Abstract
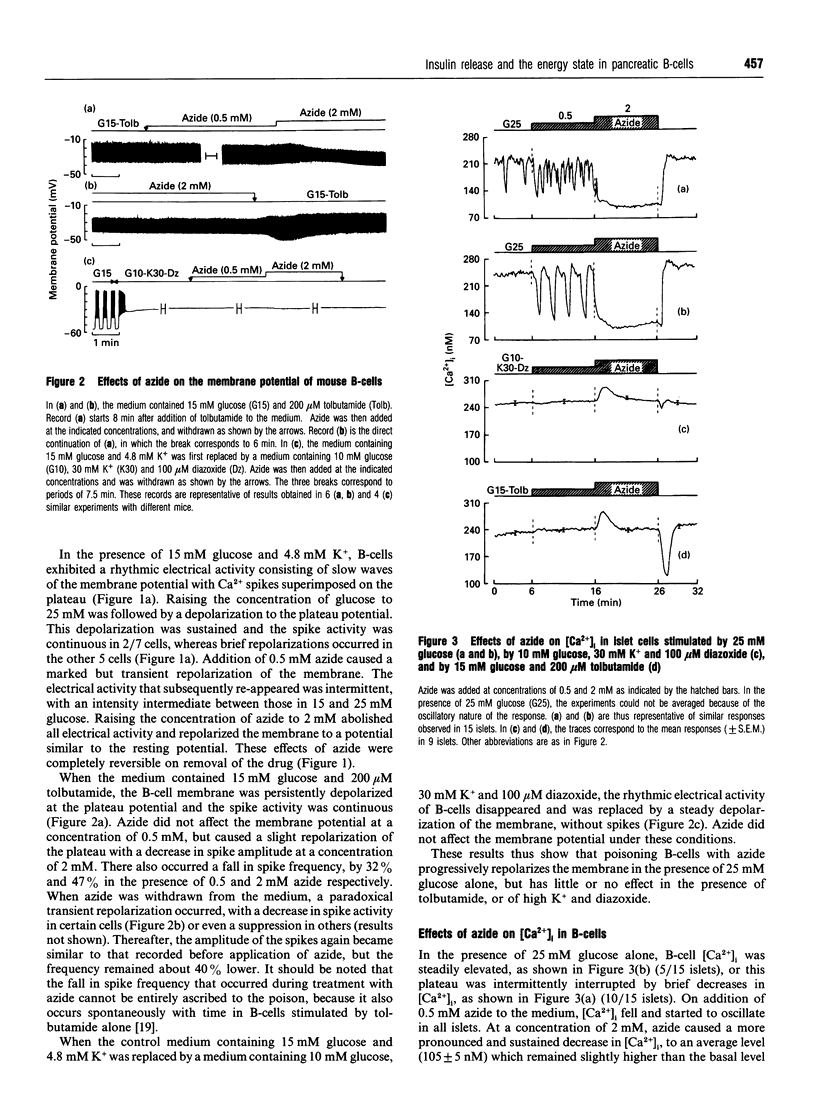
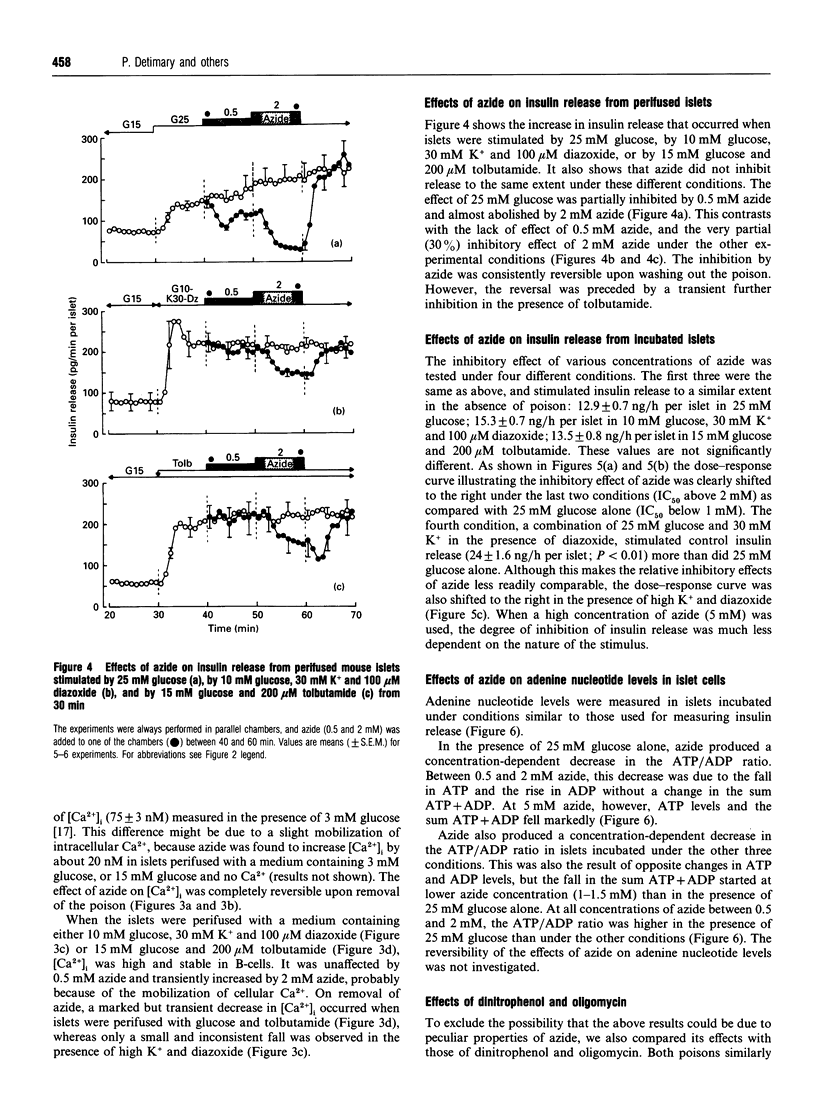
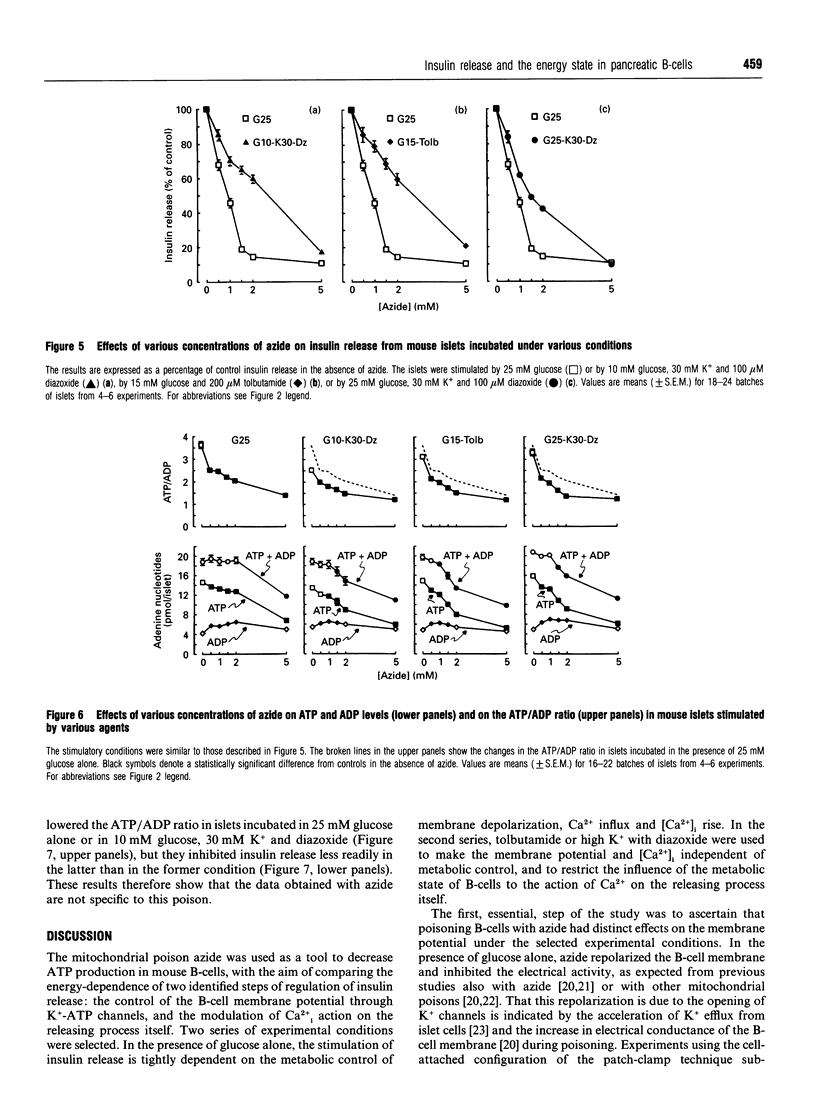
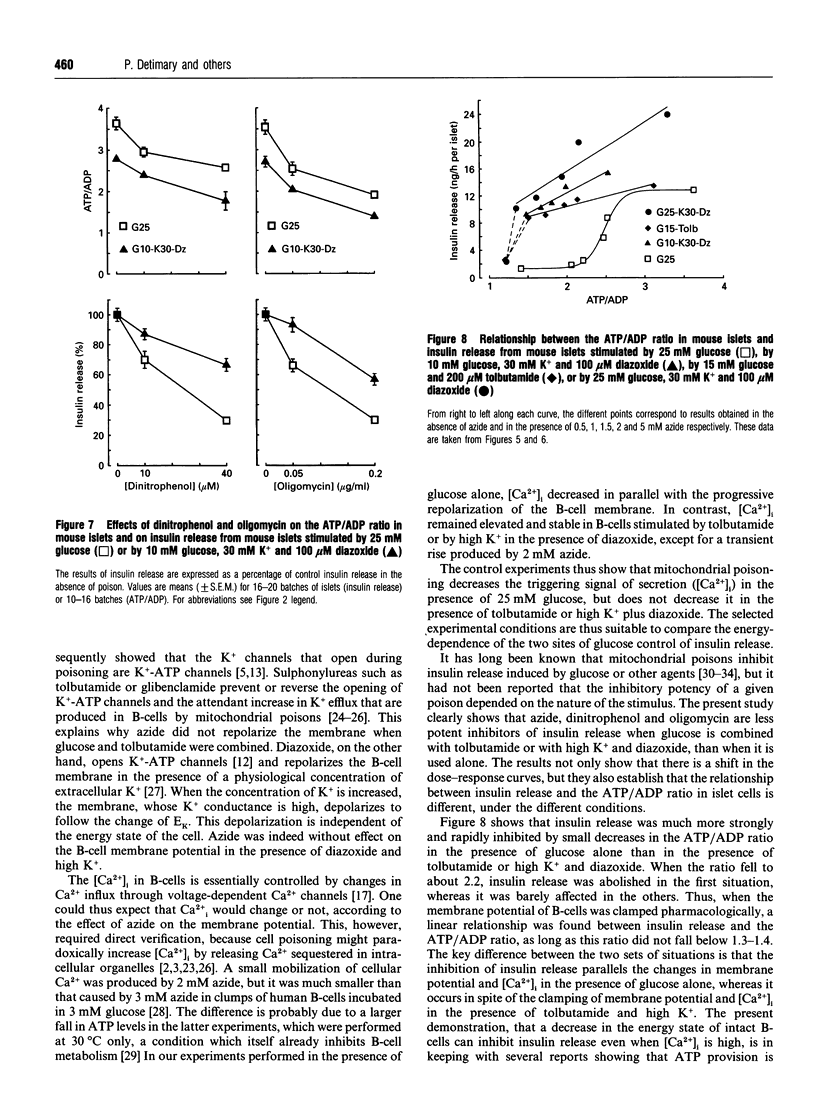
The energy state of pancreatic B-cells may influence insulin release at several steps of stimulus-secretion coupling. By closing ATP-sensitive K+ channels (K(+)-ATP channels), a rise in the ATP/ADP ratio may regulate the membrane potential, and hence Ca2+ influx. It may also modulate the effectiveness of Ca2+ on its intracellular targets. To assess the existence of these two roles and determine their relative importance for insulin release, we tested the effects of azide, a mitochondrial poison, on mouse B-cell function under various conditions. During stimulation by glucose alone, when K(+)-ATP channels are controlled by cellular metabolism, azide caused parallel, concentration-dependent (0.5-5 mM), membrane repolarization, decrease in cytosolic Ca2+ concentration [Ca2+]i and inhibition of insulin release. When K(+)-ATP channels were closed pharmacologically (by tolbutamide in high glucose), azide did not repolarize the membrane or decrease [Ca2+]i, and was much less effective in inhibiting insulin release. A similar resistance to azide was observed when K(+)-ATP channels were opened by diazoxide, and high K+ was used to depolarize the membrane and raise [Ca2+]i. In contrast, azide similarly decreased ATP levels and increased ADP levels, thereby lowering the ATP/ADP ratio under all conditions. In conclusion, lowering the