Abstract
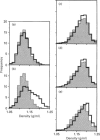
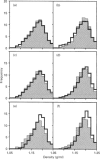
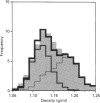
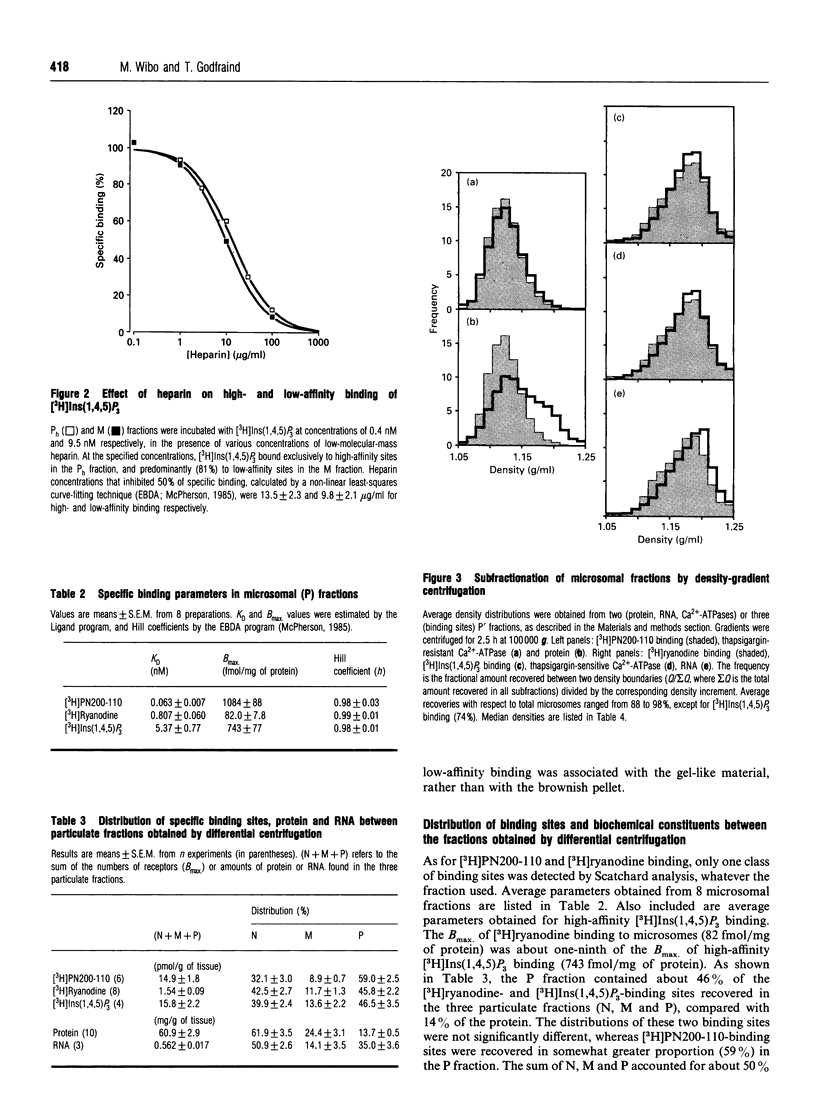
[3H]Ins(1,4,5)P3- and [3H]ryanodine-binding sites were characterized in membrane fractions from guinea-pig intestinal smooth muscle (longitudinal layer) and their subcellular localization was investigated by analytical cell-fractionation techniques. Fractions collected at low centrifugal fields (N and M fractions) contained predominantly low-affinity [3H]Ins(1,4,5)P3-binding sites (KD 80 nM), whereas microsomal (P) fractions contained only high-affinity binding sites (KD 5 nM). Total sedimentable high-affinity binding sites of [3H]Ins(1,4,5)P3 were 9-10-fold more numerous than those of [3H]ryanodine. Both high-affinity binding sites were purified in microsomal fractions, and their sub-microsomal distribution patterns after isopycnic density-gradient centrifugation were similar to those of presumed endoplasmic reticulum (ER) constituents, indicating that Ins(1,4,5)P3 and ryanodine receptors were localized primarily in ER and probably associated with rough as well as smooth ER. However, the stoichiometric ratio of Ins(1,4,5)P3 to ryanodine receptors was distinctly higher in high-density RNA-rich subfractions than in low-density RNA-poor subfractions, suggesting that Ins(1,4,5)P3 receptors were somewhat concentrated in the ribosome-coated portions of ER. The low overall stoichiometric ratio of ryanodine to Ins(1,4,5)P3 receptors in intestinal smooth muscle (1:9-10) might explain, at least partly, the existence of a Ca(2+)-storage compartment devoid of ryanodine-sensitive Ca2+ channels, but equipped with Ins(1,4,5)P3-sensitive channels, in saponin-permeabilized smooth-muscle cells [Iino, Kobayashi and Endo (1988) Biochem. Biophys. Res. Commun. 152, 417-422].
