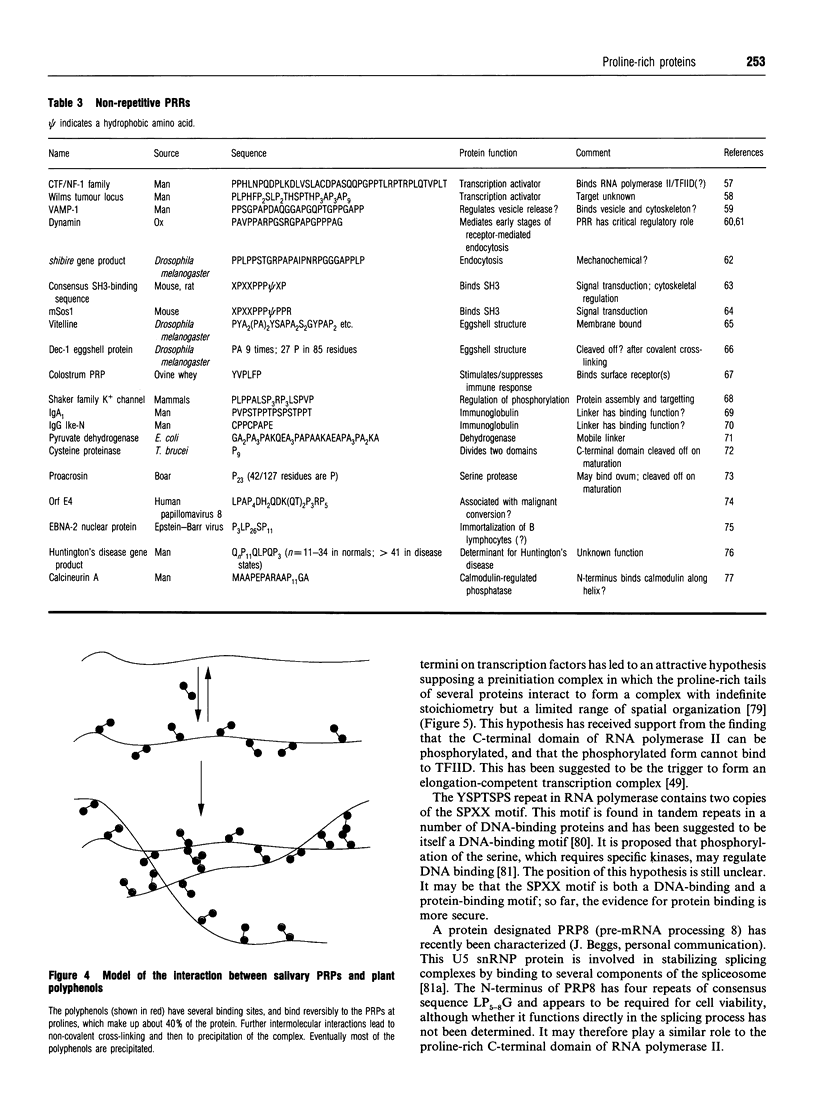
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abillon E., Bremier L., Cardinaud R. Conformational calculations on the Ala14-Pro27 LC1 segment of rabbit skeletal myosin. Biochim Biophys Acta. 1990 Mar 1;1037(3):394–400. doi: 10.1016/0167-4838(90)90042-e. [DOI] [PubMed] [Google Scholar]
- Adham I. M., Klemm U., Maier W. M., Hoyer-Fender S., Tsaousidou S., Engel W. Molecular cloning of preproacrosin and analysis of its expression pattern in spermatogenesis. Eur J Biochem. 1989 Jul 1;182(3):563–568. doi: 10.1111/j.1432-1033.1989.tb14864.x. [DOI] [PubMed] [Google Scholar]
- Adzhubei A. A., Sternberg M. J. Left-handed polyproline II helices commonly occur in globular proteins. J Mol Biol. 1993 Jan 20;229(2):472–493. doi: 10.1006/jmbi.1993.1047. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian R., Lakshminarayanan A. V., Sabesan M. N., Tegoni G., Venkatesan K., Ramachandran G. N. Studies on the conformation of amino acids. VI. Conformation of the proline ring as observed in crystal structures of amino acids and peptides. Int J Protein Res. 1971;3(1):25–33. [PubMed] [Google Scholar]
- Baron M., Norman D. G., Campbell I. D. Protein modules. Trends Biochem Sci. 1991 Jan;16(1):13–17. doi: 10.1016/0968-0004(91)90009-k. [DOI] [PubMed] [Google Scholar]
- Benfenati F., Valtorta F., Greengard P. Computer modeling of synapsin I binding to synaptic vesicles and F-actin: implications for regulation of neurotransmitter release. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):575–579. doi: 10.1073/pnas.88.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F., Valtorta F., Rubenstein J. L., Gorelick F. S., Greengard P., Czernik A. J. Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature. 1992 Oct 1;359(6394):417–420. doi: 10.1038/359417a0. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Salivary proline-rich proteins. Mol Cell Biochem. 1982 Jun 11;45(2):83–99. doi: 10.1007/BF00223503. [DOI] [PubMed] [Google Scholar]
- Berbers G. A., Hoekman W. A., Bloemendal H., de Jong W. W., Kleinschmidt T., Braunitzer G. Proline- and alanine-rich N-terminal extension of the basic bovine beta-crystallin B1 chains. FEBS Lett. 1983 Sep 19;161(2):225–229. doi: 10.1016/0014-5793(83)81013-8. [DOI] [PubMed] [Google Scholar]
- Bhandari D. G., Levine B. A., Trayer I. P., Yeadon M. E. 1H-NMR study of mobility and conformational constraints within the proline-rich N-terminal of the LC1 alkali light chain of skeletal myosin. Correlation with similar segments in other protein systems. Eur J Biochem. 1986 Oct 15;160(2):349–356. doi: 10.1111/j.1432-1033.1986.tb09978.x. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Pitts J. E., Tickle I. J., Wood S. P., Wu C. W. X-ray analysis (1. 4-A resolution) of avian pancreatic polypeptide: Small globular protein hormone. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4175–4179. doi: 10.1073/pnas.78.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker G. W., Gout I., Downing A. K., Driscoll P. C., Boyd J., Waterfield M. D., Campbell I. D. Solution structure and ligand-binding site of the SH3 domain of the p85 alpha subunit of phosphatidylinositol 3-kinase. Cell. 1993 May 21;73(4):813–822. doi: 10.1016/0092-8674(93)90259-s. [DOI] [PubMed] [Google Scholar]