ATP/ADP ratio in B-cells can inhibit insulin release even when [Ca2+]i remains high. However, this distal step is much more resistant to a decrease in the energy state of B-cells than is the control of membrane potential by K(+)-ATP channels. Generation of the signal triggering insulin release, high [Ca2+]i, through metabolic control of membrane potential requires a higher global ATP/ADP ratio than does activation of the secretory process itself.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleyassine H. Energy requirements for insulin release from rat pancreas in vitro. Endocrinology. 1970 Jul;87(1):84–89. doi: 10.1210/endo-87-1-84. [DOI] [PubMed] [Google Scholar]
- Ali L., Grapengiesser E., Gylfe E., Hellman B., Lund P. E. Free and bound sodium in pancreatic beta-cells exposed to glucose and tolbutamide. Biochem Biophys Res Commun. 1989 Oct 16;164(1):212–218. doi: 10.1016/0006-291x(89)91704-x. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Bozem M., Henquin J. C. Glucose modulation of spike activity independently from changes in slow waves of membrane potential in mouse B-cells. Pflugers Arch. 1988 Dec;413(2):147–152. doi: 10.1007/BF00582524. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draznin B. Intracellular calcium, insulin secretion, and action. Am J Med. 1988 Nov 28;85(5A):44–58. doi: 10.1016/0002-9343(88)90397-x. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Escolar J. C., Hoo-Paris R., Castex C., Sutter B. C. Effect of low temperatures on glucose-induced insulin secretion and glucose metabolism in isolated pancreatic islets of the rat. J Endocrinol. 1990 Apr;125(1):45–51. doi: 10.1677/joe.0.1250045. [DOI] [PubMed] [Google Scholar]