Full text
PDF


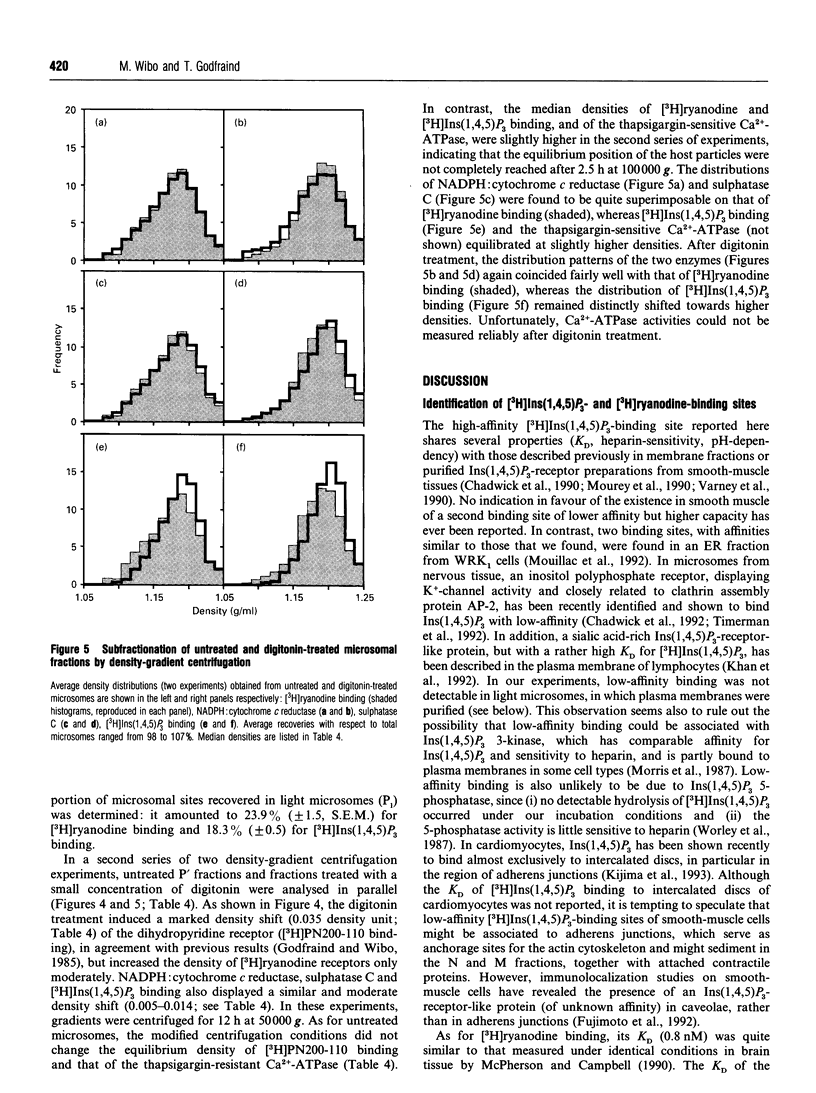
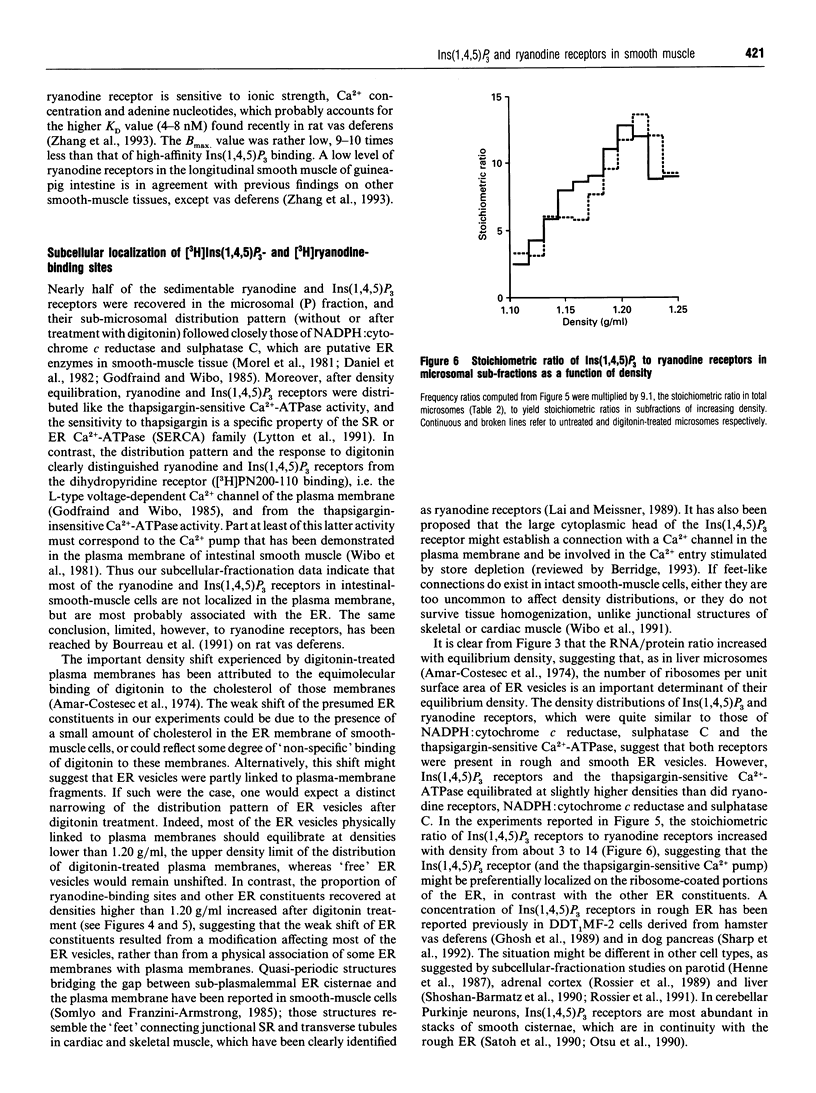
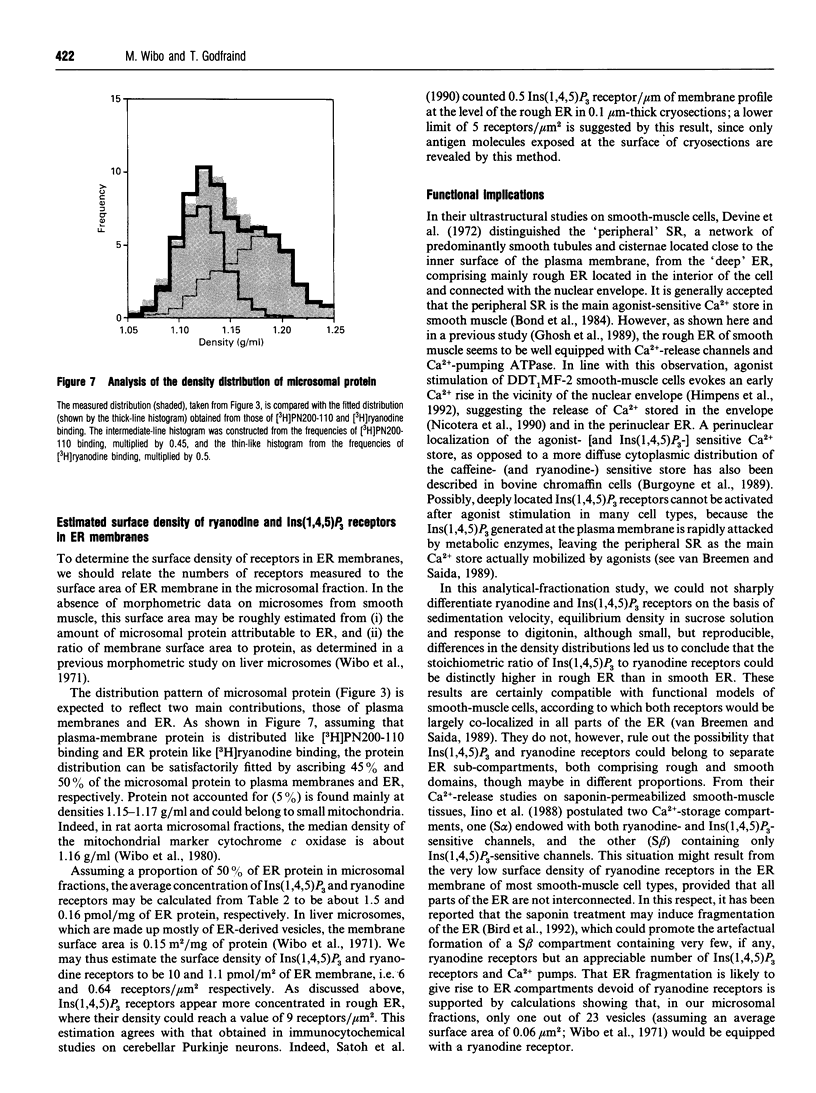

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Wibo M., Thinès-Sempoux D., Beaufay H., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. IV. Biochemical, physical, and morphological modifications of microsomal components induced by digitonin, EDTA, and pyrophosphate. J Cell Biol. 1974 Sep;62(3):717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentle L. A., Dutta S., Metcoff J. The sequential enzymatic determination of DNA and RNA. Anal Biochem. 1981 Sep 1;116(1):5–16. doi: 10.1016/0003-2697(81)90314-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bird G. J., Obie J. F., Putney J. W., Jr Functional homogeneity of the non-mitochondrial Ca2+ pool in intact mouse lacrimal acinar cells. J Biol Chem. 1992 Sep 15;267(26):18382–18386. [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreau J. P., Zhang Z. D., Low A. M., Kwan C. Y., Daniel E. E. Ryanodine and the adrenergic, purinergic stimulation in the rat vas deferens smooth muscle: functional and radioligand binding studies. J Pharmacol Exp Ther. 1991 Mar;256(3):1063–1071. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Timerman A. P., Saito A., Mayrleitner M., Schindler H., Fleischer S. Structural and functional characterization of an inositol polyphosphate receptor from cerebellum. J Biol Chem. 1992 Feb 15;267(5):3473–3481. [PubMed] [Google Scholar]
- Daniel E. E., Grover A. K., Kwan C. Y. Isolation and properties of plasma membrane from smooth muscle. Fed Proc. 1982 Oct;41(12):2898–2904. [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Kaba A. Blockade or reversal of the contraction induced by calcium and adrenaline in depolarized arterial smooth muscle. Br J Pharmacol. 1969 Jul;36(3):549–560. doi: 10.1111/j.1476-5381.1969.