- Brewer S., Tolley M., Trayer I. P., Barr G. C., Dorman C. J., Hannavy K., Higgins C. F., Evans J. S., Levine B. A., Wormald M. R. Structure and function of X-Pro dipeptide repeats in the TonB proteins of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1990 Dec 20;216(4):883–895. doi: 10.1016/S0022-2836(99)80008-4. [DOI] [PubMed] [Google Scholar]
- Brown J. D., Beggs J. D. Roles of PRP8 protein in the assembly of splicing complexes. EMBO J. 1992 Oct;11(10):3721–3729. doi: 10.1002/j.1460-2075.1992.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Elliott T., Elvin J., Bastin J., Rammensee H. G., Townsend A. The binding affinity and dissociation rates of peptides for class I major histocompatibility complex molecules. Eur J Immunol. 1991 Sep;21(9):2069–2075. doi: 10.1002/eji.1830210915. [DOI] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaillier P. Pest sequences in nuclear proteins. Int J Biochem. 1993 Apr;25(4):479–482. doi: 10.1016/0020-711x(93)90653-v. [DOI] [PubMed] [Google Scholar]
- Cicchetti P., Mayer B. J., Thiel G., Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992 Aug 7;257(5071):803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- Collins C. A. Dynamin: a novel microtubule-associated GTPase. Trends Cell Biol. 1991 Aug;1(2-3):57–60. doi: 10.1016/0962-8924(91)90090-v. [DOI] [PubMed] [Google Scholar]
- Corden J. L. Tails of RNA polymerase II. Trends Biochem Sci. 1990 Oct;15(10):383–387. doi: 10.1016/0968-0004(90)90236-5. [DOI] [PubMed] [Google Scholar]
- Darbon H., Bernassau J. M., Deleuze C., Chenu J., Roussel A., Cambillau C. Solution conformation of human neuropeptide Y by 1H nuclear magnetic resonance and restrained molecular dynamics. Eur J Biochem. 1992 Oct 15;209(2):765–771. doi: 10.1111/j.1432-1033.1992.tb17346.x. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Greengard P. Synapsin I: a synaptic vesicle-associated neuronal phosphoprotein. Biochem Pharmacol. 1986 Dec 15;35(24):4349–4357. doi: 10.1016/0006-2952(86)90747-1. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Bovey F. A., Carver J. P., Blout E. R. Nuclear magnetic resonance evidence for cis-peptide bonds in proline oligomers. J Am Chem Soc. 1970 Oct 21;92(21):6191–6198. doi: 10.1021/ja00724a016. [DOI] [PubMed] [Google Scholar]
- Dukor R. K., Keiderling T. A., Gut V. Vibrational circular dichroism spectra of unblocked proline oligomers. Int J Pept Protein Res. 1991 Sep;38(3):198–203. doi: 10.1111/j.1399-3011.1991.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Dukor R. K., Keiderling T. A. Reassessment of the random coil conformation: vibrational CD study of proline oligopeptides and related polypeptides. Biopolymers. 1991 Dec;31(14):1747–1761. doi: 10.1002/bip.360311409. [DOI] [PubMed] [Google Scholar]
- Eichinger D. J., Arnot D. E., Tam J. P., Nussenzweig V., Enea V. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Mol Cell Biol. 1986 Nov;6(11):3965–3972. doi: 10.1128/mcb.6.11.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H., Mengele R., Wenzl S., Engel J., Sumper M. The extracellular matrix of Volvox carteri: molecular structure of the cellular compartment. J Cell Biol. 1989 Dec;109(6 Pt 2):3493–3501. doi: 10.1083/jcb.109.6.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions: I. A Structural Investigation of Cell Wall Hydroxyproline-rich Glycoproteins Which Accumulate in Fungus-infected Plants. Plant Physiol. 