Abstract
Background
Idiopathic intracranial hypertension (IIH) is a neurological disorder characterized by increased intracranial pressure. Whilst lumbar puncture (LP) is necessary for the diagnosis of IIH, its therapeutic effect remains unclear. Our aim was to evaluate the therapeutic effect of a single LP in people with IIH (pwIIH).
Methods
In this prospective observational study, we analysed short-term neurological and ophthalmological outcomes in pwIIH before, one (D1) and seven days (D7) after the LP. The primary outcome was the change in papilledema degree from baseline. Secondary outcomes included visual outcomes, morphological changes in optical coherence tomography (peripapillary retinal nerve fibre layer [pRNFL] thickness and ganglion cell layer [GCL] volume) and transbulbar sonography (arachnoid optic nerve sheath diameter [AONSD]), and headache outcomes (peak and median headache severity and burden related to headache).
Results
We included 30 pwIIH (mean age 32.8 years [SD 8.4], 93.3% female, median cerebrospinal fluid [CSF] opening pressure 33.0 cmCSF [IQR 26.9–35.3], median body mass index (BMI) 34.8 kg/m2 [IQR 30.9–40.9]). The median papilledema grading at baseline was 2 (Friedman DI (1999) Pseudotumor cerebri. Neurosurg Clin N Am 10(4):609–621 viii); (Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ (2019) The expanding burden of idiopathic intracranial hypertension. Eye Lond Engl 33(3):478–485); (Ab D, Gt L, Nj V, Sl G, Ml M, Nj N et al. (2007) Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol [Internet]. Apr [cited 2024 Jun 2];143(4). https://pubmed.ncbi.nlm.nih.gov/17386271/) and was significantly reduced at D7 (2 [1–2], p = 0.011). Median pRNFL thickness had decreased significantly at D7 (-9 μm [-62.5, -1.3], p = 0.035), with pRNFL thickness at baseline being associated with the pRNFL change (F(1,11) = 18.79, p = 0.001). Mean AONSD had decreased significantly at both D1 (-0.74 mm [0.14], p < 0.001) and D7 (-0.65 mm [0.17], p = 0.01), with AONSD at baseline being associated with the change in AONSD at both time points (D1: β= -0.89, 95% CI -1.37, -0.42, p = 0.002; D7: β= -0.85, 95% CI -1.42, -0.28, p = 0.007). Peak headache severity was slightly lower at D7 (-1/10 [-3, 0], p = 0.026), whereas median headache severity and headache burden remained unchanged.
Conclusions
This short-term follow-up study in pwIIH undergoing a single LP suggests a moderate effect on ophthalmological but not headache outcomes. The usefulness of LP as a therapeutic measure in IIH remains controversial and should likely be reserved for patients with limited treatment options, e.g., in pregnancy or intolerability to medication.
Keywords: Idiopathic intracranial hypertension, Lumbar puncture, Headache, Visual outcome
Background
Idiopathic intracranial hypertension (IIH) is a neurological disorder characterized by increased intracranial pressure (ICP) most commonly affecting young obese women [1]. It frequently causes chronic headaches and papilledema with a risk of permanent vision loss. Its incidence is increasing in parallel with rising obesity rates worldwide [2], as even modest weight gain is associated with an increased risk of developing IIH [3]. Several mechanisms behind increased ICP in IIH have been proposed, including cerebrospinal fluid (CSF) overproduction, reduced resorption, and/or increased venous sinus pressure [4], although whether the latter is the primary cause or a secondary effect remains controversial. Treatment approaches in functionally stable patients are mostly based on weight loss and the reduction of CSF production with carbonic anhydrase inhibitors.
Lumbar puncture (LP) is an essential part in the diagnostic workup of IIH to confirm the elevated ICP by measuring CSF opening pressure (OP), and to exclude secondary causes of the latter [5]. Presumptive diagnosis of IIH based on typical clinical features alone, i.e., high body mass index (BMI), bilateral optic disc abnormalities and/or neuroimaging findings of increased ICP, can result in overdiagnosis of IIH and unnecessary treatment [6]. Furthermore, LP is used for treatment, especially in case of fulminant IIH with severe papilledema, resulting in a partial resolution of both papilledema and headache [7, 8]. However, the benefit of repeated LP is questionable, as the reduction of ICP after LP is only temporary, and reliable data on its therapeutic efficacy, especially in patients with mild to moderate papilledema, are still lacking [9]. Furthermore, the underlying mechanism driving the IIH-associated headache appears to be less ICP-dependent [7], as a direct correlation between headache intensity and ICP has never been demonstrated, and more than half of people with IIH (pwIIH) experience persistence of headache after resolution of papilledema and normalization of ICP [8].
The aim of this study was to evaluate the therapeutic effect of a single LP on neurological and ophthalmological outcomes in a cohort of newly diagnosed pwIIH.
Methods
Study cohort and study design
For this prospective observational study, we included newly diagnosed pwIIH according to revised Friedman criteria from an ongoing prospective observational cohort study (Vienna Idiopathic Intracranial Hypertension Biomarker study [VIIH-BIO]), jointly conducted by the Department of Neurology and the Department of Ophthalmology at the Medical University of Vienna starting in January 2021.