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Godfraind T., Wibo M. Subcellular localization of [3H]-nitrendipine binding sites in guinea-pig ileal smooth muscle. Br J Pharmacol. 1985 Jun;85(2):335–340. doi: 10.1111/j.1476-5381.1985.tb08866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne V., Piiper A., Söling H. D. Inositol 1,4,5-trisphosphate and 5'-GTP induce calcium release from different intracellular pools. FEBS Lett. 1987 Jun 22;218(1):153–158. doi: 10.1016/0014-5793(87)81037-2. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Himpens B., De Smedt H., Casteels R. Kinetics of nucleocytoplasmic Ca2+ transients in DDT1 MF-2 smooth muscle cells. Am J Physiol. 1992 Nov;263(5 Pt 1):C978–C985. doi: 10.1152/ajpcell.1992.263.5.C978. [DOI] [PubMed] [Google Scholar]
- Iino M., Kobayashi T., Endo M. Use of ryanodine for functional removal of the calcium store in smooth muscle cells of the guinea-pig. Biochem Biophys Res Commun. 1988 Apr 15;152(1):417–422. doi: 10.1016/s0006-291x(88)80730-7. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kajikuri J., Kuriyama H. Characteristic features of noradrenaline-induced Ca2+ mobilization and tension in arterial smooth muscle of the rabbit. J Physiol. 1992 Nov;457:297–314. doi: 10.1113/jphysiol.1992.sp019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Snyder S. H. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2849–2853. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima Y., Saito A., Jetton T. L., Magnuson M. A., Fleischer S. Different intracellular localization of inositol 1,4,5-trisphosphate and ryanodine receptors in cardiomyocytes. J Biol Chem. 1993 Feb 15;268(5):3499–3506. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai F. A., Meissner G. The muscle ryanodine receptor and its intrinsic Ca2+ channel activity. J Bioenerg Biomembr. 1989 Apr;21(2):227–246. doi: 10.1007/BF00812070. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Mayrleitner M., Chadwick C. C., Timerman A. P., Fleischer S., Schindler H. Purified IP3 receptor from smooth muscle forms an IP3 gated and heparin sensitive Ca2+ channel in planar bilayers. Cell Calcium. 