Abstract
Background and purpose.
Clinically localized Merkel cell carcinoma (MCC) has been associated with high rates of disease relapse. This study examines how primary tumor anatomic site drives patterns of care and outcomes in a large cohort treated in the contemporary era.
Materials and methods.
Patterns of care and associated outcomes were evaluated for clinically Stage I-II MCC patients treated at our institution with adjuvant radiation therapy (RT) to the primary site and/or regional nodal basin as a component of their curative intent therapy between 2014–2021.
Results.
Of 80 patients who met inclusion criteria, the primary tumor anatomic site was head and neck (HN) for 42 (53%) and non-head and neck (NHN) for 38 (47%). Primary tumor risk factors were similar between cohorts. Fewer patients with HN tumors had wide local excision (WLE; HN-81% vs. NHN-100% p<0.01). Of those undergoing WLE, patients with HN tumors received higher dose adjuvant RT (>50 Gy: HN-70% vs. NHN-8%; p<0.01). Patients with HN tumors were less likely to undergo sentinel lymph node biopsy (HN-62%vs. NHN-100%; p<0.01) and more likely to have elective nodal RT (HN-48% vs. NHN-0%). Despite varying management strategies, there was no significant difference in local recurrence-free survival (3-yr LRFS HN-94% vs. NHN-94%; p=0.97), nodal recurrence-free survival (3-yr NRFS HN-89% vs. NHN-85%; p=0.71) or overall recurrence-free survival (3-yr RFS 73% HN vs. 80% NHN; p=0.44).
Conclusions.
Among patients with primary MCC who had RT as a component of their initial treatment strategy, anatomically-driven heterogeneous treatment approaches were associated with equally excellent locoregional disease control.
Keywords: Merkel cell carcinoma, radiation therapy, surgery, anatomic site
Introduction
Merkel cell carcinoma (MCC) is a rare, cutaneous, neuroendocrine malignancy with rising incidence.1 MCC often presents as a rapidly growing, cutaneous or subcutaneous nodule on the head and neck (HN) or sun-exposed non-HN (NHN) areas.2 Beyond UV exposure, risk factors include older age, being immunocompromised, polyomavirus infection, and previous history of cutaneous malignancy.3–5 The majority of MCC patients are diagnosed with locally-confined disease.5 Such stage I-II patients are often treated surgically with wide local excision (WLE) and sentinel lymph node biopsy (SLNB). With surgery alone, many patients with localized MCC will develop locoregional progression.6 As a result, radiation therapy (RT) to the primary site or regional nodal basin is typically recommended in the setting of high-risk features.7
MCC is a particularly radiosensitive malignancy for which adjuvant or definitive RT to the primary tumor bed and/or draining nodal basin promotes locoregional disease control.8–10 However, the optimal RT approach in terms of which patients and/or target(s) to treat, and with what dose, is not clearly established. Specific questions include how anatomic site, primary tumor pathologic features, and SLNB findings should influence the RT plan. While excision with wide margins remains a mainstay of primary management for stage I-II MCC, addition of adjuvant RT for high-risk disease results in excellent local control independent of surgical margin size.11–13 Previously reported risk factors for locoregional recurrence include larger primary tumor size, lymphovascular invasion, immunosuppression, and primaries of the HN anatomic site.14,15 In the presence of high-risk features, excision followed by adjuvant RT can optimize disease control.14
Our institution serves as a large volume referral center for patients diagnosed with MCC. Historically, MCC and other skin cancers of the HN were often managed differently from those of the extremity and trunk, given the morbidity associated with wide excisions of cosmetically and functionally challenging regions. To combat surgical limitations in the HN region, individualized multidisciplinary approaches for HN MCC often include narrow margin excision with more aggressive primary site RT. Furthermore, concerns regarding a higher false-negative rate for sentinel node biopsy in the HN region have led to different patterns of surgical nodal evaluation and nodal irradiation. Therefore, in this study, we compared patterns of care and patterns of recurrence for clinically lymph node negative (cLN(−)) HN vs. NHN MCC patients who received RT to the primary site and/or regional nodal basin at our institution as a component of their initial treatment strategy to evaluate the clinicopathological factors associated with outcome.
Methods and Materials
Study Design and Study Population
This retrospective study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center (MDACC). MCC cases evaluated at MDACC were identified by searching Department of Pathology records from 01/2014 to 03/2021, a coherent treatment era at our institution where both HN and NHN surgeons commonly performed SLNB for MCC. Patients were included if they had clinical stage I-II MCC and received curative-intent RT at our institution to the primary site and/or draining nodal basin with at least 1 follow-up visit after locoregional therapy completion (Figure 1). Patients were further stratified based on MCC anatomic site: HN primary tumors were defined as disease above the clavicles and NHN primary tumors were defined as disease below the level of the clavicles.
Figure 1. Consort Diagram.