Data were collected at the following time points: before LP (D0), one (D1) and seven days (D7) after LP. Disease duration was defined as the time between the onset of symptoms and the diagnostic LP. Briefly, standardized VIIH-BIO case reports include demographic data, body weight, disease specific parameters, as well as documentation of diagnostic and therapeutic procedures. Headache intensity is evaluated using headache diaries recorded in months prior to LP, including the Headache Impact Test-6 (HIT-6), monthly headache days (MHD) and headache severity quantified by the 11-point Numeric Rating Scale (NRS). A clinically meaningful change in headache severity was defined as a change of ≥ 2 in the NRS score [10, 11]. The HIT-6 includes six items that assess the functions most affected by headache, such as daily activities, social functioning, psychological state, pain, fatigue and attention. Each item is rated with five responses (always, very often, sometimes, never, or rarely) [12]. The total HIT-6 score ranges from 36 to 78, with higher scores reflecting more severe impact [13]. All examinations are performed by specialized neurologists and neuro-ophthalmologists. All pwIIH are treated according to best practice based on the recommendation of weight loss and pharmacological treatment with acetazolamide, topiramate and/or furosemide following the LP [14]. For the present study, only pwIIH with stable pharmacological treatment (no change in medication dosage or regimen) during the observation period (from D0 to D7) were included.
Lumbar puncture
A standardized diagnostic LP was performed using a Quincke type point, 20GA 3.50 IN, 0.9 × 88 mm (Braun Melsungen AG, Germany) spinal needle with a spinal manometer (Rocket Medical®, UK). LP was performed in the left lateral decubitus position with the legs repositioned after dural puncture to reduce abdominal compression. A stabilised CSF OP reading was recorded as fluctuations in the manometer settled. After measuring CSF OP, CSF was drained until a target of 10–15 cm CSF-OP was reached (maximum of 50 ml CSF drained). CSF closing pressure was recorded prior to removal of the spinal needle.
Ophthalmological assessment
Fundoscopy was executed using the Frisén staging scale to rate papilledema severity and categorize the swelling of optic discs from stage 0 (no papilledema) to stage 5 (severe papilledema) [15].
Best-corrected visual acuity was assessed using Sloan charts at distance after subjective refraction. Results were given in logarithm of the minimum angle of resolution (logMAR). Meaningful change was defined as ≥ 0.2 logMAR [16].
Automated visual field testing (Humphrey Field Analyzer, Carl Zeiss Meditec, Jena, Germany), using 30 − 2 Swedish Interactive Threshold Algorithm (SITA) standard protocols, was performed, quantifying the mean deviation (MD) in decibels (dB) of all test locations compared to age-matched controls and defining abnormal perimetry as an MD lower than − 2 dB [14, 15].
Optical coherence tomography (OCT) imaging was performed without pupil dilation in both eyes of each person using the same spectral-domain OCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany; software Heidelberg eye explorer software version 6.9a) adhering to the OSCAR-IB quality control criteria and describing findings in accordance with the APOSTEL criteria [17, 18]. For peripapillary retinal nerve fiber layer (pRNFL) measurement, a 12° (3.4 mm) ring scan centered on the optic nerve head was used (1536 A-scans, automatic real-time tracking [ART]: 100 averaged frames) [19]. Ganglion cell layer (GCL) volume was measured by means of a 20°×20° macular volume scan (centered on the macula with 512 A-scans and 25 B-scans aligned vertically with 16 averaged frames). Volume values characterize the GCL volume of the circular area centered around the foveola corresponding to the circular grid defined by the Early Treatment Diabetic Retinopathy Study [20]. Image processing was semiautomated using the built-in proprietary software for automated layer segmentation and manual correction of obvious errors. Measurements of worse eyes were used for statistical analysis, i.e., higher pRNFL thickness as a marker of edema and lower GCL volume as a marker of neuronal cell loss.
Transbulbar sonography (ABSolu, Quantel Medical, Cournon d’Auvergne, France) was performed after topical anesthesia with oxybuprocaine eye drops. Optic nerve sheaths were qualitatively assessed by transverse and longitudinal B-scans using a 20 MHz annular probe placed temporally on the eyeball to verify the presence of a bat sign indicating perineural CSF congestion. For quantitative measurement of arachnoid optic nerve sheath diameter (AONSD) standardized amplitude modulation, (A-scan) echography with tissue sensitivity settings was performed, placing the 8 MHz parallel beam A-scan probe on the temporal eye equator in primary gaze position [21, 22]. Standardized A-scan echography enables a more accurate measurement of AONSD than caliper measurements from B-scans, which lack standardized settings and are influenced by the applied signal gain. At least two measurements were taken within 3 mm of the posterior bulb wall, and the highest was documented as the diameter. Abnormal AONSD was defined as ≥ 4.50 mm.
Study outcomes
The primary outcome was the change in papilledema degree on Frisén scale at D1/D7 compared to D0.
