. Some providers highlighted that newly diagnosed individuals may still view an HIV diagnosis as: “a death sentence.” As one provider described among his predominantly Latino patients: “[they] are still stuck with what they knew about HIV from the 80 s and 90s … they have this idea that becoming positive for HIV is like a death sentence. And they are going to develop up all of these symptoms like, loss of weight” (Nonprescribing provider). These providers noted that it usually takes multiple interactions for new information to stick with people newly diagnosed with HIV; one compared hearing an HIV diagnosis to first learning you have cancer:
It's kinda like hearing a cancer diagnosis. Right? Like, you say the word cancer, and then nothing else you say is just, ‘[Wah wah wah wah]…’ Like, nothing else means anything, and you have to explain things multiple times just ‘cause of the shock and psychological trauma of stuff like that. So there's a chance that not only will patients have a lot of questions, but they might need multiple iterations to, kind of, absorb it. (Prescribing provider)
Describing the stigmatized experience of people newly diagnosed with HIV, many providers saw the benefits of the RTI program to reduce stigma in multiple ways. First, patients could hear about Undetectable = Untransmittable (U = U) closer to their diagnosis with the program nurse compared to hearing this message at the first clinic appointment. Second, because the RTI program would ensure labs are ready for the first clinic appointment, providers could reinforce treatment goals with people using laboratory data, as described by one nonprescribing provider:
…can have a conversation with patients about their lab values—especially the viral load and the CD4, and watching those trend in the appropriate direction, I think that gives patients a tangible goal. (Nonprescribing provider)
Despite some concern about linkage to care mentioned earlier, many providers see the potential benefit of RTI in increasing linkage to care if the program can help people overcome the anxiety of going to their first HIV care appointment. Providers described how people are nervous to come to the first clinic appointment and how the ability of the program nurse to see people in the community may reduce some anxiety. If the visit goes well, some believe the established rapport between the program nurse and the person newly diagnosed may transfer to HCPs, as described by one provider: “If it goes well, it could be the first experience with this thing [HIV care] that they were so scared of that it might actually be okay” (Prescribing provider).
Many providers, particularly prescribing providers, identified another benefit of RTI: to send a more urgent message to people to begin HIV treatment now compared to starting treatment after completing lab work. Some providers shared that many people newly diagnosed with HIV want to start immediately at the first clinic visit, saying: “Just give me anything that is gonna keep me safe” (Nonprescribing provider). Providers explained that delaying the start of treatment sends the wrong message to people that their diagnosis is not a big deal:
Yes, you’ve got this super important disease, um, and you’re gonna see me in 4 weeks, and we’re gonna follow up your labs and then start meds. (Prescribing provider)
And some worry that the “see me in 4 weeks” message might affect future treatment adherence, as described below:
People kinda got that mixed message about having a severe disease, ‘Oh, it's okay for us to wait a month before we start you on treatment.’ You know? That sets the stage for them thinking that treatment is not that important. (Prescribing provider)
Others worry the messaging of delaying HIV treatment, if not explained properly, leads to confusion and anxiety for people with HIV who did want to start treatment right away. One provider described how this looks in particular for people of color:
There are communities of color, who may actually believe that they were receiving inferior care because we’ve scheduled their visit to be that far out from when they were first diagnosed… especially if we’re not giving them the justification for the decisions that we’re making about when they get their care. (Prescribing provider)
Specific to the NC RAPID program—where people newly diagnosed with HIV will be offered the opportunity to take their first dose with the program nurse—some providers saw the importance of reinforcing a message of urgency. As one provider shared, this “ups the urgency of the situation,” going on to explain how they hope to see this translate to better retention in care:
‘Here. I’ll watch you take your first dose’ … that should install an extra sense of urgency in getting the HIV under control- which is something that I would hope would then lead to more retention and care. So staying with the clinical care and staying on the meds ‘cause they, kind of, regard it as an emergent situation. (Prescribing provider)
Perceived Benefits for Providers: “You’re Making Our Job Easier”
