Abstract
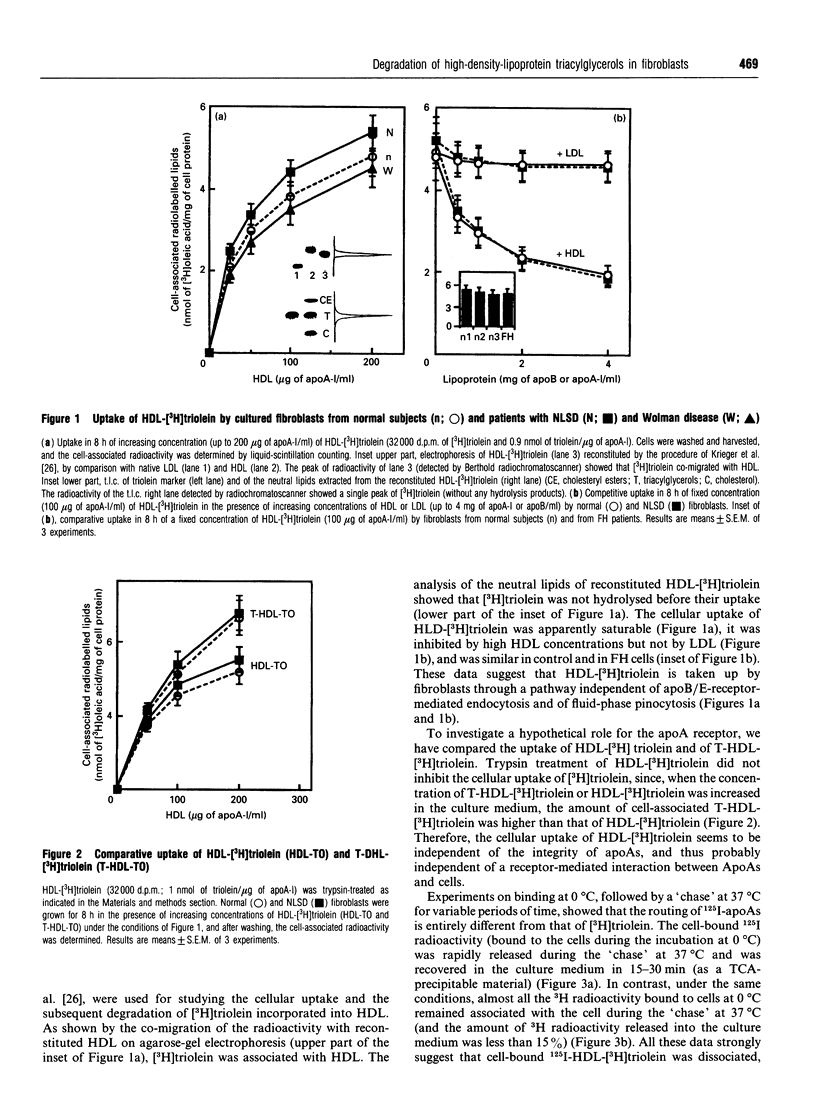
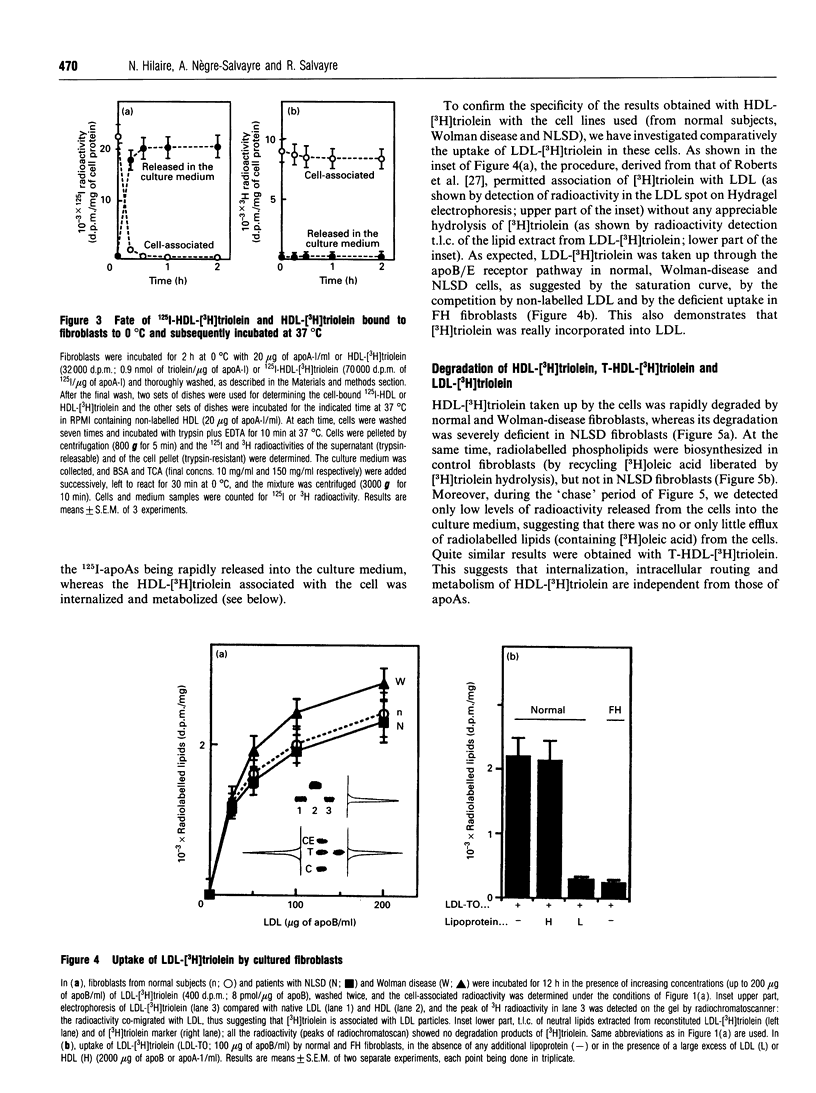
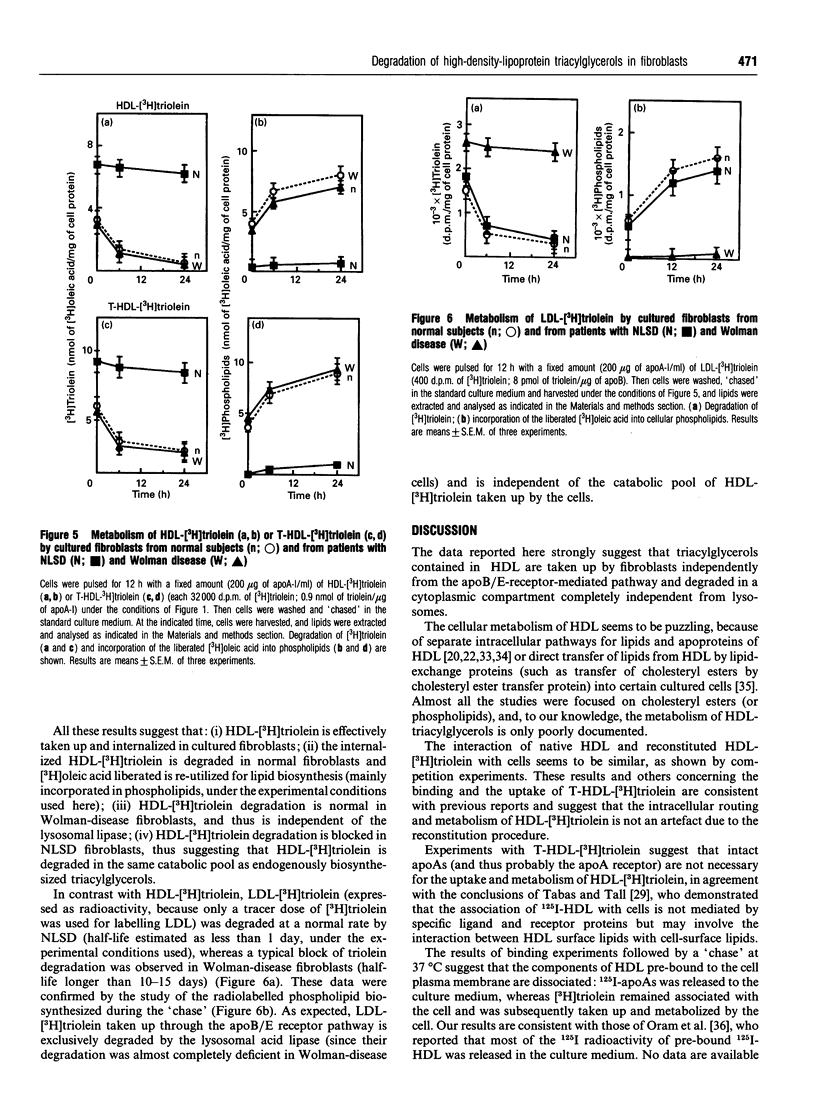
High-density lipoprotein (HDL)-[3H]triolein (i.e. [3H]triolein incorporated into reconstituted HDL) was taken up by cultured fibroblasts through an apparently saturable process, competitively inhibited by non-labelled HDL and independent of the LDL receptor. Using 125I-HDL and HDL-[3H]triolein, binding experiments (at 0 degrees C) followed by a short-time 'chase' at 37 degrees C showed that 125I radioactivity was rapidly released in the culture medium (as trichloroacetic acid-precipitable material), whereas 3H radioactivity remained associated with the cell. The cell-associated HDL-[3H]triolein was rapidly degraded in normal fibroblasts, and the liberated [3H]oleic acid was incorporated into newly biosynthesized phospholipids. In Wolman-disease fibroblasts HDL-[3H]triolein was degraded at a normal rate, and thus independently of the lysosomal compartment. In contrast, the degradation of HDL-[3H]triolein was blocked in fibroblasts from Neutral Lipid Storage Disease (NLSD), similarly to that of endogenously biosynthesized triacylglycerols [Radom, Salvayre, Nègre, Maret and Douste-Blazy (1987) Eur. J. Biochem. 164, 703-708]. Trypsin-treated HDL-[3H]triolein was also taken up by cells and degraded quite similarly to HDL-[3H]triolein. In conclusion, all these data taken together suggest that HDL-[3H]triolein is: (i) associated with the cell through a process independent of intact apolipoprotein (apo) As, thus probably independent of an apoA-receptor-mediated uptake; (ii) internalized by cells, whereas 125I-apoAs are released in the culture medium; (iii) directed to the same non-lysosomal catabolic pool (blocked in NLSD) as for endogenously biosynthesized triacylglycerols.