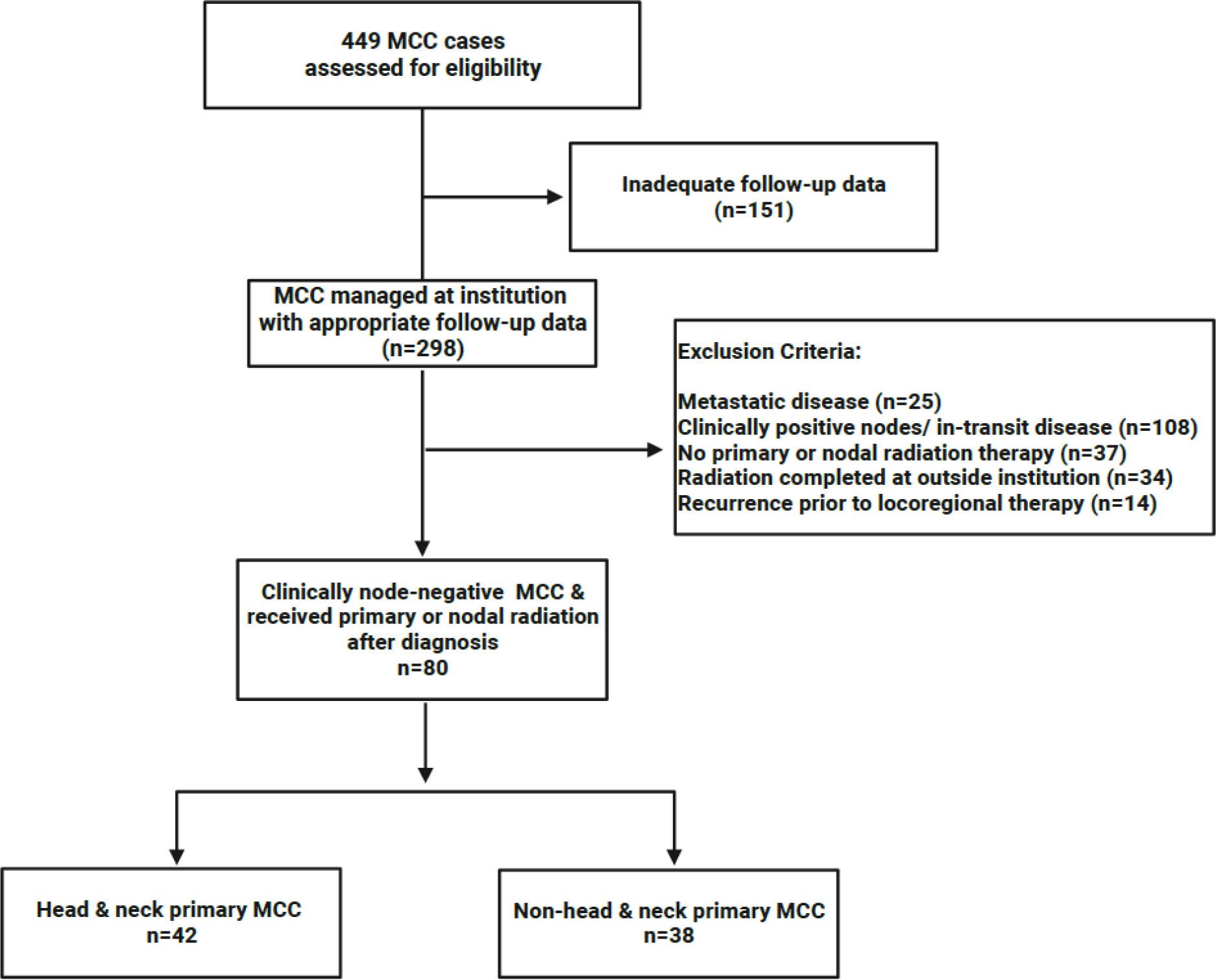
illustrating how the 80 patients described in this cohort were identified from all MCC cases at our institution.
Data Collection
Clinicopathological characteristics of patients and their disease were extracted from the electronic medical record, including: date of birth; date of diagnosis; sex; race; immunocompromised status; prior history of malignancy; features of primary MCC including size and location; SLN features; and details of any local, regional and/or systemic treatments. Patients were considered immunocompromised if they had another malignancy and were receiving chemotherapy at the time of MCC diagnosis, were receiving immunosuppressive drugs due to history of transplantation or autoimmune disease, had HIV/AIDS, and/or had a hematologic malignancy with reduced lymphocyte counts. Surgeries were considered to be a WLE if documentation was consistent with >1 cm surgical margins. Dates of recurrence (local, regional, nodal and/or distant), and last follow-up were also recorded. Local recurrence was defined as recurrence within 2 cm of the primary site, including new clinically detected satellite lesions. Regional recurrence was defined as development of in-transit disease proximal to the draining lymph node basin (>2 cm from the primary site), while nodal recurrence was defined as disease within the draining regional lymph node basin, or in the case of HN MCC, development of disease within any nodal basin above the clavicles. Locoregional recurrence included local, regional and nodal recurrences.
Statistical Analysis
HN and NHN cohorts were assessed for clinicopathological characteristics, treatment received, and outcomes. Descriptive statistics were used to characterize clinicopathological parameters of each cohort. Median and interquartile range were determined for continuous variables; frequency and percentage were used for categorical variables. The Wilcoxon rank sum test was used to compare nonparametric continuous variables. The χ2 or Fisher exact tests were used to compare categorical variables.