A common theme among many providers was that the first clinic visit would be very different with a person who had already talked with a nurse and started medications. Many believed their jobs would be easier because people would be coming with a greater “knowledge base” of HIV medications: “If they’re already on meds, then they have a little bit of an understanding of the value of what those medications can do” (Nonprescribing provider). Also, providers reported that having laboratory data ready to review on the first visit would shift the initial conversation from explaining HIV treatment options to discussing adherence and side effects in more detail. One provider described having this time to discuss with people how their medication is going as “adding value” to the first clinic appointment. Others saw their jobs becoming easier because instead of initiating treatment, they saw themselves as reinforcing the treatment:
You’re making our job easier because we already have a knowledge base that we can build upon … we can just kinda reinforce the knowledge. (Nonprescribing provider)
One nonprescribing provider wondered if her job would become easier because fewer people would be out of care. After sharing her experience with burn-out due to the high number of people out of care, she described the potential of the program nurse to secure more contact information:
They [RTI nurse] have really good locating information, which will help us if and when that person falls out of care because that tends to be a big problem because sometimes people are so transient, I mean, just even getting the emergency contact, or a number where a person hangs out at, which is something that the NC Rapid nurse may be able to collect when they’re in their intake. (Nonprescribing provider)
Furthermore, for providers, the ability to help people newly diagnosed with HIV take immediate action may lead to a psychological benefit for themselves, as one provider described:
I wonder if there's a psychological benefit for providers, a feeling of accomplishment, a feeling of doing right by your patients …. it feels good to me when I’m seeing somebody for a new diagnosis, and that day saying, ‘Yeah. Today we’re also gonna start you on therapy.’ (Prescribing provider)
The Appropriate RTI Candidate: “Is this person basically healthy except for the HIV?”
Given the concerns providers shared about starting RTI among people newly diagnosed presenting with serious comorbidities (eg, kidney issues, active hepatitis infection), several providers saw the appropriate RTI candidate as young, healthy, and psychologically ready to begin HIV therapy:
…younger people because just the odds of having some underlying comorbidity are less…[and] kids, are likely to be already vaccinated and immune to Hepatitis-B, so that would be less of a concern in that population. (Prescribing provider)
Further, providers discussed the advantages of RTI for reducing community transmission of HIV among young people, a population they see as more sexually active:
But then one other issue here is why are we doing rapid initiation? Part of it is the public health benefit of rapidly initiating therapy, where we can get the viral load down so that if they are sexually active then we can prevent new transmission by starting therapy immediately. And I think that that benefit is gonna be more prominent, more likely to occur in a younger person, who may not be in a monogamous relationship. (Prescribing provider)
A few nonprescribing providers also identified young people to be ideal candidates; however, they were less focused on the initial health status of the person, as prescribing providers were, and more interested in the potential of RTI to capitalize on the “instant gratifying” ethos of the younger population and support the messaging around becoming undetectable (ie, U = U):
The younger folks like that instant gratification … Like, if you have something that's so life-saving that can make you undetectable, why not get started as soon as possible, I think is the way we try to frame it with some of our younger folks. (Nonprescribing provider)
Beyond younger people, many providers saw the ideal candidate as someone who passes a checklist of concerning comorbidities. As explained by one prescribing provider, “I think I would like to see some evaluation … maybe running through a checklist.” Echoing this sentiment, another provider saw the checklist as an opportunity to “pump the brakes” and address those concerns prior to RTI initiation:
I would hope that there is a point in the interview with the [patient] that you can just pump the brakes and say, ‘You know what? We need to address these issues first … maybe even before they come to the ID clinic, have them go to a mental health provider.’ (Prescribing provider)
While most providers saw the ideal person as healthy, one provider believed the ideal candidate to be someone that has “more advanced disease … the ones who are crossing that 200 threshold [CD4 count].” This provider believes the medical risks are worth the potential decrease in community spread:
Maybe patients that are at high risk for actually transmitting the virus to others? Getting them undetectable earlier could be beneficial, but not necessarily at immediate risk of complications of their disease, I believe. (Prescribing provider)