1979 Aug;64(2):314–319. doi: 10.1104/pp.64.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R. Collagen: molecular diversity in the body's protein scaffold. Science. 1980 Mar 21;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Fahnestock S. R., Alexander P., Nagle J., Filpula D. Gene for an immunoglobulin-binding protein from a group G streptococcus. J Bacteriol. 1986 Sep;167(3):870–880. doi: 10.1128/jb.167.3.870-880.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell H. M., Jr Models for casein micelle formation. J Dairy Sci. 1973 Sep;56(9):1195–1206. doi: 10.3168/jds.S0022-0302(73)85335-4. [DOI] [PubMed] [Google Scholar]
- Feigl G., Gram M., Pongs O. A member of the steroid hormone receptor gene family is expressed in the 20-OH-ecdysone inducible puff 75B in Drosophila melanogaster. Nucleic Acids Res. 1989 Sep 25;17(18):7167–7178. doi: 10.1093/nar/17.18.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J. M., Tatham A. S., Shewry P. R. The structure of a high-Mr subunit of durum-wheat (Triticum durum) gluten. Biochem J. 1987 Oct 1;247(1):215–221. doi: 10.1042/bj2470215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G., Weeds A. G. The amino-acid sequence of the alkali light chains of rabbit skeletal-muscle myosin. Eur J Biochem. 1974 May 15;44(2):317–334. doi: 10.1111/j.1432-1033.1974.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Iftner T., Weninger J., Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986 May;58(2):626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisser S., Braun V. The tonB gene of Serratia marcescens: sequence, activity and partial complementation of Escherichia coli tonB mutants. Mol Microbiol. 1991 Nov;5(11):2777–2787. doi: 10.1111/j.1365-2958.1991.tb01986.x. [DOI] [PubMed] [Google Scholar]
- Gendler S. J., Lancaster C. A., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E. N., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990 Sep 5;265(25):15286–15293. [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Gigliotti S., Graziani F., De Ponti L., Rafti F., Manzi A., Lavorgna G., Gargiulo G., Malva C. Sex-, tissue-, and stage-specific expression of a vitelline membrane protein gene from region 32 of the second chromosome of Drosophila melanogaster. Dev Genet. 1989;10(1):33–41. doi: 10.1002/dvg.1020100106. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr, Warren R. A. Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991 Jun;55(2):303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grathwohl C., Wüthrich K. The X-Pro peptide bond as an nmr probe for conformational studies of flexible linear peptides. Biopolymers. 1976 Oct;15(10):2025–2041. doi: 10.1002/bip.1976.360151012. [DOI] [PubMed] [Google Scholar]
- Green J. D., Perham R. N., Ullrich S. J., Appella E. Conformational studies of the interdomain linker peptides in the dihydrolipoyl acetyltransferase component of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Biol Chem. 1992 Nov 25;267(33):23484–23488. [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman A. E., Butler L. G. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981 May 10;256(9):4494–4497. [PubMed] [Google Scholar]