1991 Jul;12(7):505–514. doi: 10.1016/0143-4160(91)90032-a. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. Solubilization and biochemical characterization of the high affinity [3H]ryanodine receptor from rabbit brain membranes. J Biol Chem. 1990 Oct 25;265(30):18454–18460. [PubMed] [Google Scholar]
- Meldolesi J., Madeddu L., Pozzan T. Intracellular Ca2+ storage organelles in non-muscle cells: heterogeneity and functional assignment. Biochim Biophys Acta. 1990 Nov 12;1055(2):130–140. doi: 10.1016/0167-4889(90)90113-r. [DOI] [PubMed] [Google Scholar]
- Menetski J. P., Bear D. G., Kowalczykowski S. C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci U S A. 1990 Jan;87(1):21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Wibo M., Godfraind T. A calmodulin-stimulated Ca2+ pump in rat aorta plasma membranes. Biochim Biophys Acta. 1981 Jun 9;644(1):82–88. doi: 10.1016/0005-2736(81)90061-4. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Downes C. P., Harden T. K., Michell R. H. Turkey erythrocytes possess a membrane-associated inositol 1,4,5-trisphosphate 3-kinase that is activated by Ca2+ in the presence of calmodulin. Biochem J. 1987 Dec 1;248(2):489–493. doi: 10.1042/bj2480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillac B., Devilliers G., Jard S., Guillon G. Pharmacological characterization of inositol 1,4,5-trisphosphate binding sites: relation to Ca2+ release. Eur J Pharmacol. 1992 Mar 12;225(3):179–193. doi: 10.1016/0922-4106(92)90019-r. [DOI] [PubMed] [Google Scholar]
- Mourey R. J., Verma A., Supattapone S., Snyder S. H. Purification and characterization of the inositol 1,4,5- trisphosphate receptor protein from rat vas deferens. Biochem J. 1990 Dec 1;272(2):383–389. doi: 10.1042/bj2720383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu H., Yamamoto A., Maeda N., Mikoshiba K., Tashiro Y. Immunogold localization of inositol 1, 4, 5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells using three monoclonal antibodies. Cell Struct Funct. 