Time to event calculations were performed from the date of completion of locoregional therapy (RT and/or surgery). Local recurrence-free survival (LRFS) was defined as the proportion of patients alive without local recurrence (within 2 cm of the irradiated field). Regional recurrence-free survival (RRFS) was defined as the proportion of patients alive without regional (i.e., in-transit disease) recurrence. Nodal recurrence-free survival (NRFS) was defined as the proportion of patients alive without regional nodal recurrence. Distant-metastasis-free survival (DMFS) was defined as the proportion of patients alive after locoregional therapy without distant metastatic disease. Recurrence-free survival (RFS) was defined as the proportion of patients alive without recurrence. Merkel cell specific survival (MCSS) was defined as the proportion of patients who had not died from MCC. The Kaplan-Meier method was used to estimate actuarial rates of LRFS, RRFS, NRFS, DMFS, RFS and MCSS. Log-rank tests were used to compare factors associated with clinical outcome. Variables were considered to show a statistically significant association if p<0.05. To construct multivariable Cox proportional hazards models, variables with an association defined as p<0.20 were initially included. Backward stepwise selection was then used to refine the multivariable models. Statistical analysis was performed in SAS Enterprise Guide 7.15 (SAS Inc., Cary, NC).
Results
Eighty patients were included with 42 (53%) having HN primary disease and 38 (47%) having NHN primary disease (Table 1). HN patients were slightly older (median age 70 vs. 66, p=0.02) and had a male predominance (76% vs. 53%, p=0.03). Race/ethnicity, immunocompromised status, and prior history of cancer were similar between cohorts. Similar T-category distribution, numbers of mitoses, and rates of lymphovascular invasion were also observed between HN and NHN cohorts.
Table 1.
Clinicopathological variables among patients with cLN(-) MCC by primary anatomic site
| HN (n=42) | NHN (n=38) | ||||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | p-value | |
|
| |||||
| Age at diagnosis, years, median [IQR] | 70 [66–77] | 66 [57–73] | 0.020 | ||
| Sex | |||||
| Male | 32 | 76% | 20 | 53% | 0.027 |
| Female | 10 | 24% | 18 | 47% | |
| Race/Ethnicity | 0.91 | ||||
| White | 38 | 91% | 33 | 86% | |
| Hispanic | 2 | 5% | 3 | 8% | |
| Black | 1 | 2% | 1 | 3% | |
| Asian | 1 | 2% | 1 | 3% | |
| Immunocompromised | 7 | 17% | 7 | 18% | 0.84 |
| Prior history of cancer | 28 | 67% | 25 | 66% | 0.93 |
| History of skin cancer | 23 | 55% | 17 | 45% | 0.37 |
|
| |||||
| Primary tumor features | |||||
| Mitoses/mm2, median, [IQR] | 29 [15–60] | 33 [19–52] | 0.72 | ||
| T-stage | 0.78 | ||||
| T1 | 31 | 74% | 27 | 71% | |
| T2+ | 11 | 26% | 11 | 29% | |
| Lymphovascular invasion | 16 | 38% | 19 | 50% | 0.24 |
| Nodal status | |||||
| Nodal evaluation by SLNB or LND | 26 | 62% | 38 | 100% | <0.001 |
| 1 node positive | 6 | 23% | 10 | 26% | 0.78 |
| 2–3 nodes positive | 4 | 15% | 5 | 13% | |
| >3 nodes positive | 1 | 4% | 0 | 0% | |
Patterns of care differed significantly between HN and NHN patients (Table 2). All NHN patients (n=38) underwent WLE of their primary tumor, compared to only 81% (n=34) of HN patients (p=0.006). All eight HN patients who did not undergo WLE had excisional biopsies with positive microscopic margins but no gross residual disease.
Table 2.
Treatment variables among patients with cLN(-) MCC by primary anatomic site
| HN (n=42) | NHN (n=38) | ||||
|---|---|---|---|---|---|
|
| |||||
| Surgical Strategy | n | % | n | % | p-value |
|
| |||||
| Wide local excision | 34 | 81% | 38 | 100% | 0.006 |
| Surgical nodal evaluation | |||||
| SLNB | 26 | 62% | 38 | 100% | <0.001 |
| SLN Positive (among SLNBs) | 11 | 42% | 15 | 39% | 0.99 |
| CLND (among +SLNs) | 0 | 0% | 1 | 7% | 0.58 |
|
| |||||
| Radiation Therapy | |||||
|
| |||||
| n=34 | n=38 | ||||
| Adjuvant primary RT (WLE) | 33 | 97% | 38 | 100% | 0.47 |
| ≤50 Gy | 10 | 30% | 35 | 92% | <0.001 |
| 51–56 Gy | 13 | 40% | 2 | 5% | |
| >56 Gy | 10 | 30% | 1 | 3% | |
| n=8 | n=0 | ||||
| Definitive primary RT (no WLE) | 8 | 100% | 0 | 0% | |
| < 60 Gy | 3 | 37% | |||
| ≥ 60 Gy | 5 | 63% | |||
| n=11 | n=14 | ||||
| Adjuvant nodal RT (for +SLNB, no CLND) | 11 | 100% | 9 | 64% | 0.024 |
| 50 Gy | 4 | 36% | 7 | 78% | 0.092 |
| > 50 Gy | 7 | 64% | 2 | 22% | |
| n= 31 | n=23 | ||||
| Elective nodal RT (no nodal eval or -SLNB) | 15 | 48% | 0 | 0% | |
| ≤ 50 Gy | 12 | 80% | |||
| 51–59.4 Gy | 2 | 13% | |||
| > 60 Gy | 1 | 7% | |||
Of the patients who received adjuvant primary site RT, HN patients received higher overall RT doses (patients receiving >50 Gy: 70% HN (n=23) vs. 8% NHN (n=3), p<0.01). Overall, margin-positive resections, including patients who had excisional biopsy without formal wide excision, were more common in the HN cohort (33% HN (n=14) vs. 5% NHN (n=2), p=0.002). Higher primary site RT doses were used in the HN cohort regardless of margin status (margin-negative patients receiving >50 Gy: 68% HN (n=19/28) vs. 9% NHN (n=3/35), p<0.01; margin-positive patients receiving >50 Gy: 92% HN (n=12/13) vs. 0% NHN pts, p=0.03). For the eight HN patients who did not undergo WLE, definitive primary site RT was delivered: three received less than 60 Gy and five received 60 Gy or greater. Primary site radiation field size was typically 3–5 cm around the excision/primary site. NHN patients were typically treated with 3 cm RT fields while HN patients were typically treated with 4–5 cm fields when anatomically feasible. Electron radiotherapy was used for 84% (n=32) of NHN patient primary site treatments and 49% (n=18) of HN patient primary site treatments in the context of HN primary sites more commonly being adjacent to treated nodal basins which were typically treated with photon radiotherapy (usually IMRT/VMAT). Across the board 2 Gy/fraction delivered daily was used with the exception of 2 patients receiving elective nodal RT who received 1.7–1.8 Gy/fraction.