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeeny C. M., Rifici V. A., Eder H. A. The uptake of the apoprotein and cholesteryl ester of high-density lipoproteins by the perfused rat liver. Biochim Biophys Acta. 1987 Jan 13;917(1):9–17. doi: 10.1016/0005-2760(87)90277-3. [DOI] [PubMed] [Google Scholar]
- Bachorik P. S., Franklin F. A., Jr, Virgil D. G., Kwiterovich P. O., Jr Reversible high affinity uptake of apo E-free high density lipoproteins in cultured pig hepatocytes. Arteriosclerosis. 1985 Mar-Apr;5(2):142–152. doi: 10.1161/01.atv.5.2.142. [DOI] [PubMed] [Google Scholar]
- Biesbroeck R., Oram J. F., Albers J. J., Bierman E. L. Specific high-affinity binding of high density lipoproteins to cultured human skin fibroblasts and arterial smooth muscle cells. J Clin Invest. 1983 Mar;71(3):525–539. doi: 10.1172/JCI110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanarin I., Patel A., Slavin G., Wills E. J., Andrews T. M., Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975 Mar 8;1(5957):553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapus C., Sari H., Sémériva M., Desnuelle P. Role of colipase in the interfacial adsorption of pancreatic lipase at hydrophilic interfaces. FEBS Lett. 1975 Oct 15;58(1):155–158. doi: 10.1016/0014-5793(75)80247-x. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. AB variant of infantile GM2 gangliosidosis: deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato S., Garavaglia B., Strisciuglio P., Borrone C., Andria G. Multisystem triglyceride storage disease is due to a specific defect in the degradation of endocellularly synthesized triglycerides. Neurology. 1988 Jul;38(7):1107–1110. doi: 10.1212/wnl.38.7.1107. [DOI] [PubMed] [Google Scholar]