- Hall M. D., Hoon M. A., Ryba N. J., Pottinger J. D., Keen J. N., Saibil H. R., Findlay J. B. Molecular cloning and primary structure of squid (Loligo forbesi) rhodopsin, a phospholipase C-directed G-protein-linked receptor. Biochem J. 1991 Feb 15;274(Pt 1):35–40. doi: 10.1042/bj2740035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannavy K., Barr G. C., Dorman C. J., Adamson J., Mazengera L. R., Gallagher M. P., Evans J. S., Levine B. A., Trayer I. P., Higgins C. F. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J Mol Biol. 1990 Dec 20;216(4):897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- Hedén L. O., Frithz E., Lindahl G. Molecular characterization of an IgA receptor from group B streptococci: sequence of the gene, identification of a proline-rich region with unique structure and isolation of N-terminal fragments with IgA-binding capacity. Eur J Immunol. 1991 Jun;21(6):1481–1490. doi: 10.1002/eji.1830210623. [DOI] [PubMed] [Google Scholar]
- Hejtmancik J. F., Thompson M. A., Wistow G., Piatigorsky J. cDNA and deduced protein sequence for the beta B1-crystallin polypeptide of the chicken lens. Conservation of the PAPA sequence. J Biol Chem. 1986 Jan 15;261(2):982–987. [PubMed] [Google Scholar]
- Helbecque N., Loucheux-Lefebvre M. H. Critical chain length for polyproline-II structure formation in H-Gly-(Pro)n-OH. Int J Pept Protein Res. 1982 Jan;19(1):94–101. doi: 10.1111/j.1399-3011.1982.tb03028.x. [DOI] [PubMed] [Google Scholar]
- Hurley J. H., Mason D. A., Matthews B. W. Flexible-geometry conformational energy maps for the amino acid residue preceding a proline. Biopolymers. 1992 Nov;32(11):1443–1446. doi: 10.1002/bip.360321104. [DOI] [PubMed] [Google Scholar]
- Janusz M., Wieczorek Z., Spiegel K., Kubik A., Szewczuk Z., Siemion I., Lisowski J. Immunoregulatory properties of synthetic peptides, fragments of a proline-rich polypeptide (PRP) from ovine colostrum. Mol Immunol. 1987 Oct;24(10):1029–1031. doi: 10.1016/0161-5890(87)90069-1. [DOI] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Koleske A. J., Buratowski S., Nonet M., Young R. A. A novel transcription factor reveals a functional link between the RNA polymerase II CTD and TFIID. Cell. 1992 May 29;69(5):883–894. doi: 10.1016/0092-8674(92)90298-q. [DOI] [PubMed] [Google Scholar]
- Laurent B. C., Treitel M. A., Carlson M. The SNF5 protein of Saccharomyces cerevisiae is a glutamine- and proline-rich transcriptional activator that affects expression of a broad spectrum of genes. Mol Cell Biol. 1990 Nov;10(11):5616–5625. doi: 10.1128/mcb.10.11.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layfield R., Bannister A. J., Pierce E. J., McDonald C. J. cDNA clones for mouse parotid proline-rich proteins. mRNA regulation by isoprenaline and the nucleotide sequence of proline-rich protein cDNA MP5. Eur J Biochem. 1992 Mar 1;204(2):591–597. doi: 10.1111/j.1432-1033.1992.tb16672.x. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Momany F. A., Scheraga H. A. Chain reversals in proteins. Biochim Biophys Acta. 1973 Apr 20;303(2):211–229. doi: 10.1016/0005-2795(73)90350-4. [DOI] [PubMed] [Google Scholar]