Secondary outcomes assessed at D1 and D7 and compared to D0 comprised:
visual change: increase/decrease of visual acuity by ≥ 0.2 logMAR and/or mean deviation by ≥ 2.0 dB in static threshold perimetry,
change in pRNFL thickness and GCL volume,
change in AONSD,
change in headache presence and its improvement or worsening according to headache severity (a change of ≥ 2 in the NRS score) from D0 to D7 compared to the month before LP.
Standard protocol approvals, registrations, patient consents, and reporting
The study was approved by the ethics committee of the Medical University Vienna (ethical approval number: 2216/2020). Written informed consent was obtained from all participants. This study adheres to the reporting guidelines outlined within the ‘Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement’.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data-clearing committee of the Medical University Vienna.
Statistical analysis
Statistical analysis was performed using the statistical package of SPSS version 29.0.2.0. (Armonk, NY, USA). Categorial variables were expressed in absolute frequencies and percentages, continuous parametric variables as mean and standard deviation (SD) and continuous non-parametric variables as median with interquartile range (IQR) as appropriate. Continuous variables were tested for normal distribution using the Shapiro-Wilk-Test.
Repeated measures ANOVA, Friedman test and Wilcoxon Signed Ranks Test were performed for pairwise comparisons as appropriate, adjusted for age, sex, BMI, disease duration and CSF OP. Ophthalmological outcomes were investigated in a stepwise manner, additionally adjusted for [1] papilledema degree, [2] pRNFL thickness, [3] GCL volume, and [4] AONSD at baseline as appropriate. Linear mixed effects models were used to evaluate effects of covariates on clinical and ophthalmological outcomes.
Prespecified sensitivity analyses to determine the potential confounding influence were performed with the same statistical analysis setup excluding pwIIH without papilledema (IIH-WOP). Missing values due to missed study visits, missing information in the source document and/or data unavailability (lack of cooperation during the examination) were reported at each step. Significance level was set at a two-sided p-value < 0.05 with hierarchical Bonferroni correction for multiple testing according to the order of secondary outcomes.
Results
We included 30 pwIIH (mean age 32.8 [SD 8.4], 93.3% female, median CSF OP 33.0 cmCSF [IQR 26.9–35.3], median BMI 34.8 kg/m2 [IQR 30.9–40.9]), with a median disease duration prior to diagnostic LP of 3 months [IQR 2–7.5]. At baseline, 20 pwIIH [66.7%] suffered from headache with median MHD of 13.5 [IQR 10–28] and a mean HIT-6 score of 55.5 points [SD 10.8]; 12 [40.0%] pwIIH had chronic headache. The inclusion/exclusion process is shown in Fig. 1. The demographics of the study cohort at baseline are described in Table 1.
Fig. 1.
Flowchart of the inclusion/exclusion process. IIH: idiopathic intracranial hypertension, ONSF: optic nerve sheath fenestration
Table 1.
Cohort characteristics at baseline
| Study cohort (n = 30) | |
|---|---|
| Femalea | 28 (93.3) |
| Ageb | 32.8 (8.4) |
| Disease duration (months)c | 3 (2–7.5) |
| CSF opening pressure (cmCSF)c | 33.0 (26.9–35.3) |
| Volume of CSF drained (ml)d | 29 (10–40) |
| BMI (kg/m2)c | 34.8 (30.9–40.9) |
| IIH-WOPa | 5 (16.7) |
| Visual disturbancesa | 22 (73.3) |
| Tinnitusa | 15 (50.0) |
| Therapy after diagnostic LP | |
| Acetazolamidea | 29 (96.7) |
| Median acetazolamide dosage (mg)c | 1,000 (500–1,000) |
| Topiramatea | 1 (3.3) |
| Topiramate dosage (mg) | 50 |
| Headache | |
| Headache presencea | 20 (66.7) |
| MHDc | 13.5 (10–28) |
| Chronic headachea | 12 (40.0) |
| Median headache severity (NRS)b | 5 (3.8–6) |
| Peak headache severity (NRS)c | 8.5 (7–10) |
| HIT-6b | 55.5 (10.8) |
| Ophthalmological parameters | |
| Frisén-Scalec | 2 (1–3) |
| Visual acuity of worse eye (logMAR)c | 0.00 (-0.08, 0.00) |
| Decreased visual acuitya | 2 (6.7) |
| Visual field mean deviation of worse eye (dB)c | -1.84 (-5.27, -0.57) |
| Abnormal visual fielda | 14 (46.7) |
| pRNFL thickness of the worse eye (µm)c | 145.5 (97.8–221.5) |
| GCL volume of the worse eye (mm3)c | 1.05 (1.0-1.16) |
| Presence of a bat sign in echographya | 28 (93.3) |
| Abnormal AONSDa | 29 (96.7) |
| AONSD of the worse eyeb | 5.85 (0.75) |
aNumber (percentage), bMean (standard deviation), cMedian (interquartile range), dMedian (range)
AONSD: arachnoid optic nerve sheath diameter, BMI: body mass index, CSF: cerebrospinal fluid, GCL: ganglion cell layer, IIH-WOP: idiopathic intracranial hypertension without papilledema, LP: lumbar puncture, MHD: monthly headache days, NRS: Numeric Rating Scale, pRNFL: peripapillary retinal nerve fiber layer
Primary outcome
At baseline, 25 [83.3%] pwIIH presented with papilledema with a median Frisén grade of 2 [1–3]. While papilledema persisted in 17/21 [81.0%] and 20/24 [83.3%] pwIIH at D1 and D7, respectively, Frisén grading had only improved at D7 (Table 2). None of the covariates (age, sex, BMI, disease duration, CSF OP, papilledema degree at baseline) had a significant effect on change in Frisén grade.