Surgical nodal evaluation with SLNB was less common among HN patients compared to NHN patients (HN-62% vs. NHN-100%, p<0.01). Among SLNBs performed, rates of sentinel node positivity were similar between groups (HN-42%, n=11/26 vs. NHN-39%, n=15/38, p=0.99). No HN patient received CLND, while the one (of 15) NHN patient who underwent CLND had one positive node without extracapsular extension among the 27 nodes removed, and did not receive nodal RT.
For patients who had positive sentinel nodes and did not undergo CLND, RT to the draining nodal basin was utilized more frequently in the HN group (100% HN, n=11/11 vs. 64% NHN, n=9/14 p=0.04). HN patients showed a trend towards higher adjuvant nodal RT doses (patients receiving >50 Gy: HN-64% vs. NHN-22%, p=0.092). Among the HN patients who did not undergo nodal evaluation (n=16), 75% received adjuvant nodal RT (primarily to lower doses with 75%, n=9/12 receiving ≤50Gy). Elective nodal RT was administered to 6 HN patients after negative SLNB, also primarily to lower doses (80% receiving ≤ 50 Gy). Meanwhile, elective nodal RT was not performed for any NHN patients, all of whom had a negative SLNB.
Neoadjuvant therapies were rarely utilized in this cLN(−) MCC population: two HN patients and no NHN patients received systemic therapy before locoregional therapy. Neoadjuvant immunotherapy (pembrolizumab) was utilized in one HN patient for a rapidly growing, T4 eyelid tumor, while chemotherapy (cisplatin/etoposide) was given to a second HN patient for a 4 cm, exophytic eyelid tumor. Following neoadjuvant therapy, both underwent WLE and SLNB (both with positive nodes) as well as adjuvant primary site and nodal RT.
Adjuvant systemic therapy was used in 12% (n=5/42) of HN patients after resection and 8% (n=3/38) of NHN patients, for either SLNB-positivity, positive margins, or other high-risk features. Four HN patients received chemotherapy (platinum and etoposide) and one received immunotherapy (avelumab). Three NHN patients received adjuvant chemotherapy (platinum and etoposide) and one received immunotherapy (avelumab).
Median follow-up was 35 months (IQR 22–57) for patients alive at last follow-up in the overall cohort and was not significantly different between HN and NHN cohorts (HN-median 30 months, IQR 21–49 vs. NHN median 37 months, IQR 27–59, p=0.15). Overall, 21% of patients thus far recurred at any time and similar rates were seen between the HN and NHN cohorts (24% vs. 18%, p= 0.60). The first site of recurrence among those who relapsed was similar between HN and NHN subgroups (locoregional: 20% vs. 14%; distant: 30% vs. 29%). Among patients with regional nodal relapse (n=9), 89% (n=8/9) had isolated nodal disease that was targeted for further treatment, while one patient in the NHN group had a synchronous distant metastasis at the time of relapse. Correspondingly, 3-year event rates for all oncologic outcomes evaluated were similar between HN and NHN cohorts (Table 3). Of note, overall survival showed a trend towards being worse for HN patients (3-yr OS 75% HN vs. 86% NHN, p=0.06) but this was in the context of HN patients being older (median 70 years (IQR 66–77) HN vs. 66 years (IQR 57–73, p=0.02), such that the similar MCC-specific survival was thought to be more reflective of the effect of MCC on survival outcomes.
Table 3.