- Li L. J., Dougan G., Novotny P., Charles I. G. P.70 pertactin, an outer-membrane protein from Bordetella parapertussis: cloning, nucleotide sequence and surface expression in Escherichia coli. Mol Microbiol. 1991 Feb;5(2):409–417. doi: 10.1111/j.1365-2958.1991.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Li X. B., Kieliszewski M., Lamport D. T. A chenopod extensin lacks repetitive tetrahydroxyproline blocks. Plant Physiol. 1990 Feb;92(2):327–333. doi: 10.1104/pp.92.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. S., Low T. L., Infante A., Putnam F. W. Complete covalent structure of a human IgA1 immunoglobulin. Science. 1976 Sep 10;193(4257):1017–1020. doi: 10.1126/science.821146. [DOI] [PubMed] [Google Scholar]
- MacArthur M. W., Thornton J. M. Influence of proline residues on protein conformation. J Mol Biol. 1991 Mar 20;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- Machado R. S., Guest J. R., Williamson M. P. Mobility in pyruvate dehydrogenase complexes with multiple lipoyl domains. FEBS Lett. 1993 Jun 1;323(3):243–246. doi: 10.1016/0014-5793(93)81349-5. [DOI] [PubMed] [Google Scholar]
- Mann K., Schäfer W., Thoenes U., Messerschmidt A., Mehrabian Z., Nalbandyan R. The amino acid sequence of a type I copper protein with an unusual serine- and hydroxyproline-rich C-terminal domain isolated from cucumber peelings. FEBS Lett. 1992 Dec 21;314(3):220–223. doi: 10.1016/0014-5793(92)81475-2. [DOI] [PubMed] [Google Scholar]
- McCaffery C. A., DeGennaro L. J. Determination and analysis of the primary structure of the nerve terminal specific phosphoprotein, synapsin I. EMBO J. 1986 Dec 1;5(12):3167–3173. doi: 10.1002/j.1460-2075.1986.tb04625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack T., Vega-Saenz de Miera E. C., Rudy B. Molecular cloning of a member of a third class of Shaker-family K+ channel genes in mammals. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5227–5231. doi: 10.1073/pnas.87.13.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Mehansho H., Butler L. G., Carlson D. M. Dietary tannins and salivary proline-rich proteins: interactions, induction, and defense mechanisms. Annu Rev Nutr. 1987;7:423–440. doi: 10.1146/annurev.nu.07.070187.002231. [DOI] [PubMed] [Google Scholar]
- Meluh P. B., Rose M. D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990 Mar 23;60(6):1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Morris A. L., MacArthur M. W., Hutchinson E. G., Thornton J. M. Stereochemical quality of protein structure coordinates. Proteins. 1992 Apr;12(4):345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- Mottram J. C., North M. J., Barry J. D., Coombs G. H. A cysteine proteinase cDNA from Trypanosoma brucei predicts an enzyme with an unusual C-terminal extension. FEBS Lett. 1989 Dec 4;258(2):211–215. doi: 10.1016/0014-5793(89)81655-2. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Noble M., Pauptit R., Wierenga R., Saraste M. Crystal structure of a Src-homology 3 (SH3) domain. Nature. 1992 Oct 29;359(6398):851–855. doi: 10.1038/359851a0. [DOI] [PubMed] [Google Scholar]
- Nicholson H., Tronrud D. E., Becktel W. J., Matthews B. W. Analysis of the effectiveness of proline substitutions and glycine replacements in increasing the stability of phage T4 lysozyme. Biopolymers. 1992 Nov;32(11):1431–1441. doi: 10.1002/bip.360321103. [DOI] [PubMed] [Google Scholar]
- Noegel A. A., Gerisch G., Lottspeich F., Schleicher M. A protein with homology to the C-terminal repeat sequence of Octopus rhodopsin and synaptophysin is a member of a multigene family in Dictyostelium discoideum. FEBS Lett. 1990 Jun 18;266(1-2):118–122. doi: 10.1016/0014-5793(90)81521-o. [DOI] [PubMed] [Google Scholar]