Table 2.
Ophthalmological and neurological outcomes during the observation period
| D0 | D1 |
p-value (D0 vs. D1) |
D7 |
p-value (D0 vs. D7) |
|
|---|---|---|---|---|---|
| Ophthalmological outcomes | |||||
| Frisen-Scaleb | 2 (1–3) | 2 (1–2) | 0.102 | 1 (0–1) | 0.011 |
| Visual acuity of worse eye (logMAR)b | 0.00 (-0.08, 0.00) | -0.08 (-0.09, 0.00) | 0.786 | -0.08 (-0.08, 0.00) | 0.959 |
| Visual field mean deviation of worse eye (dB)b | -1.84 (-5.27, -0.57) | -1.53 (-3.70, -0.17) | 0.077 | -1.67 (-4.37, -0.54) | 0.521 |
| Change in pRNFL thickness (µm)b | n.a. | -3 (-9, 0) | 0.511 | -9 (-62.5, -1.3) | 0.035 |
| Change in GCL volume (mm3)b | n.a. | 0.00 (-0.02, 0.01) | > 0.999 | -0.01 (-0.03, 0.01) | 0.534 |
| Change in AONSD (mm)c | n.a. | -0.74 (0.14) | < 0.001 | -0.65 (0.17) | 0.010 |
| Neurological outcomes | |||||
| Headache presencea | 20/30 (66.7) | n.a. | n.a. | 13/26 (50.0) | 0.059 |
| Peak headache severity (NRS)b | 8.5 (7–10) | n.a. | n.a. | 7.5 (5.3–9.8) | 0.026 |
| Median headache severity (NRS)c | 4.3 (0.3) | n.a. | n.a. | 3.3 (0.8) | > 0.999 |
| Presence of visual disturbancesa | 22/30 (73.3) | n.a. | n.a. | 8/22 (36.4) | 0.011 |
| Tinnitus presencea | 15/30 (50.0) | n.a. | n.a. | 7/22 (31.8) | 0.046 |
aNumber (percentage), bMedian (interquartile range), cMean (standard deviation)
Secondary outcomes
Ophthalmological outcomes
Visual acuity had improved in 1/21 (4.8%) and 3/25 (12.0%) pwIIH at D1 and D7, respectively, with one (4.0%) patient experiencing a worsening of visual acuity at D7. There was no significant difference in visual acuity between time points, and none of the covariates (age, sex, BMI, disease duration, CSF OP, visual acuity at baseline) significantly affected the outcome (Table 2). Similarly, visual field had improved significantly in 4/21 (19.0%) and 5/23 (21.7%) pwIIH at D1 and D7, respectively, while two (8.7%) pwIIH experienced a worsening in visual field at D7. However, no significant difference in visual field MD was seen between time points, and none of the covariates (age, sex, BMI, disease duration, CSF OP, visual field MD at baseline) significantly affected the outcome (Table 2).
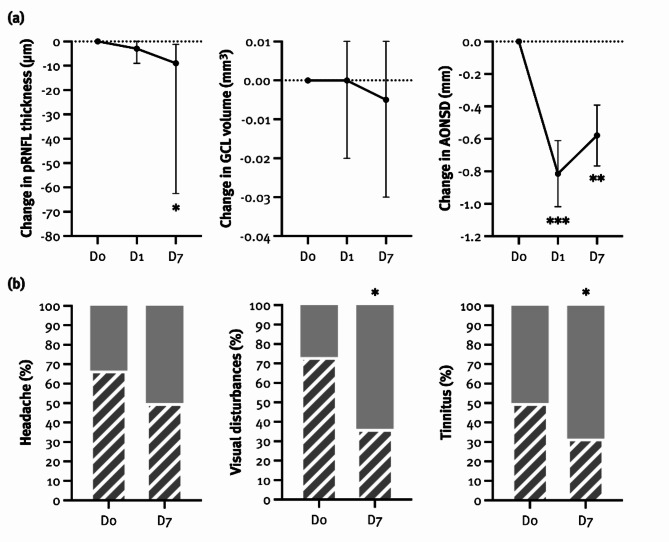
Median pRNFL thickness had decreased significantly at D7, with pRNFL thickness at baseline being associated with the pRNFL change (F(1,11) = 18.79, p = 0.001), explaining 63.1% of its variance (Table 2; Fig. 2). Interestingly, CSF OP also showed a trend towards association with pRNFL change (F(1,11) = 4.08, p = 0.068). In contrast, median GCL volume had not changed significantly at D1 or D7, and none of the covariates (age, sex, BMI, disease duration, CSF OP, GCL volume at baseline) had a significant effect on the outcome (Table 2; Fig. 2).