Survival and recurrence rates among patients with MCC by primary anatomic site
| All Patients (n=80) | HN (n=42) | NHN (n=38) | P | |
|---|---|---|---|---|
|
| ||||
| Median follow up (IQR), Months | 34.5 (22–57) | 30.1 (21–49) | 37.0 (27–59) | 0.15 |
|
| ||||
| 3-year event rate (%) | ||||
|
| ||||
| Local recurrence-free survival | 94 | 94 | 94 | 0.97 |
| Nodal recurrence-free survival | 87 | 89 | 85 | 0.71 |
| Regional recurrence-free survival | 88 | 82 | 94 | 0.14 |
| Distant metastasis-free survival | 90 | 92 | 89 | 0.69 |
| Recurrence-free survival | 76 | 73 | 80 | 0.44 |
| MCC-specific survival | 90 | 88 | 91 | 0.69 |
| Overall survival | 81 | 75 | 86 | 0.06 |
On univariate analysis of clinicopathological factors (Supplementary Table 1), larger tumor size (T2+ vs. T1 category) was a significant predictor of worse outcomes. This included worse LRFS (3-yr LRFS 85% (95% CI: 59–95%) vs. 98% (95% CI: 85–99%), p=0.04) and worse overall RFS (3-yr RFS 51% (95% CI: 28–70% vs. 86% (95% CI: 74–93%)), p<0.01). No evaluated variables were associated with nodal recurrence-free survival, including whether SLNB was performed (86% when performed vs. 93% when not performed, p=0.58) or whether elective nodal RT was delivered (93% with vs. 86% without, p=0.56). Being immunocompromised was associated with worse RRFS (3-yr RRFS 68% (95% CI: 35–87%) vs. 92% (95% CI: 80–97%), p<0.01) and MCSS (3-yr MCSS 66% (95% CI: 33–86%) vs. 95% (95% CI: 85–98%), p<0.01). Primary tumor site was not associated with any evaluated outcome. Figure 2 highlights the lack of significant difference in RFS or MCSS by anatomic site but does illustrate the effect of tumor size and immunocompromise on these outcomes.
Figure 2. Predictors of improved MCC-specific and recurrence-free survival.
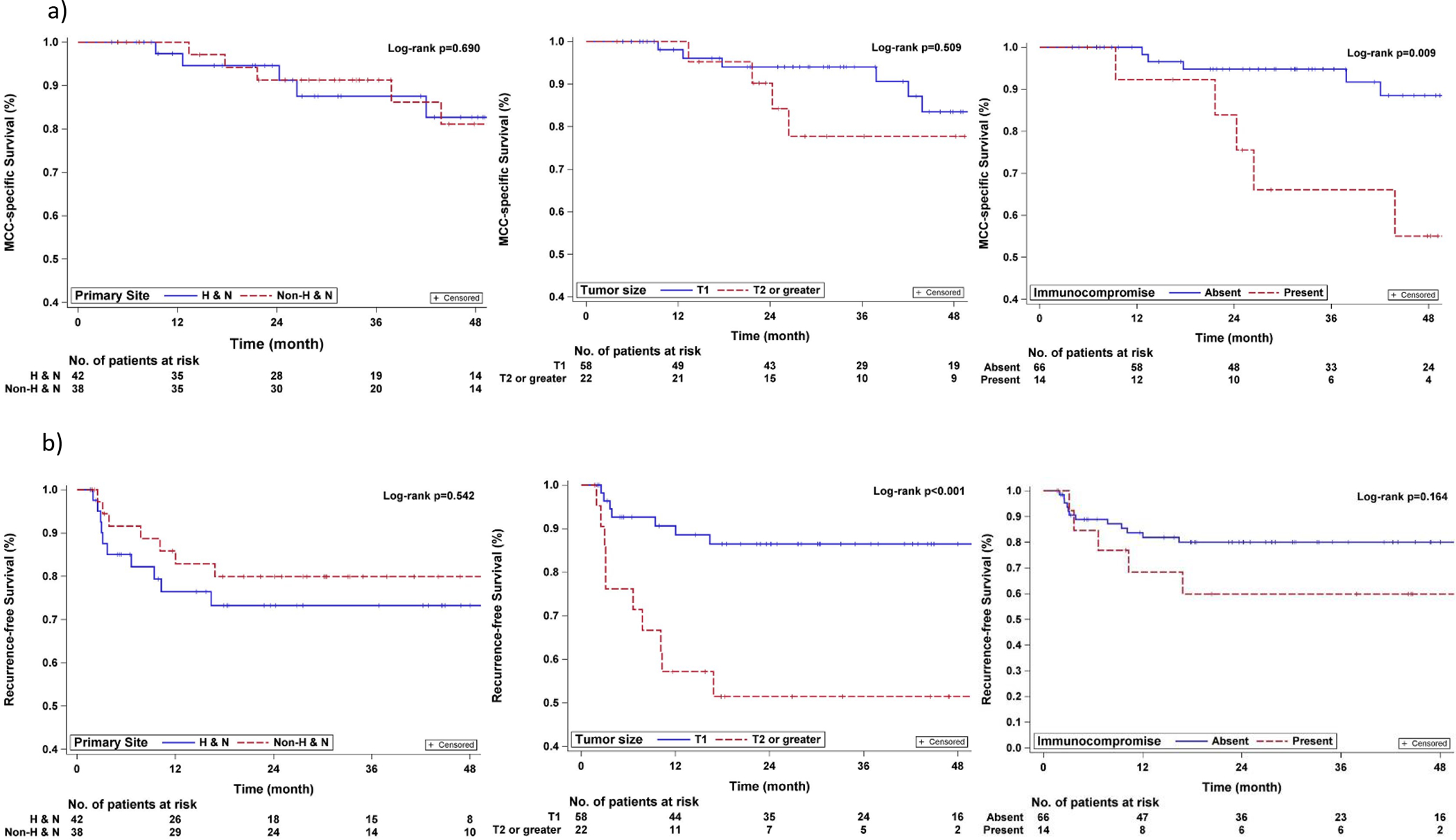
(a) MCCS for HN vs. NHN patients, (b) MCCS for patients with T1 vs. T2 or greater primary tumors, (c) MCSS for patients without vs. with immunocompromise, (d) RFS for HN vs. NHN patients, (e) RFS for patients with T1 vs. T2 or greater primary tumors, (f) RFS for patients without vs. with immunocompromise.