The final characteristic providers identified for the ideal candidate was the importance of psychological readiness to begin treatment and attend clinic appointments. A few providers saw buy-in to be the defining candidate characteristic, regardless of health status. Notably, all physicians described how they would first assess buy-in and withhold RTI if there was not “individual willingness to be on therapy.” Here one provider describes how they would first assess readiness:
…mostly through a conversation about how they feel about taking pills. And what their schedule is like. How they think they could fit it- where they would fit it in. How worried they are about side effects and things like that. (Prescribing provider)
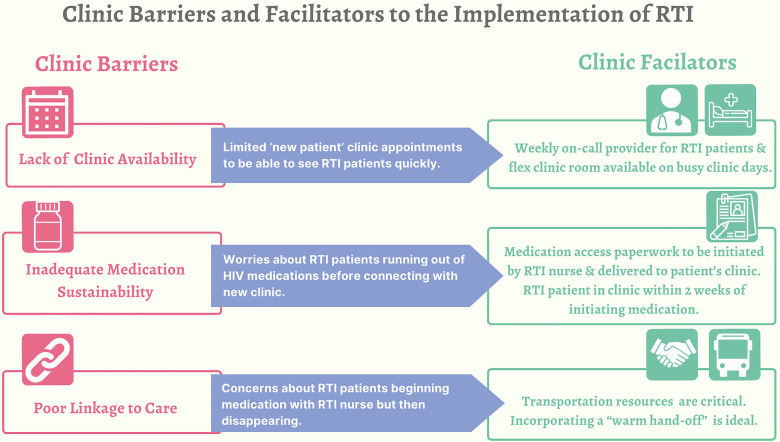
Barriers and Facilitators to Clinic Implementation of NC RAPID
Providers identified three clinic barriers and facilitators to the implementation of RTI (Figure 3). First, providers were concerned about clinic appointment availability. Many described the current challenges of limited, new clinic appointments and worried about being able to meet the demand of people on the RTI program who need to be seen within 1–14 days of starting medications:
The way we currently have it set up, there's a number of new patient appointments available every week but they do fill … so you guys call and say, ‘Hey, we have a new person. We’re starting meds today. And we really need to get them in.’ We’re gonna need to figure out where we work people in if there doesn’t happen to be a spot open. (Prescribing provider)
This same provider believed a facilitator to meet the demand of the RTI program would be to have a physician on call each week responsible for seeing new people. Another provider offered the same solution and said they could see the clinic having a dedicated “flex room” to help with space constraints particularly on busy clinic days. Among prescribing providers, many saw the importance of increasing clinic availability while noting that physicians would be motivated to meet this demand, as illustrated by one prescriber: “many of us are happy to, kind of, make extra time when needed for somebody who's newly diagnosed.” (Prescribing provider)
Figure 3.
Clinic barriers and facilitators to the implementation of RTI.
RTI, rapid treatment initiation.
The second most described barrier to RTI implementation was the clinic's ability to ensure medication sustainability. As one provider described, “the clock is ticking, ‘cause the patient's on meds.” This same provider described how RTI could place an increased burden on the clinic or, more specifically, the patient navigator to finalize the medication paperwork quickly, particularly if people show up “swallowing their last dose.” To facilitate this process and ensure medication sustainability, many providers agreed with the RTI program's linkage to care goal of 1–14 days to give clinics the most time to process paperwork:
I’d probably want a 2-week overlap or so, so that I’d have some wiggle room. ‘Cause again, people aren’t perfect. People are human. Is everybody gonna show up with all their documents and everything signed, and every ‘i’ dotted and ‘t’ crossed? Eh. I’d like to think so, but realistically, we know the answer is ‘no.’ Right? So we need to build in some-some redundancy and some cushion into the system so that we can address the real-world stuff. You know? (Prescribing provider)
The final barrier was a concern that people may start medications and not show up for their first visit. Providers worried that clinics would not have the capacity or the appropriate staff to be able to search for these people. As one provider shared:
… what is the process for tracking down that person and getting them into care? Even if it's not in the original location where they were first seen for the Rapid Start [program] … you’re talking about having the actual personnel available to … travel to places where they many not feel comfortable walking into a person's home. (Prescribing provider)
To facilitate linkage to care, some providers stressed the importance of transportation resources to and from clinic and suggested the RTI program add a fourth step to help facilitate people accessing these resources. Providers suggested the program nurse call the clinic and have the person talk to an HCP for a “warm hand-off”:
It would be nice if when the patient is meeting with the nurse, if they do a conference call or speaker phone call to the [patient] navigator so that they can have a joint conversation. (Nonprescribing provider)
Discussion
The goal of this qualitative study was to better understand HCP's views of a rapid treatment initiation program that would initiate HIV therapy before the first HIV care appointment of persons newly diagnosed with HIV and thus closer to date of diagnosis. Awareness of RTI was high among all provider types. Acceptability of RTI as an approach was, in general, supported, but providers reported concerns about beginning an ART regimen before reviewing labs and that linkage to care may not occur in some instances. However, providers identified the value of an RTI program to reduce stigma, provide more opportunities for counseling, and potentially encourage linkage to care. Overall providers see the appropriate candidate for RTI to be young and healthy. HCPs believed their jobs would be easier if people were already on medication at the initial HIV care visit, but worried about clinic capacity to meet new appointment demands and ensure medication sustainability.