1990 Jun;15(3):163–173. doi: 10.1247/csf.15.163. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Bird G. S., Putney J. W., Jr Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through the actin microfilaments. Biochem J. 1991 Mar 15;274(Pt 3):643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier M. F., Capponi A. M., Vallotton M. B. The inositol 1,4,5-trisphosphate-binding site in adrenal cortical cells is distinct from the endoplasmic reticulum. J Biol Chem. 1989 Aug 25;264(24):14078–14084. [PubMed] [Google Scholar]
- Satoh T., Ross C. A., Villa A., Supattapone S., Pozzan T., Snyder S. H., Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990 Aug;111(2):615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. H., Snyder S. H., Nigam S. K. Inositol 1,4,5-trisphosphate receptors. Localization in epithelial tissue. J Biol Chem. 1992 Apr 15;267(11):7444–7449. [PubMed] [Google Scholar]
- Shoshan-Barmatz V., Zhang G. H., Garretson L., Kraus-Friedmann N. Distinct ryanodine- and inositol 1,4,5-trisphosphate-binding sites in hepatic microsomes. Biochem J. 1990 Jun 15;268(3):699–705. doi: 10.1042/bj2680699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. An assay for sarcoplasmic reticulum Ca2(+)-ATPase activity in muscle homogenates. Anal Biochem. 1990 Dec;191(2):321–331. doi: 10.1016/0003-2697(90)90226-y. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Franzini-Armstrong C. New views of smooth muscle structure using freezing, deep-etching and rotary shadowing. Experientia. 1985 Jul 15;41(7):841–856. doi: 10.1007/BF01970000. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timerman A. P., Mayrleitner M. M., Lukas T. J., Chadwick C. C., Saito A., Watterson D. M., Schindler H., Fleischer S. Inositol polyphosphate receptor and clathrin assembly protein AP-2 are related proteins that form potassium-selective ion channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8976–8980. doi: 10.1073/pnas.89.19.8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney M. A., Rivera J., Lopez Bernal A., Watson S. P. Are there subtypes of the inositol 1,4,5-trisphosphate receptor? Biochem J. 1990 Jul 1;269(1):211–216. doi: 10.1042/bj2690211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Amar-Costesec A., Berthet J., Beaufay H. Electron microscope examination of subcellular fractions. 3. Quantitative analysis of the microsomal fraction isolated from rat liver. J Cell Biol. 1971 Oct;51(1):52–71. doi: 10.1083/jcb.51.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Bravo G., Godfraind T. Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res. 1991 Mar;68(3):662–673. doi: 10.1161/01.res.68.3.662. [DOI] [PubMed] [Google Scholar]
- Wibo M., Duong A. T., Godfraind T. Subcellular location of semicarbazide-sensitive amine oxidase in rat aorta. Eur J Biochem. 1980 Nov;112(1):87–94. doi: 10.1111/j.1432-1033.1980.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Wibo M., Morel N., Godfraind T. Differentiation of Ca2+ pumps linked to plasma membrane and endoplasmic reticulum in the microsomal fraction from intestinal smooth muscle. Biochim Biophys Acta. 1981 Dec 21;649(3):651–660. doi: 10.1016/0005-2736(81)90170-x. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]
- Yamazawa T., Iino M., Endo M. Presence of functionally different compartments of the Ca2+ store in single intestinal smooth muscle cells. FEBS Lett. 1992 Apr 20;301(2):181–184. doi: 10.1016/0014-5793(92)81243-f. [DOI] [PubMed] [Google Scholar]
- Zhang Z. D., Kwan C. Y., Daniel E. E. Subcellular-membrane characterization of [3H]ryanodine-binding sites in smooth muscle. Biochem J. 1993 Feb 15;290(Pt 1):259–266. doi: 10.1042/bj2900259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]