On multivariable analyses, being immunocompromised was independently associated with worse RRFS (HR 5.4, 95% CI [1.3–22.3], p=0.02) but was no longer significantly associated with MCSS. Larger tumor size (T2+ vs T1) was also independently associated with worse RFS (HR 4.3, 95% CI [1.6–11.4], p<0.01).
Discussion
This study illustrates that despite considerable variation in locoregional therapy approach for clinical stage I-II HN vs. NHN MCC at our institution, both cohorts overall had excellent locoregional control, consistent with previous reports.9,10,16,17 Overall, HN patients less frequently underwent WLE and were more frequently considered for definitive primary site RT. Despite this difference, HN and NHN patients had similar excellent 94% 3-year LRFS. While only 62% of HN patients had formal nodal evaluation with SLNB, HN patients were more frequently considered for elective nodal RT. Again, both HN and NHN patients experienced similarly favorable 3-year (>85%) NRFS. While MCC is known to have high rates of distant metastasis, which is known to be associated with poor outcomes, locoregional disease control is an important endpoint in the context of the potential morbidity of local, regional or nodal recurrence, which can frequently be multifocal, aggressive, and depending on location, and prior therapy, challenging to salvage.16,18–20
The rationale for the significant variation in treatment of HN vs. NHN patients in this study were multifactorial. At out institution, HN MCC patients are most commonly managed by our multidisciplinary Head & Neck Oncology Team while NHN MCC patients are typically managed by out multidisciplinary Melanoma and Cutaneous Oncology Team. Given the challenging anatomy of HN patients with critical structures often immediately adjacent to primary tumor sites or the morbidity of larger resections in terms of patient disfigurement, the HN multidisciplinary team has historically opted for smaller resections with RT serving as a tool to maximize local disease control. In addition, given the complexity of the HN lymphatic system there had previously been concern regarding the reliability of SLNB to accurately assess the entire nodal basin, such that elective nodal RT was historically more common. More recently, however, as data has accumulated regarding the value of SLNB across anatomic sites, SLNB has been applied more commonly with less elective nodal RT (except in cases where treating the primary site would necessarily radiate a portion of the nodal basin, in which case the relevant nodal levels may still be included).
Consistent with our observed practice variation by primary MCC anatomic site for cLN(−) patients, National Comprehensive Cancer Network (NCCN) guidelines recommend that multidisciplinary management be based on primary site risk factors. For patients without baseline high-risk factors (>1 cm size, immunosuppression, HN site, lymphovascular invasion), WLE alone (1–2 cm margins) is recommended, but in the presence of risk factors discovered following surgery, consideration of RT is recommended. For patients with high-risk factors known prior to resection, individualized-margin excision with adjuvant RT is recommended regardless of excisional approach. For all patients, SLNB is typically recommended with CLND and/or adjuvant nodal RT for positive SLNs. While observation of the nodal basin is typical after negative SLNB, NCCN does note that elective nodal RT can be considered for patients at high risk for false-negative SLNB.
Previous reports of MCC have typically grouped HN and NHN anatomic sites together and have also demonstrated variability in locoregional MCC practice patterns. A Massachusetts General Hospital (MGH) study reported 48% of patients with pathological stage I-IIIa MCC (n=52/109) were managed with surgery alone, while a study of cLN(−) MCC patients from Memorial Sloan-Kettering Cancer Center (MSKCC) observed 20% (n=55/270) received adjuvant primary RT and 16% (n= 42/270) received adjuvant nodal RT. Despite these variations in treatment, locoregional control remained favorable (>84%) in both studies.21,22 In a retrospective review from the British Columbia Cancer Agency, 37 patients with stage I-III MCC were treated with biopsy alone with macroscopic margins and definitive primary RT and demonstrated 5-year locoregional control of 90%.23 In a previous series from our center focused on 106 stage I-III HN patients treated between 1988–2011, 83% of patients received adjuvant primary RT and 17% received definitive primary RT after positive-margin biopsy.24 Despite nearly 40% of these patients having stage III disease and 17% having gross residual disease at the time of RT, 5-year locoregional control rates were 96% and 96%, respectively. In our current series of clinically stage I-II MCC patients treated between 2014–2021 with RT to the primary site and/or regional nodal basin at our institution as a component of their initial treatment strategy, despite significant differences in surgical and RT practice patterns between HN and NHN patients, similar excellent locoregional control rates were achieved in both cohorts. Though it is important to note that the current analysis required adjuvant RT as an inclusion criterion.