Although a few providers reported worries about the safety of RTI, research shows RTI programs are safe, increase viral suppression at 12 months, and can lower the risk of tuberculosis and severe bacterial infections.16,35 Further case reports show that BIC/FTC/TAF can safely be used in people with advanced kidney disease 36 and CD4 count, viral load, hepatitis B status, or genotype do not need to be known prior to initiation. To alleviate concerns among providers, in particular prescribing physicians, RTI programs could consider developing a checklist of medical contraindications.
Compared to safety, evidence that RTI programs improve linkage to care evidence is more mixed. Systematic reviews of several RTI programs report increased retention in care at 8- and 12-month timepoints,16,35 suggesting rapid initiation simplifies and reduces the number of clinic visits, and reduces barriers to care (ie, transportation) for PLWH.19,21 However, a few international studies have reported decreases in retention in care among those who completed same-day RTI compared to those who initiated at later timepoints.37–39 Authors of these studies suggest additional counseling and adherence support would allow people enough time to accept their HIV positive status and need to initiate life-long therapy—a finding echoed here among providers in this sample and a key component (Step 2) of the NC RAPID program. A positive first encounter with a provider for a newly diagnosed PLWH can lead to successful HIV treatment outcomes. 40 Furthermore, reviewing potential barriers to care ahead of time, such as transportation challenges or work schedules, can increase linkage to care. Enriquez et al developed a comprehensive checklist of perceived barriers to HIV care that could be used during an RTI program's counseling session to improve retention in care. 41
Same-day RTI at the first HIV care clinic visit can be labor intensive. Pilcher et al reported that same-day RTI was a 3–4 h visit. 21 People newly diagnosed with HIV often lacked health insurance, faced housing insecurities, and needed substance use or mental health treatment, requiring HCPs to address competing needs while initiating HIV therapy. While they found the program feasible after adoption in Ward 86, 96% of people started immediate-ART, 42 the authors warned more research will be needed to create optimal implementation systems given the barriers many people with HIV face.21,42 Similarly, a randomized controlled trial in South Africa comparing same-day ART initiation to standard of care (ART dispensed after 3 to 5 additional clinic visits over 2-4 week period) reported that among the 48 people who did not initiate same day RTI, 13% (6/48) were due to insufficient time to complete all steps on the same day. 19
In contrast, the RTI model we described to providers did not take place at the first clinic visit, but instead before that visit. As such, providers believed this RTI program would make their jobs easier. Providers reported that people already on ART at their first HIV care clinic visit would have a different knowledge foundation—compared to people not on ARTs at that first visit—allowing the provider to shift the discussion from initiating treatment to reinforcing and discussing treatment sustainability. Additionally, providers were excited about the potential of the RTI program to draw labs prior to the first care visit. Providers stated that having laboratory data ready to review at the first HIV care visit would free up opportunities to discuss adherence challenges and side effects. Despite seeing their jobs as being easier, providers identified appointment availability as a key clinic barrier that will require creative solutions such as flex-rooms and on-call providers. For future replication of the RTI model described here, additional staff time to conduct the 3-step process needs to be considered.
Finally, providers described the benefit of an RTI program to shift the messaging from, “Yes, you’ve got this super important disease and you’re gonna see me in four weeks” to “Today we’re gonna start you on therapy.” Providers believed this shift in messaging could improve treatment adherence and viral suppression, which is supported above with increased viral suppression at 12 months for most RTI programs. 43 Further qualitative research to better understand the psychological benefits of RTI on treatment adherence and its effect on other barriers, such as stigma and fear of disclosure, is needed. Future research should consider how implementation barriers and facilitators for RTI programs may differ for newly approved treatment modalities.