- Okabayashi H., Isemura T., Sakakibara S. Steric structure of L-proline oligopeptides. II. Far-ultraviolet absorption spectra and optical rotations of L-proline oligopeptides. Biopolymers. 1968;6(3):323–330. doi: 10.1002/bip.1968.360060307. [DOI] [PubMed] [Google Scholar]
- Page M. I., Jencks W. P. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1678–1683. doi: 10.1073/pnas.68.8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotou G., Waterfield M. D. Phosphatidyl-inositol 3-kinase: a key enzyme in diverse signalling processes. Trends Cell Biol. 1992 Dec;2(12):358–360. doi: 10.1016/0962-8924(92)90042-l. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. E., Laue E. D., Perham R. N., Martin S. R., Appella E. Conformational flexibility and folding of synthetic peptides representing an interdomain segment of polypeptide chain in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Biol Chem. 1989 Jan 15;264(2):767–775. [PubMed] [Google Scholar]
- Raz R., Crétin C., Puigdomènech P., Martínez-Izquierdo J. A. The sequence of a hydroxyproline-rich glycoprotein gene from Sorghum vulgare. Plant Mol Biol. 1991 Feb;16(2):365–367. doi: 10.1007/BF00020571. [DOI] [PubMed] [Google Scholar]
- Reid K. B. Amino acid sequence of the N-terminal forty-two amino acid residues of the C chain of subcomponent C1q of the first component of human complement. Biochem J. 1977 Feb 1;161(2):247–251. doi: 10.1042/bj1610247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Roditi I., Schwarz H., Pearson T. W., Beecroft R. P., Liu M. K., Richardson J. P., Bühring H. J., Pleiss J., Bülow R., Williams R. O. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989 Feb;108(2):737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Russell G. C., Guest J. R. Sequence similarities within the family of dihydrolipoamide acyltransferases and discovery of a previously unidentified fungal enzyme. Biochim Biophys Acta. 1991 Jan 29;1076(2):225–232. doi: 10.1016/0167-4838(91)90271-z. [DOI] [PubMed] [Google Scholar]
- Sankararamakrishnan R., Vishveshwara S. Characterization of proline-containing alpha-helix (helix F model of bacteriorhodopsin) by molecular dynamics studies. Proteins. 1993 Jan;15(1):26–41. doi: 10.1002/prot.340150105. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Yokoyama E., Kuroiwa A. Specific DNA binding of the two chicken Deformed family homeodomain proteins, Chox-1.4 and Chox-a. Nucleic Acids Res. 1990 Apr 11;18(7):1739–1747. doi: 10.1093/nar/18.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. How receptor tyrosine kinases activate Ras. Trends Biochem Sci. 1993 Aug;18(8):273–275. doi: 10.1016/0968-0004(93)90031-h. [DOI] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sohma H., Yazawa M., Yagi K., Ebashi S. Histone H1 kinase specific to the SPKK motif. J Biochem. 1990 Sep;108(3):356–364. doi: 10.1093/oxfordjournals.jbchem.a123206. [DOI] [PubMed] [Google Scholar]
- Takagi T., Suzuki M., Baba T., Minegishi K., Sasaki S. Complete amino acid sequence of amelogenin in developing bovine enamel. Biochem Biophys Res Commun. 1984 Jun 15;121(2):592–597. doi: 10.1016/0006-291x(84)90223-7. [DOI] [PubMed] [Google Scholar]