Fig. 2.
Ophthalmological (a) and neurological (b) outcomes in pwIIH before and after lumbar puncture (LP). Neurological outcomes were assessed as the change in their presence from D0 to D7 compared to the month before LP. AONSD: arachnoid optic nerve sheath diameter, GCL: ganglion cell layer, pRNFL: peripapillary retinal nerve fiber layer, D0: before LP, D1: one day after LP, D7: seven days after LP
*p < 0.05, **p < 0.01, ***p < 0.001
Mean AONSD was significantly lower at both timepoints, with AONSD at baseline being associated with change AONSD change (D1: β= -0.89, 95% CI -1.37, -0.42, p = 0.002; D7: β= -0.85, 95% CI -1.42, -0.28, p = 0.007), explaining 68.9% of its variance (Table 2; Fig. 2). Again, CSF OP showed a trend towards association with AONSD change at D1 (β = 0.06, 95% CI 0.00, 0.12, p = 0.057) but not at D7.
We found a correlation between the volume of CSF drained and the CSF OP (rs=0.40, p = 0.03) but not with any other ophthalmological outcomes (pRNFL thickness, AONSD).
Neurological outcomes
Headache presence was not significantly reduced at D7 (Table 2; Fig. 2). However, its persistence was associated with disease duration (F(1,13) = 2.65, p = 0.026). Peak headache severity, measured by NRS, was significantly reduced at D7, whereas median headache severity was not (Table 2). At D7, 8 (30.8%) and 10 (38.5%) pwIIH reported an improvement in peak and median headache severity, respectively. Postdural puncture headache was reported by 21/26 (80.8%) pwIIH. None of the covariates (age, sex, BMI, disease duration, CSF OP, MHD) significantly affected the outcome.
The presence of visual disturbances was significantly reduced at D7 but none of the covariates (age, sex, BMI, disease duration, CSF OP) had a significant effect on the outcome (Table 2; Fig. 2). Tinnitus presence also significantly decreased at D7, which was associated with higher BMI (F(1,9) = 8.15, p = 0.019), and tended to be more common in older participants (F(1,9) = 4.66, p = 0.059) and those with longer disease duration (F(1,9) = 3.92, p = 0.079) (Table 2; Fig. 2). At D7, tinnitus had improved in 2/7 (28.6%), worsened in 2/7 (28.6%) and remained unchanged in 3/7 (42.8%) pwIIH.
Sensitivity analyses removing pwIIH-WOP did not significantly change the overall results or the impact of individual variables.
Discussion
In the present study, we evaluated the short-term therapeutic effect of a single LP in a prospective cohort of pwIIH. We found that a single LP has a moderate effect on ophthalmological, e.g., papilledema, pRNFL thickness and AONSD, but not headache outcomes.
The pathophysiological mechanism behind increased ICP in IIH is not well understood, with CSF overproduction, its reduced absorption, and/or increased cerebral venous pressure comprising plausible and potentially over-lapping mechanisms. As CSF is passively reabsorbed into the intracranial venous sinus via the arachnoid granulations, transverse sinus stenosis may impair venous drainage, resulting in cerebral venous hypertension and impaired CSF absorption [23]. Some studies have suggested that a single LP may lead to a sudden reopening of collapsed transverse sinuses due to the reduction of ICP, resulting in improvement of IIH symptoms [5, 7, 24, 25].
Papilledema can be reliably detected and monitored by measuring the pRNFL thickness, which increases as a result of axoplasmic flow stasis in the retinal ganglion cell axons at the optic nerve head [26–28]. A decrease in pRNFL thickness can indicate either improvement of papilledema or axonal loss. On the other hand, measurement of GCL volume or thickness is not affected by axonal swelling and allows earlier direct quantification of neuroaxonal damage during acute exacerbation of IIH (compared to the pRNFL thickness). The lack of any significant change in the GCL volume in our study is not surprising as the observation period was short and the degree of papilledema was at most moderate. The significant decrease in pRNFL thickness observed after seven days therefore most likely reflects the reduction in axonal flow stasis as a result of ICP-relieving LP.
Transbulbar sonography is a useful imaging technique in the diagnosis and monitoring of increased ICP. The latter leads to an expansion of the perineural subarachnoid space, resulting in enlargement of the perioptic nerve sheath [29, 30]. Transbulbar sonography has high resolution and is therefore sensitive to changes in ICP. In our study, a single LP had a moderate effect on AONSD without significantly affecting functional outcomes. However, the decrease in AONSD at D7 is still indicative of a decrease in ICP, which is particularly important in the initial therapy monitoring, where few or no detectable changes in other ophthalmological parameters are expected. While fundoscopy with the Frisén scale remains the most practical approach for clinical evaluation of papilledema, our findings suggest that AONSD may be more sensitive for detecting early ICP changes. However, our study was not design to compare the superiority of these methods in assessing papilledema, and further research is required to provide more definitive answers. Also, although AONSD presents a promising tool, data on its association with degree of papilledema degree are still limited, and its routine clinical use is constrained by the lack of standardization and availability.