- Gembal M., Detimary P., Gilon P., Gao Z. Y., Henquin J. C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J Clin Invest. 1993 Mar;91(3):871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembal M., Gilon P., Henquin J. C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992 Apr;89(4):1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K. D., Gee W. M., Hammoud A., McDaniel M. L., Falke L. C., Misler S. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic B-cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1119–C1127. doi: 10.1152/ajpcell.1989.257.6.C1119. [DOI] [PubMed] [Google Scholar]
- Gilon P., Henquin J. C. Activation of muscarinic receptors increases the concentration of free Na+ in mouse pancreatic B-cells. FEBS Lett. 1993 Jan 11;315(3):353–356. doi: 10.1016/0014-5793(93)81193-4. [DOI] [PubMed] [Google Scholar]
- Gilon P., Henquin J. C. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem. 1992 Oct 15;267(29):20713–20720. [PubMed] [Google Scholar]
- Henquin J. C. Adenosine triphosphate-sensitive K+ channels may not be the sole regulators of glucose-induced electrical activity in pancreatic B-cells. Endocrinology. 1992 Jul;131(1):127–131. doi: 10.1210/endo.131.1.1611991. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem Pharmacol. 1982 Apr 1;31(7):1407–1415. doi: 10.1016/0006-2952(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. The electrogenic sodium-potassium pump of mouse pancreatic B-cells. J Physiol. 1982 Nov;332:529–552. doi: 10.1113/jphysiol.1982.sp014429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Fatherazi S., Peter-Riesch B., Corkey B. E., Cook D. L. Two sites for adenine-nucleotide regulation of ATP-sensitive potassium channels in mouse pancreatic beta-cells and HIT cells. J Membr Biol. 1992 Sep;129(3):287–295. doi: 10.1007/BF00232910. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Stutchfield J., Howell S. L. Effects of Ca2+ and a phorbol ester on insulin secretion from islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1985 Oct 21;191(1):102–106. doi: 10.1016/0014-5793(85)81002-4. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Meissner H. P. Membrane potential measurements in pancreatic beta cells with intracellular microelectrodes. Methods Enzymol. 1990;192:235–246. doi: 10.1016/0076-6879(90)92073-m. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The interaction of various inhibitors and stimuli of insulin release studied with rabbit pancreas in vitro. Biochem J. 1969 Jul;113(3):473–479. doi: 10.1042/bj1130473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S., Barnett D. W., Falke L. C. Effects of metabolic inhibition by sodium azide on stimulus-secretion coupling in B cells of human islets of Langerhans. Pflugers Arch. 1992 Jun;421(2-3):289–291. doi: 10.1007/BF00374842. [DOI] [PubMed] [Google Scholar]