This study has several limitations. First, our sample size (n = 10) was small and only included providers from NC, which may limit the generalizability of the findings. However, we did feel that we reached saturation on all themes even with a small sample. Second, providers for this study were recruited from clinics where NC RAPID was to be implemented. This may have led to a selection bias of providers who are more familiar with, or have more favorable views of, RTI programs. Third, the NC RAPID program had to be modified due to the COVID-19 pandemic, so provider perspectives on the original protocol are limited. However, there seems to have been strong support from providers about the potential benefits of the program nurse going into the field as originally planned. Lastly, this paper does not include perspectives from people living with HIV, which may limit our understanding of how the program may be received.
Conclusion
This study interviewed a diverse group of HCPs to explore their perceptions of a rapid treatment initiation program that initiates HIV therapy before the first HIV care appointment. Providers expressed concerns about safety and linkage to care of such programs, but they recognized the value added by reducing stigma, providing more opportunities for counseling, and addressing linkage to care issues before they arise. Providers believed their jobs would be easier if people were already on medication at the initial HIV care visit, but worried about clinic capacity to meet new appointment demands and ensure medication sustainability. Despite these concerns, providers saw the benefits of an RTI program to shift the messaging and improve treatment adherence and viral suppression. This study suggests that RTI programs may be acceptable among HIV care providers in the US South, though several barriers to implementation and scale-up still exist. Additionally, more research is needed to better understand the psychological benefits of RTI for people newly diagnosed with HIV.
Supplemental Material
Supplemental material, sj-docx-1-jia-10.1177_23259582241269919 for Provider Perspectives on Rapid Treatment Initiation Among People Newly Diagnosed With HIV: A New Message of “Urgency”? by Breana J. Uhrig Castonguay, Noah Mancuso, Sarah Hatcher, Sable Watson, Eunice Okumu, Rica Abbott, Carol E. Golin, Victoria Mobley, Erika Samoff, Heidi Swygard, Candice J. McNeil and Cynthia L. Gay in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Supplemental material, sj-docx-2-jia-10.1177_23259582241269919 for Provider Perspectives on Rapid Treatment Initiation Among People Newly Diagnosed With HIV: A New Message of “Urgency”? by Breana J. Uhrig Castonguay, Noah Mancuso, Sarah Hatcher, Sable Watson, Eunice Okumu, Rica Abbott, Carol E. Golin, Victoria Mobley, Erika Samoff, Heidi Swygard, Candice J. McNeil and Cynthia L. Gay in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Acknowledgments
All research was implemented and analyzed by the UNC and Wake Forest teams. This work was also supported by the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410).
Footnotes
Author Contributions: Conceptualization—all; methodology—Heidi, Cindy, Carol, Breana, and Eunice; formal analysis—Breana, Noah, Sarah, Eunice, and Sable; writing—original draft—Breana, Noah, and Sarah; writing—review and editing—all; project administration—Rica; funding acquisition—Heidi, Breana, and Carol.
CEG served in a consulting role for Gilead and received research support from Gilead. HS reports being a full-time employee of ViiV Healthcare. CJM has received grants, contracts and/or participated in clinical trials with Becton Dickinson, Biomedical Advanced Research and Development Authority/GlaxoSmithKline, Centers for Disease Control and Prevention, Cepheid, Hologic, Lupin, The National Association of County and City Health Officials, and the National Institutes of Health, and is on the advisory board for Talis Biomedical paid to her employer Wake Forest University School of Medicine. CLG has received research support from ViiV Healthcare, Moderna and Novavax. All other authors have no conflicts to disclose.
Funding: Interviewers received oral consent from all study participants, and participants were compensated for their time. We continued interviewing until data saturation was reached. This study was approved by The University of North Carolina in Chapel Hill (IRB study number: 19-1482). This project was funded under the Gilead Investigator Sponsored Research program (Grant Number IN-US-380-5507).
ORCID iDs: Breana J. Uhrig Castonguay https://orcid.org/0000-0001-6046-6353
Noah Mancuso https://orcid.org/0000-0002-4442-9293
Sarah Hatcher https://orcid.org/0009-0003-9800-4789
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Global HIV & AIDS statistics — Fact sheet [Internet]. cited 2022. Sep 28. Available from: https://www.unaids.org/en/resources/fact-sheet.