While IIH can be a deleterious condition leading to visual worsening or even blindness, it is also associated with reduced quality of life due to severe headache. In this regard, we found a reduction in peak headache severity following a single LP, but this was offset by a lack of change in mean headache severity and headache burden. Overall, the benefit of a single LP in terms of headache improvement appears to be moderate at best and partially offset by post-dural puncture headache [7]. The lack of association between headache outcomes and the CSF OP strengthens the hypothesis that headache disability in IIH is less dependent on ICP [31], with patients with the greatest headache severity benefiting the most from therapeutic LP [7]. Furthermore, headache persistence after LP was associated with disease duration, meaning that people with longer disease duration and a possible headache chronification, benefited least from therapeutic LP. These observations imply that specific neuronal mechanisms, such as sensitization of nociceptive trigeminal pathways involving calcitonin gene-related peptide (CGRP)-related mechanisms [32, 33], may occur after a prolonged period of increased ICP, preventing recovery once its cause has been treated. However, it needs to be acknowledged that observations of headache over a short observation period need to be interpreted cautiously as they are prone to be confounded by coincidental variation, regression to the mean and – not least – placebo effect following LP.
There are some additional limitations acknowledged to this study. The relatively small sample size (due to the rarity of IIH) and a short follow-up are partially mitigated by the standardized data collection and thorough quality control. However, long-term effects of therapeutic LP are a priori unlikely and a longer follow-up period would have been confounded by changes to pharmacological treatment. The participants were treated following best practice recommendations and their treatment remained stable throughout the observation period, but naturally varied between individuals, potentially introducing bias. Moreover, statistical models were not adjusted for pharmacological treatment, presenting a potential bias of overcalling the therapeutic role of LP. As some authors argue that pwIIH-WOP should be excluded or studied separately, we performed prespecified sensitivity analyses excluding pwIIH-WOP, which did not significantly change the results.
Conclusions
A single LP has a moderate effect on ophthalmological but not headache outcomes, highlighting an additional therapeutic aspect of mandatory diagnostic LP. As its effects are likely to be short-lived, the benefit of repeated LP is still controversial and should be limited to pwIIH with vision-threatening papilledema and limited therapeutic options, e.g., in pregnancy or intolerance to pharmacological IIH treatment. To achieve complete resolution of papilledema, pharmacological treatment and weight loss are the most important long-term treatment strategies. Importantly, headache does not represent a suitable indication for therapeutic LP as it does not appear to be adequately affected by this intervention, hence other approaches focusing on headache treatment should be considered.
Acknowledgements
We thank all the VIIH investigators, clinical research staff, and especially all participants for helping to collect these data. The named individuals were not compensated for their help.
VIIH investigators in alphabetical order: Bsteh, Gabriel (Department of Neurology, Medical University of Vienna); Grechenig, Christoph (Department of Ophthalmology, Medical University of Vienna); Kircher, Karl (Department of Ophthalmology, Medical University of Vienna); Krajnc, Nik (Department of Neurology, Medical University of Vienna); Macher, Stefan (Department of Neurology, Medical University of Vienna); Michl, Martin (Department of Ophthalmology, Medical University of Vienna), Mitsch, Christoph (Department of Ophthalmology, Medical University of Vienna); Pemp, Berthold (Department of Ophthalmology, Medical University of Vienna); Reitner, Andreas (Department of Ophthalmology, Medical University of Vienna); Wöber, Christian (Department of Neurology, Medical University of Vienna); Zebenholzer, Karin (Department of Neurology, Medical University of Vienna).
Abbreviations
- ANOVA
analysis of variance
- AONSD
arachnoid optic nerve sheath diameter
- ART
automatic real time tracking
- BMI
body mass index
- CGRP
calcitonin gene-related peptide
- CSF
cerebrospinal fluid
- GCL
ganglion cell layer
- HIT-6
Headache Impact Test-6
- ICP
intracranial pressure
- IQR
interquartile range
- IIH
idiopathic intracranial hypertension
- IIH-WOP
idiopathic intracranial hypertension without papilledema
- LP
lumbar puncture
- logMAR
logarithm of the minimum angle of resolution
- MD
mean deviation
- MHD
monthly headache days
- NRS
Numeric Rating Scale
- OCT
optic coherence tomography
- ONSF
optic nerve sheath fenestration
- OP
opening pressure
- pRNFL
peripapillary retinal nerve fiber layer
- pwIIH
people with idiopathic intracranial hypertension
- pwIIH-WOP
people with idiopathic intracranial hypertension without papilledema
- SD
standard deviation
- SITA
Swedish Interactive Threshold Algorithm
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- VIIH-BIO
Vienna Idiopathic Intracranial Hypertension Biomarker study
Author contributions
SZ: acquisition of data, data management, statistical analysis and interpretation of data, drafting of manuscript. NK: acquisition of data, data management, statistical analysis and interpretation of data, drafting of manuscript. SM: acquisition of data, interpretation of data, critical revision of manuscript for intellectual content. MM: acquisition of data, critical revision of manuscript for intellectual content. NM: acquisition of data, interpretation of data, critical revision of manuscript for intellectual content. CM: acquisition of data, critical revision of manuscript for intellectual content. WM: acquisition of data, interpretation of data, critical revision of manuscript for intellectual content. KN: acquisition of data, interpretation of data, critical revision of manuscript for intellectual content. CW: acquisition of data, interpretation of data, critical revision of manuscript for intellectual content. BP: acquisition of data, interpretation of data, study supervision, critical revision of manuscript for intellectual content. GBs: study concept and design, acquisition of data, interpretation of data, study supervision, critical revision of manuscript for intellectual content.
