Abstract
Malonyl-CoA synthetase catalyses the formation of malonyl-CoA directly from malonate and CoA with hydrolysis of ATP into AMP and PP1. The catalytic mechanism of malonyl-CoA synthetase from Bradyrhizobium japonicum was investigated by steady-state kinetics. Initial-velocity studies and the product-inhibition studies with AMP and PPi strongly suggested ordered Bi Uni Uni Bi Ping Pong Ter Ter system as the most probable steady-state kinetic mechanism of malonyl-CoA synthetase. Michaelis constants were 61 microM, 260 microM and 42 microM for ATP, malonate and CoA respectively, and the value for Vmax, was 11.2 microM/min. The t.l.c. analysis of the 32P-labelled products in a reaction mixture containing [gamma-32P]ATP in the absence of CoA showed that PPi was produced after the sequential addition of ATP and malonate. Formation of malonyl-AMP, suggested as an intermediate in the kinetically deduced mechanism, was confirmed by the analysis of 31P-n.m.r. spectra of an AMP product isolated from the 18O-transfer experiment using [18O]malonate. The 31P-n.m.r. signal of the AMP product appeared at 0.024 p.p.m. apart from that of [16O4]AMP, indicating that one atom of 18O transferred from [18O]malonate to AMP through the formation of malonyl-AMP. Formation of malonyl-AMP was also