- Tatham A. S., Drake A. F., Shewry P. R. A conformational study of a glutamine- and proline-rich cereal seed protein, C hordein. Biochem J. 1985 Mar 1;226(2):557–562. doi: 10.1042/bj2260557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Scheller R. H. Molecular biology of synaptic vesicle-associated proteins. Trends Neurosci. 1988 Jun;11(6):241–242. doi: 10.1016/0166-2236(88)90098-7. [DOI] [PubMed] [Google Scholar]
- Turner S. L., Russell G. C., Williamson M. P., Guest J. R. Restructuring an interdomain linker in the dihydrolipoamide acetyltransferase component of the pyruvate dehydrogenase complex of Escherichia coli. Protein Eng. 1993 Jan;6(1):101–108. doi: 10.1093/protein/6.1.101. [DOI] [PubMed] [Google Scholar]
- Usheva A., Maldonado E., Goldring A., Lu H., Houbavi C., Reinberg D., Aloni Y. Specific interaction between the nonphosphorylated form of RNA polymerase II and the TATA-binding protein. Cell. 1992 May 29;69(5):871–881. doi: 10.1016/0092-8674(92)90297-p. [DOI] [PubMed] [Google Scholar]
- Veis A., Nawrot C. F. Basicity differences among peptide bonds. J Am Chem Soc. 1970 Jul 1;92(13):3910–3914. doi: 10.1021/ja00716a013. [DOI] [PubMed] [Google Scholar]
- Waring G. L., Hawley R. J., Schoenfeld T. Multiple proteins are produced from the dec-1 eggshell gene in Drosophila by alternative RNA splicing and proteolytic cleavage events. Dev Biol. 1990 Nov;142(1):1–12. doi: 10.1016/0012-1606(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Whitney R. M., Brunner J. R., Ebner K. E., Farrell H. M., Jr, Josephson R. V., Morr C. V., Swaisgood H. E. Nomemclature of the proteins of cow's milk: fourth revision. J Dairy Sci. 1976 May;59(5):795–815. doi: 10.3168/jds.s0022-0302(76)84280-4. [DOI] [PubMed] [Google Scholar]
- Williams D. H., Searle M. S., Mackay J. P., Gerhard U., Maplestone R. A. Toward an estimation of binding constants in aqueous solution: studies of associations of vancomycin group antibiotics. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1172–1178. doi: 10.1073/pnas.90.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. A., Deber C. M. Proline residues in transmembrane helices: structural or dynamic role? Biochemistry. 1991 Sep 17;30(37):8919–8923. doi: 10.1021/bi00101a001. [DOI] [PubMed] [Google Scholar]
- Williamson G., Belshaw N. J., Williamson M. P. O-glycosylation in Aspergillus glucoamylase. Conformation and role in binding. Biochem J. 1992 Mar 1;282(Pt 2):423–428. doi: 10.1042/bj2820423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton J. C., Drummond M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989 May;2(7):535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- Yu H., Rosen M. K., Shin T. B., Seidel-Dugan C., Brugge J. S., Schreiber S. L. Solution structure of the SH3 domain of Src and identification of its ligand-binding site. Science. 1992 Dec 4;258(5088):1665–1668. doi: 10.1126/science.1280858. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Meyerowitz E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991 May 30;351(6325):411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Redelmeier T. E., Damke H., Tisdale E. J., Meyerowitz E. M., Schmid S. L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993 Aug;122(3):553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]