- 2.JC2225_UNAIDS_datatables_en_1.pdf [Internet]. cited 2022. Sep 28. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2225_UNAIDS_datatables_en_1.pdf.
- 3.World Health Organization. 2017. [cited 2023 Sep 20]. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy [Internet]. Available from: https://www.who.int/publications-detail-redirect/9789241550062.
- 4.Temprano ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808‐822. [DOI] [PubMed] [Google Scholar]
- 5.Zolopa AR, Andersen J, Komarow L, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLOS ONE. 2009;4(5):e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insight Start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2018 [cited 2019 Jul]. Understanding the HIV care continuum [Internet]. Available from: https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf. [Google Scholar]
- 9.HIVinfo.NIH.gov [Internet]. cited 2022. Sep 30. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available from: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/early-acute-and-recent-hiv-infection.
- 10.CDC. Centers for Disease Control and Prevention. [cited 2022 Sep 28]. HIV in the Southern United States. Available from: https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf.
- 11.Brewer RA, Chrestman S, Mukherjee S, et al. Exploring the correlates of linkage to HIV medical care among persons living with HIV infection (PLWH) in the deep south: results and lessons learned from the Louisiana positive charge initiative. AIDS Behav. 2018;22(8):2615‐2626. [DOI] [PubMed] [Google Scholar]
- 12.Parker K, Horowitz JM, Brown A, Fry R, Cohn D, Igielnik R. 4. Views of problems facing urban, suburban and rural communities [Internet]. Pew Research Center’s Social & Demographic Trends Project. 2018. [cited 2022 Sep 28]. Available from: https://www.pewresearch.org/social-trends/2018/05/22/views-of-problems-facing-urban-suburban-and-rural-communities/. [Google Scholar]
- 13.Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129(6):611‐620. [DOI] [PubMed] [Google Scholar]
- 14.Sprague C, Simon SE. Understanding HIV care delays in the US south and the role of the social-level in HIV care engagement/retention: a qualitative study. Int J Equity Health. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seña AC, Donovan J, Swygard H, et al. The North Carolina HIV bridge counselor program: outcomes from a statewide level intervention to link and reengage HIV-infected persons in care in the south. J Acquir Immune Defic Syndr. 2017;76(1):e7‐e14. [DOI] [PubMed] [Google Scholar]
- 16.Mateo-Urdiales A, Johnson S, Smith R, Nachega JB, Eshun-Wilson I. Rapid initiation of antiretroviral therapy for people living with HIV. Cochrane Database Syst Rev. 2019. Jun 17;6(6):CD012962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA. 2018;319(11):1103‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan AK, Kanike E, Bedell R, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in option B+ prevention of mother-to-child transmission services at antenatal care in Zomba district, Malawi. J Int AIDS Soc. 2016;19(1):20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr. 2017;74(1):44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd MA, Boffito M, Castagna A, Estrada V. Rapid initiation of antiretroviral therapy at HIV diagnosis: definition, process, knowledge gaps. HIV Med. 2019. Mar;20(Suppl 1):3‐11. [DOI] [PubMed] [Google Scholar]
- 23.Semitala FC, Camlin CS, Wallenta J, et al. Understanding uptake of an intervention to accelerate antiretroviral therapy initiation in Uganda via qualitative inquiry. J Int AIDS Soc. 2017. Dec;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halperin J, Butler I, Conner K, et al. Linkage and antiretroviral therapy within 72 hours at a federally qualified health center in New Orleans. AIDS Patient Care STDS. 2018. Feb;32(2):39‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colasanti J, Sumitani J, Mehta CC, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the southern United States. Open Forum Infect Dis. 2018;5(6):ofy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez AE, Wawrzyniak AJ, Tookes HE, et al. Implementation of an immediate HIV treatment initiation program in a public/academic medical center in the U.S. South: the Miami test and treat rapid response program. AIDS Behav. 2019;23(Suppl 3):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomillia CES, Backus KV, Brock JB, Melvin SC, Parham JJ, Mena LA. Rapid antiretroviral therapy (ART) initiation at a community-based clinic in Jackson, MS. AIDS Res Ther. 2020;17(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathela P, Jamison K, Braunstein SL, et al. Initiating antiretroviral treatment for newly diagnosed HIV patients in sexual health clinics greatly improves timeliness of viral suppression. AIDS. 2021;35(11):1805‐1812. [DOI] [PubMed] [Google Scholar]
- 29.Patel ND, Dallas RH, Knapp KM, Flynn PM, Gaur AH. Rapid start of antiretroviral therapy in youth diagnosed with HIV infection. Pediatr Infect Dis J. 2021;40(2):147‐150. [DOI] [PubMed] [Google Scholar]
- 30.Pettit AC, Pichon LC, Ahonkhai AA, et al. Comprehensive process mapping and qualitative interviews to inform implementation of rapid linkage to HIV care programs in a mid-sized urban setting in the southern United States. JAIDS J Acquir Immune Defic Syndr. 2022;90(1):S56‐S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapid ART Initiation - Clinical Guidelines Program [Internet]. cited 2024. Mar 28. Available from: https://www.hivguidelines.org/guideline/hiv-art-rapid/.