Funding
There was no funding to this research.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data-clearing committee of the Medical University Vienna.
Declarations
Competing interests
Sina Zaic: declares no conflict of interest relevant to this study.
Nik Krajnc: has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen-Cilag, Merck, Novartis, Roche and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Stefan Macher: declares no conflict of interest relevant to this study.
Martin Michl: declares no conflict of interest relevant to this study.
Nina Müller: declares no conflict of interest relevant to this study.
Christoph Mitsch: declares no conflict of interest relevant to this study.
Wolfgang Marik: declares no conflict of interest relevant to this study.
Klaus Novak: declares no conflict of interest relevant to this study.
Christian Wöber: has received honoraria consultancy/speaking from Apomedica, Curelator.
Berthold Pemp: declares no conflict of interest relevant to this study.
Gabriel Bsteh: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi-Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sina Zaic and Nik Krajnc contributed equally and share first authorship.
Berthold Pemp and Gabriel Bsteh contributed equally and share senior authorship.
References
- 1.Friedman DI (1999) Pseudotumor Cerebri. Neurosurg Clin N Am 10(4):609–621 viii 10.1016/S1042-3680(18)30161-X [DOI] [PubMed] [Google Scholar]
- 2.Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ (2019) The expanding burden of idiopathic intracranial hypertension. Eye Lond Engl 33(3):478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ab D, Gt L, Nj V, Sl G, Ml M, Nj N et al (2007) Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol [Internet]. Apr [cited 2024 Jun 2];143(4). https://pubmed.ncbi.nlm.nih.gov/17386271/ [DOI] [PubMed]
- 4.Burkett JG, Ailani J (2018) An up to date review of Pseudotumor Cerebri Syndrome. Curr Neurol Neurosci Rep 18(6):33 10.1007/s11910-018-0839-1 [DOI] [PubMed] [Google Scholar]
- 5.Friedman DI, Liu GT, Digre KB (2013) Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 81(13):1159–1165 10.1212/WNL.0b013e3182a55f17 [DOI] [PubMed] [Google Scholar]
- 6.Moss HE, Margolin E, Lee AG, Van Stavern GP (2021) Should lumbar puncture be required to diagnose every patient with idiopathic intracranial hypertension? J neuro-ophthalmol off. J North Am Neuro-Ophthalmol Soc 41(3):379–384 10.1097/WNO.0000000000001373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiangou A, Mitchell J, Markey KA, Scotton W, Nightingale P, Botfield H et al (2019) Therapeutic lumbar puncture for headache in idiopathic intracranial hypertension: minimal gain, is it worth the pain? Cephalalgia Int J Headache 39(2):245–253 10.1177/0333102418782192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yri HM, Jensen RH (2015) Idiopathic intracranial hypertension: clinical nosography and field-testing of the ICHD diagnostic criteria. A case-control study. Cephalalgia Int J Headache 35(7):553–562 10.1177/0333102414550109 [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann J (2019) The utility of the lumbar puncture in idiopathic intracranial hypertension. Cephalalgia Int J Headache 39(2):171–172 10.1177/0333102418787301 [DOI] [PubMed] [Google Scholar]
- 10.Childs JD, Piva SR, Fritz JM (2005) Responsiveness of the Numeric Pain Rating Scale in patients with low back Pain. Spine 30(11):1331–1334 10.1097/01.brs.0000164099.92112.29 [DOI] [PubMed] [Google Scholar]
- 11.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94(2):149–158 10.1016/S0304-3959(01)00349-9 [DOI] [PubMed] [Google Scholar]
- 12.Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Garber WH, Batenhorst A et al (2003) A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 12(8):963–974 [DOI] [PubMed] [Google Scholar]
- 13.Gandek B, Alacoque J, Uzun V, Andrew-Hobbs M, Davis K (2003) Translating the short-form headache impact test (HIT-6) in 27 countries: methodological and conceptual issues. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 12(8):975–979 [DOI] [PubMed] [Google Scholar]
- 14.Pruckner P, Mitsch C, Macher S, Krajnc N, Marik W, Novak K et al (2024) The Vienna idiopathic intracranial hypertension database—An Austrian registry. Wien Klin Wochenschr 136(1–2):32–39 10.1007/s00508-023-02252-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisen L (1982) Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry 45(1):13–18 10.1136/jnnp.45.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosser DA, Cousens SN, Murdoch IE, Fitzke FW, Laidlaw DAH (2003) How sensitive to clinical change are ETDRS logMAR visual acuity measurements? Invest Ophthalmol Vis Sci 44(8):3278–3281 10.1167/iovs.02-1100 [DOI] [PubMed] [Google Scholar]