The optimal RT dose for MCC has not been definitively established. NCCN guidelines recommend delivery of 60–66 Gy for gross disease, 56–60 Gy for microscopically-positive margins, and 50–56 Gy after margin-negative resection.25 Our data (as well as others) supports that lesser surgery can be compensated for with RT.23 Particularly in functionally or cosmetically sensitive regions (such as near the eyes or lips), MCC’s high radiosensitivity may provide an opportunity to avoid the morbidity of extensive surgery with the appropriate use of RT. Our data supports this hypothesis by illustrating similar LRFS rates in HN vs. NHN MCC patients who received adjuvant RT despite 23% of HN patients not undergoing WLE.
In general, SLNB is recommended at the time of MCC resection to provide prognostic and staging information with at least one report suggesting a potential survival advantage.26 For patients with sentinel node positivity who do not undergo CLND, adjuvant nodal RT is recommended and is typically delivered at 50–56 Gy. However, for SLNB-negative patients, the role for elective nodal RT has been debated.23,27 Some argue that HN SLNB may not be as accurate due to aberrant/more complex lymphatic anatomy in the region, suggesting an increased utility of elective nodal RT; nonetheless, evidence is also conflicting.28 In our study, adjuvant nodal RT was delivered to all HN patients with positive SLNB, while elective nodal radiation was utilized in 24% of HN patients with negative SLNB and 79% of those with no nodal evaluation. In contrast, no NHN patient received elective nodal RT and adjuvant nodal RT was used less frequently. Given that 3-year NRFS was similar between HN and NHN patients, both approaches seem equivalently effective.
Regardless of approach, similar local, regional, and nodal control rates were seen in both HN and NHN patients in this study, suggesting that locoregional control may be accomplished using a multidisciplinary team approach through various surgical and RT strategies. Paralleling these locoregional control rates, we observed excellent MCSS in both cohorts. Based on these outcomes, multidisciplinary consideration of the morbidity associated with varied approaches (e.g. functional outcomes, cosmesis, lymphedema) and patient preferences are recommended to optimize patient-level outcomes.
There are important limitations of this analysis, including the retrospective evaluation from a single high-volume MCC center and the heterogeneity in medical teams caring for patients depending on primary anatomic disease site. Furthermore, this study focused only on patients who received adjuvant RT as a component of their care from 2014–2021, thus excluding the cohort of stage I/II MCC patients who were treated with surgery alone and who may have an overall more favorable risk profile than patients who received RT and were included in this study. In addition, factors that may confound the effect of locoregional therapy on locoregional recurrence events include the competing risk of death in this relatively older age patient cohort and the fact that patients who recurred at any site were more likely to receive systemic therapy, which would influence subsequent rates of locoregional control. Taken together, these limitations likely account for why the multivariable analyses did not find well established risk factors like LVI to be associated with poor prognosis. The small sample size and heterogeneity of treatment approach also makes definitive assessment of the absolute recurrence risk based on anatomic site difficult to determine. Despite these caveats, this study represents one of the largest studies to date to explore detailed patterns of care and outcomes of patients with localized MCC managed with RT as a component of their initial treatment plan. While variable practice patterns by site of primary MCC did not affect recurrence or survival outcomes, unsurprisingly, larger tumor size and immunosuppressed status were associated with higher likelihood of recurrence and worse survival. Future prospective studies are needed to evaluate the optimal surgical and RT strategies for clinically localized MCC.
Conclusions
In this retrospective review, we observed similar locoregional control rates among patients with clinically localized MCC of the HN and NHN regions who had adjuvant RT as a component of their initial treatment, despite differences in surgical and RT practice patterns. Given the exquisite radiosensitivity of MCC, this study further supports the critical role of patient-centric, multidisciplinary management in maximizing disease control while minimizing treatment-associated morbidity and adverse cosmetic and/or functional consequences.
Supplementary Material
Highlights:
For MCC patients who received RT, practice patterns varied by primary tumor site.
Patients with head & neck MCC had less surgery and more radiation therapy.
Despite practice variance by tumor site, patients had similar locoregional control.
Given similar outcomes, patient-centric approaches for management are warranted.
Funding Disclosures:
Funding to support this study was provided by the National Center for Advancing Translational Science (NCATS), 5KL2TR003168–05.
ABBREVIATIONS
- MCC
Merkel cell carcinoma
- cLN(−)
clinically lymph node negative
- RT
radiation therapy
- HN
head and neck
- NHN
non-head and neck
- WLE
wide local excision
- SLNB
sentinel lymph node biopsy
- SLN
sentinel lymph node
- CLND
completion lymph node dissection
- MCSS
Merkel cell-specific survival
- RFS
recurrence-free survival
- LC
local control
- NC
nodal control
- RC
regional control
- DMFS
distant-metastasis-free survival
Footnotes
Conflicts of Interest:
There are no conflicts of interest related to the design or execution of this study. JEG has served as a consultant and/ or on advisory board for Merck, unrelated to the content of this work.