- Misler S., Gee W. M., Gillis K. D., Scharp D. W., Falke L. C. Metabolite-regulated ATP-sensitive K+ channel in human pancreatic islet cells. Diabetes. 1989 Apr;38(4):422–427. doi: 10.2337/diab.38.4.422. [DOI] [PubMed] [Google Scholar]
- Niki I., Ashcroft F. M., Ashcroft S. J. The dependence on intracellular ATP concentration of ATP-sensitive K-channels and of Na,K-ATPase in intact HIT-T15 beta-cells. FEBS Lett. 1989 Nov 6;257(2):361–364. doi: 10.1016/0014-5793(89)81572-8. [DOI] [PubMed] [Google Scholar]
- Ohta M., Nelson D., Nelson J., Meglasson M. D., Erecińska M. Oxygen and temperature dependence of stimulated insulin secretion in isolated rat islets of Langerhans. J Biol Chem. 1990 Oct 15;265(29):17525–17532. [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T., Neighbors A. S., Pirkle J. A., Greider M. H. Use of a high voltage technique to determine the molecular requirements for exocytosis in islet cells. Diabetes. 1980 Nov;29(11):911–918. doi: 10.2337/diab.29.11.911. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Rajan A. S., Aguilar-Bryan L., Nelson D. A., Yaney G. C., Hsu W. H., Kunze D. L., Boyd A. E., 3rd Ion channels and insulin secretion. Diabetes Care. 1990 Mar;13(3):340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Wolf B. A., Colca J. R., Turk J., Florholmen J., McDaniel M. L. Regulation of Ca2+ homeostasis by islet endoplasmic reticulum and its role in insulin secretion. Am J Physiol. 1988 Feb;254(2 Pt 1):E121–E136. doi: 10.1152/ajpendo.1988.254.2.E121. [DOI] [PubMed] [Google Scholar]