confirmed through the t.l.c. analysis of reaction mixture containing [alpha-32P]ATP. These results strongly support the ordered Bi Uni Uni Bi Pin Pong Ter Ter mechanism deduced from initial-velocity and product-inhibition studies.
Full text
PDF

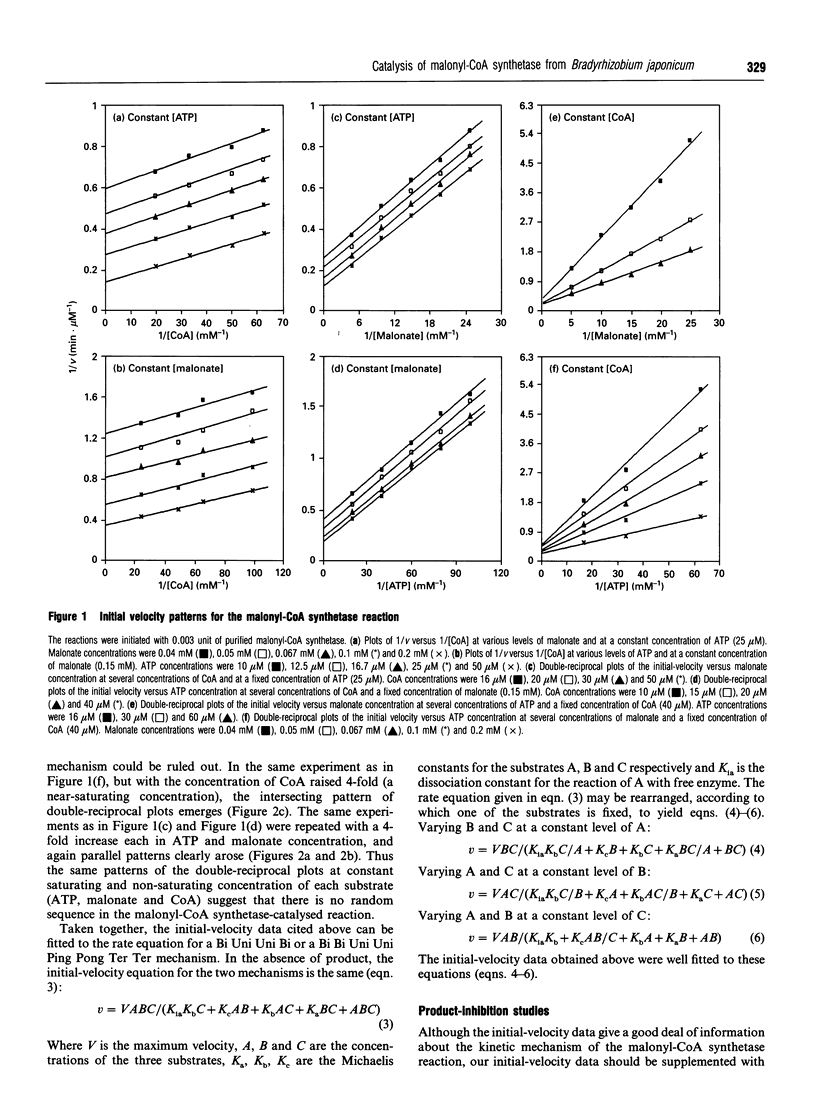
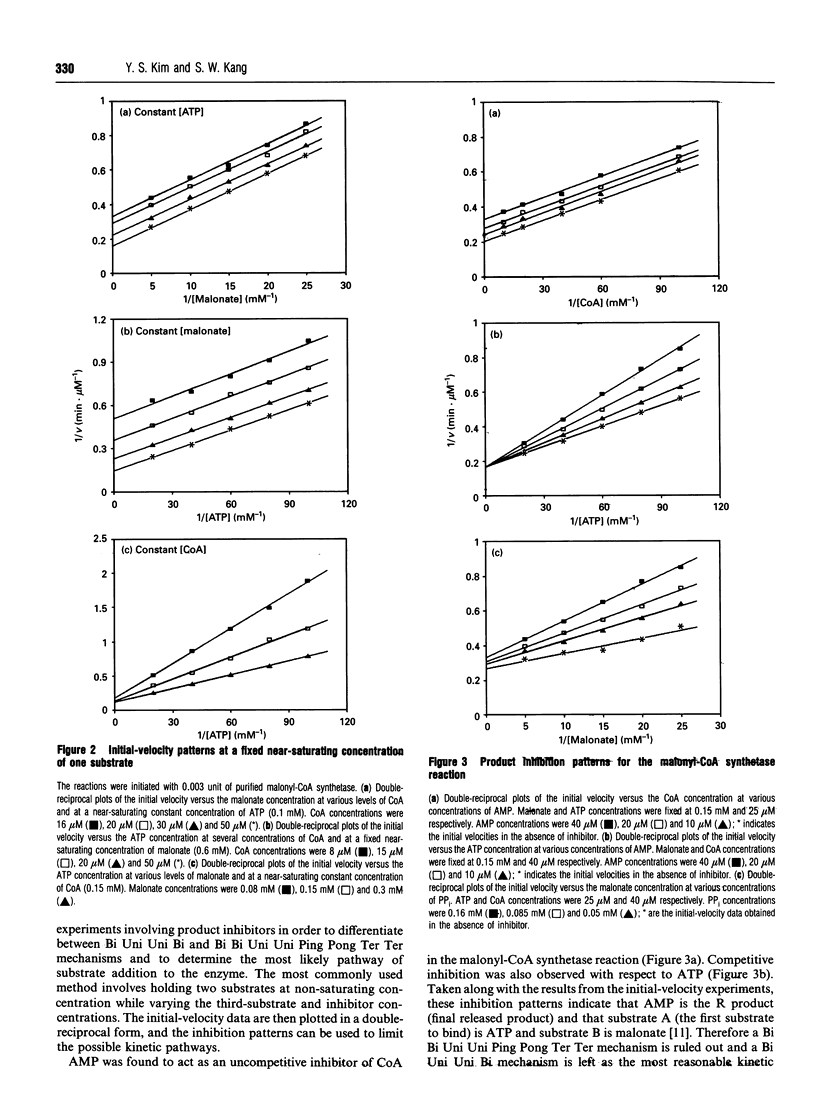

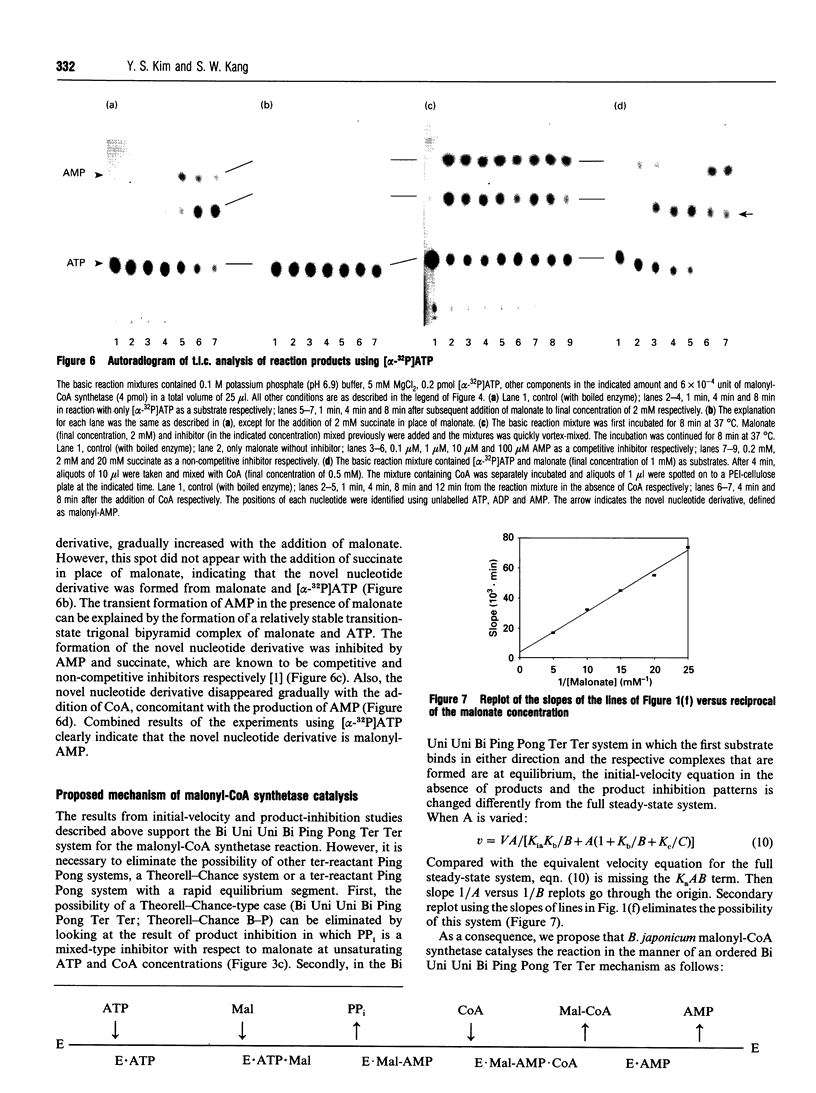

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cohn M., Hu A. Isotopic (18O) shift in 31P nuclear magnetic resonance applied to a study of enzyme-catalyzed phosphate--phosphate exchange and phosphate (oxygen)--water exchange reactions. Proc Natl Acad Sci U S A. 1978 Jan;75(1):200–203. doi: 10.1073/pnas.75.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. III. The order of substrate addition in the enzymatic reaction. J Biol Chem. 1959 Nov;234:2891–2896. [PubMed] [Google Scholar]
- HAYAISHI O. Enzymatic decarboxylation of malonic acid. J Biol Chem. 1955 Jul;215(1):125–136. [PubMed] [Google Scholar]
- Hirsh D. I. A study of the threonyl adenylate complex with threonyl transfer ribonucleic acid synthetase and its reaction with hydroxylamine. J Biol Chem. 1968 Nov 10;243(21):5731–5738. [PubMed] [Google Scholar]