- 32.Moran L, Koester KA, Le Tourneau N, et al. The rapid interaction: a qualitative study of provider approaches to implementing rapid ART. Implement Sci Commun. 2023;4(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349‐357. [DOI] [PubMed] [Google Scholar]
- 34.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334‐340. [DOI] [PubMed] [Google Scholar]
- 35.Bai R, Du J, Lv S, Hua W, Dai L, Wu H. Benefits and risks of rapid initiation of antiretroviral therapy: a systematic review and meta-analysis. Front Pharmacol. 2022. Jun 3;13:898449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidman EF, Ondrush NM. Utilization of bictegravir/emtricitabine/tenofovir alafenamide in patients with end-stage renal disease on hemodialysis. Am J Health Syst Pharm. 2023. Apr 19;80(9):e92‐e97. [DOI] [PubMed] [Google Scholar]
- 37.Kerschberger B, Boulle A, Kuwengwa R, Ciglenecki I, Schomaker M. The impact of same-day antiretroviral therapy initiation under the world health organization treat-all policy. Am J Epidemiol. 2021;190(8):1519‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: analysis of routine data. PLOS ONE. 2020;15(1):e0227572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph Davey D, Kehoe K, Serrao C, et al. Same-day antiretroviral therapy is associated with increased loss to follow-up in South African public health facilities: a prospective cohort study of patients diagnosed with HIV. J Int AIDS Soc. 2020;23(6):e25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott NA, Maskew M, Fong RM, et al. Patient perspectives of quality of the same-day antiretroviral therapy initiation process in gauteng province, South Africa: qualitative dominant mixed-methods analysis of the SLATE II trial. Patient. 2021;14(2):175‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enriquez M, Cheng AL, McKinsey D, et al. Peers keep it real: Re-engaging adults in HIV care. J Int Assoc Provid AIDS Care. 2019. Jan-Dec;18:2325958219838858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coffey S, Bacchetti P, Sachdev D, et al. RAPID antiretroviral therapy: High virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS. 2019;33(5):825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32(1):17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA. 2018;320(4):379‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jia-10.1177_23259582241269919 for Provider Perspectives on Rapid Treatment Initiation Among People Newly Diagnosed With HIV: A New Message of “Urgency”? by Breana J. Uhrig Castonguay, Noah Mancuso, Sarah Hatcher, Sable Watson, Eunice Okumu, Rica Abbott, Carol E. Golin, Victoria Mobley, Erika Samoff, Heidi Swygard, Candice J. McNeil and Cynthia L. Gay in Journal of the International Association of Providers of AIDS Care (JIAPAC)
Supplemental material, sj-docx-2-jia-10.1177_23259582241269919 for Provider Perspectives on Rapid Treatment Initiation Among People Newly Diagnosed With HIV: A New Message of “Urgency”? by Breana J. Uhrig Castonguay, Noah Mancuso, Sarah Hatcher, Sable Watson, Eunice Okumu, Rica Abbott, Carol E. Golin, Victoria Mobley, Erika Samoff, Heidi Swygard, Candice J. McNeil and Cynthia L. Gay in Journal of the International Association of Providers of AIDS Care (JIAPAC)