- 17.Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S et al (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 7(4):e34823 10.1371/journal.pone.0034823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aytulun A, Cruz-Herranz A, Aktas O, Balcer LJ, Balk L, Barboni P et al (2021) APOSTEL 2.0 recommendations for reporting quantitative Optical Coherence Tomography studies. Neurology 97(2):68–79 10.1212/WNL.0000000000012125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pemp B, Kardon RH, Kircher K, Pernicka E, Schmidt-Erfurth U, Reitner A (2013) Effectiveness of averaging strategies to reduce variance in retinal nerve fibre layer thickness measurements using spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 251(7):1841–1848 10.1007/s00417-013-2337-0 [DOI] [PubMed] [Google Scholar]
- 20.Classification of diabetic retinopathy from fluorescein angiograms (1991) ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98(5 Suppl):807–822 [PubMed] [Google Scholar]
- 21.Ossoinig KC (1975) A-scan echography and orbital disease. Mod Probl Ophthalmol 14:203–235 [PubMed] [Google Scholar]
- 22.Atta HR (1988) Imaging of the optic nerve with standardised echography. Eye Lond Engl 2(Pt 4):358–366 [DOI] [PubMed] [Google Scholar]
- 23.Biousse V, Bruce BB, Newman NJ (2012) Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 83(5):488–494 10.1136/jnnp-2011-302029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puffer RC, Mustafa W, Lanzino G (2013) Venous sinus stenting for idiopathic intracranial hypertension: a review of the literature. J Neurointerventional Surg 5(5):483–486 10.1136/neurintsurg-2012-010468 [DOI] [PubMed] [Google Scholar]
- 25.Stevens SA, Previte M, Lakin WD, Thakore NJ, Penar PL, Hamschin B (2007) Idiopathic intracranial hypertension and transverse sinus stenosis: a modelling study. Math Med Biol J IMA 24(1):85–109 10.1093/imammb/dql025 [DOI] [PubMed] [Google Scholar]
- 26.Waisbourd M, Leibovitch I, Goldenberg D, Kesler A (2011) OCT assessment of morphological changes of the optic nerve head and macula in idiopathic intracranial hypertension. Clin Neurol Neurosurg 113(10):839–843 10.1016/j.clineuro.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 27.Vartin CV, Nguyen AM, Balmitgere T, Bernard M, Tilikete C, Vighetto A (2012) Detection of mild papilloedema using spectral domain optical coherence tomography. Br J Ophthalmol 96(3):375–379 10.1136/bjo.2010.199562 [DOI] [PubMed] [Google Scholar]
- 28.OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group, Auinger P, Durbin M, Feldon S, Garvin M, Kardon R et al (2014) Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci 55(12):8173–8179 10.1167/iovs.14-14961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerscher SR, Zipfel J, Bevot A, Sollmann N, Haas-Lude K, Tellermann J et al (2023) Non-invasive quantitative approximation of intracranial pressure in Pediatric Idiopathic Intracranial Hypertension based on point-of-care Ultrasound of the Optic nerve sheath diameter. Brain Sci 14(1):32 10.3390/brainsci14010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knodel S, Roemer SN, Moslemani K, Wykrota A, Käsmann-Kellner B, Seitz B et al (2021) Sonographic and ophthalmic assessment of optic nerve in patients with idiopathic intracranial hypertension: a longitudinal study. J Neurol Sci 430:118069 10.1016/j.jns.2021.118069 [DOI] [PubMed] [Google Scholar]
- 31.Friedman DI, Quiros PA, Subramanian PS, Mejico LJ, Gao S, McDermott M et al (2017) Headache in idiopathic intracranial hypertension: findings from the idiopathic intracranial hypertension treatment trial. Headache 57(8):1195–1205 10.1111/head.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bsteh G, Macher S, Krajnc N, Pruckner P, Marik W, Mitsch C et al (2023) Idiopathic intracranial hypertension presenting with migraine phenotype is associated with unfavorable headache outcomes. Headache 63(5):601–610 10.1111/head.14478 [DOI] [PubMed] [Google Scholar]
- 33.Krajnc N, Frank F, Macher S, Michl M, Müller N, Maier S et al (2024) Plasma calcitonin gene-related peptide levels in idiopathic intracranial hypertension: an exploratory study. J Headache Pain 25(1):92 10.1186/s10194-024-01799-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data-clearing committee of the Medical University Vienna.
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the data-clearing committee of the Medical University Vienna.