- Kim Y. S., Bang S. K. Assays for malonyl-coenzyme A synthase. Anal Biochem. 1988 Apr;170(1):45–49. doi: 10.1016/0003-2697(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Chae H. Z. A model of nitrogen flow by malonamate in Rhizobium japonicum-soybean symbiosis. Biochem Biophys Res Commun. 1990 Jun 15;169(2):692–699. doi: 10.1016/0006-291x(90)90386-2. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Chae H. Z. Purification and properties of malonyl-CoA synthetase from Rhizobium japonicum. Biochem J. 1991 Feb 1;273(Pt 3):511–516. doi: 10.1042/bj2730511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen A. H., Mitzen E. J., Ammoumi A. A. Malonate metabolism in rat brain mitochondria. Biochemistry. 1974 Aug 13;13(17):3589–3595. doi: 10.1021/bi00714a029. [DOI] [PubMed] [Google Scholar]
- Lõvgren T. N., Heinonen J., Loftfield R. B. The mechanism of aminoacylation of transfer ribonucleic acid. Reactivity of enzyme-bound isoleucyl adenylate. J Biol Chem. 1975 May 25;250(10):3854–3860. [PubMed] [Google Scholar]
- STALDER K. Papierchromatographische Bestimmung einiger kurzkettiger Dicarbonsäuren im Harn. Hoppe Seylers Z Physiol Chem. 1958;311(4-6):221–226. [PubMed] [Google Scholar]
- Waldenström J. Some properties of lysyl ribonucleic acid synthetase from Escherichia coli. Eur J Biochem. 1968 Jul;5(2):239–245. doi: 10.1111/j.1432-1033.1968.tb00363.x. [DOI] [PubMed] [Google Scholar]