References
- 1.Houben R, Schrama D, and Becker JC, Molecular pathogenesis of Merkel cell carcinoma. Exp Dermatol, 2009. 18(3): p. 193–8. [DOI] [PubMed] [Google Scholar]
- 2.Schadendorf D, et al. , Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer, 2017. 71: p. 53–69. [DOI] [PubMed] [Google Scholar]
- 3.Becker JC, et al. , Merkel cell carcinoma. Nat Rev Dis Primers, 2017. 3: p. 17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaae J, et al. , Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst, 2010. 102(11): p. 793–801. [DOI] [PubMed] [Google Scholar]
- 5.Harms KL, et al. , Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol, 2016. 23(11): p. 3564–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouary T, et al. , Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol, 2012. 23(4): p. 1074–80. [DOI] [PubMed] [Google Scholar]
- 7.Perez MC, et al. , Resection Margins in Merkel Cell Carcinoma: Is a 1-cm Margin Wide Enough? Ann Surg Oncol, 2018. 25(11): p. 3334–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Servy A, et al. , Merkel cell carcinoma: value of sentinel lymph-node status and adjuvant radiation therapy. Ann Oncol, 2016. 27(5): p. 914–9. [DOI] [PubMed] [Google Scholar]
- 9.Joseph K, et al. , Patterns and predictors of relapse in Merkel cell carcinoma: Results from a population-based study. Radiother Oncol, 2022. 166: p. 110–117. [DOI] [PubMed] [Google Scholar]
- 10.Levy S, et al. , Postoperative radiotherapy in stage I-III Merkel cell carcinoma. Radiother Oncol, 2022. 166: p. 203–211. [DOI] [PubMed] [Google Scholar]
- 11.Tarabadkar ES, et al. , Narrow excision margins are appropriate for Merkel cell carcinoma when combined with adjuvant radiation: Analysis of 188 cases of localized disease and proposed management algorithm. J Am Acad Dermatol, 2021. 84(2): p. 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaouen F, et al. , Narrow resection margins are not associated with mortality or recurrence in patients with Merkel cell carcinoma: A retrospective study. J Am Acad Dermatol, 2021. 84(4): p. 921–929. [DOI] [PubMed] [Google Scholar]
- 13.Singh B, et al. , Demographics and outcomes of stage I and II Merkel cell carcinoma treated with Mohs micrographic surgery compared with wide local excision in the National Cancer Database. J Am Acad Dermatol, 2018. 79(1): p. 126–134.e3. [DOI] [PubMed] [Google Scholar]
- 14.Banks PD, et al. , Recent Insights and Advances in the Management of Merkel Cell Carcinoma. J Oncol Pract, 2016. 12(7): p. 637–46. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz JL, et al. , Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol, 2011. 29(8): p. 1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashby MA, et al. , Primary cutaneous neuroendocrine (Merkel cell or trabecular carcinoma) tumour of the skin: a radioresponsive tumour. Clin Radiol, 1989. 40(1): p. 85–7. [DOI] [PubMed] [Google Scholar]
- 17.Fang LC, et al. , Radiation monotherapy as regional treatment for lymph node-positive Merkel cell carcinoma. Cancer, 2010. 116(7): p. 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasle H, Merkel cell carcinoma: the role of primary treatment with radiotherapy. Clin Oncol (R Coll Radiol), 1991. 3(2): p. 114–6. [DOI] [PubMed] [Google Scholar]
- 19.Morrison WH, et al. , The essential role of radiation therapy in securing locoregional control of merkel cell carcinoma. International Journal of Radiation Oncology*Biology*Physics, 1990. 19(3): p. 583–591. [DOI] [PubMed] [Google Scholar]
- 20.Pacella J, et al. , The role of radiotherapy in the management of primary cutaneous neuroendocrine tumors (Merkel cell or trabecular carcinoma): experience at the Peter MacCallum Cancer Institute (Melbourne, Australia). Int J Radiat Oncol Biol Phys, 1988. 14(6): p. 1077–84. [DOI] [PubMed] [Google Scholar]
- 21.Fields RC, et al. , Recurrence after complete resection and selective use of adjuvant therapy for stage I through III Merkel cell carcinoma. Cancer, 2012. 118(13): p. 3311–20. [DOI] [PubMed] [Google Scholar]
- 22.Santamaria-Barria JA, et al. , Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol, 2013. 20(4): p. 1365–73. [DOI] [PubMed] [Google Scholar]
- 23.Harrington C and Kwan W, Outcomes of Merkel Cell Carcinoma Treated with Radiotherapy without Radical Surgical Excision. Annals of Surgical Oncology, 2014. 21(11): p. 3401–3405. [DOI] [PubMed] [Google Scholar]
- 24.Bishop AJ, et al. , Merkel cell carcinoma of the head and neck: Favorable outcomes with radiotherapy. Head Neck, 2016. 38 Suppl 1: p. E452–8. [DOI] [PubMed] [Google Scholar]
- 25.NCCN clinical practice guidelines in oncology Merkel cell carcinioma version 2.2022 [Google Scholar]
- 26.Kachare SD, et al. , Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol, 2014. 21(5): p. 1624–30. [DOI] [PubMed] [Google Scholar]
- 27.Grotz TE, et al. , Negative Sentinel Lymph Node Biopsy in Merkel Cell Carcinoma is Associated with a Low Risk of Same-Nodal-Basin Recurrences. Ann Surg Oncol, 2015. 22(12): p. 4060–6. [DOI] [PubMed] [Google Scholar]
- 28.Straker RJ 3rd, et al. , Predictors of False Negative Sentinel Lymph Node Biopsy in Clinically Localized Merkel Cell Carcinoma. Ann Surg Oncol, 2021. 28(12): p. 6995–7003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