- Dorfman M. L., Hershko C., Eisenberg S., Sagher F. Ichthyosiform dermatosis with systemic lipidosis. Arch Dermatol. 1974 Aug;110(2):261–266. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fürst W., Sandhoff K. Activator proteins and topology of lysosomal sphingolipid catabolism. Biochim Biophys Acta. 1992 Jun 5;1126(1):1–16. doi: 10.1016/0005-2760(92)90210-m. [DOI] [PubMed] [Google Scholar]
- Glass C., Pittman R. C., Weinstein D. B., Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. L., Oram J. F. Identification and characterization of a high density lipoprotein-binding protein in cell membranes by ligand blotting. J Biol Chem. 1987 Jun 5;262(16):7439–7442. [PubMed] [Google Scholar]
- Granot E., Tabas I., Tall A. R. Human plasma cholesteryl ester transfer protein enhances the transfer of cholesteryl ester from high density lipoproteins into cultured HepG2 cells. J Biol Chem. 1987 Mar 15;262(8):3482–3487. [PubMed] [Google Scholar]
- Gwynne J. T., Mahaffee D. D. Rat adrenal uptake and metabolism of high density lipoprotein cholesteryl ester. J Biol Chem. 1989 May 15;264(14):8141–8150. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitz H., Ronen I., Bilu S., Tietz A. Bile acid synthesis from HDL cholesterol and cholesterol ester by cultured chick embryo hepatocytes. Biochim Biophys Acta. 1986 Oct 3;878(3):426–434. doi: 10.1016/0005-2760(86)90252-3. [DOI] [PubMed] [Google Scholar]
- JORDANS G. H. The familial occurrence of fat containing vacuoles in the leukocytes diagnosed in two brothers suffering from dystrophia musculorum progressiva (ERB.). Acta Med Scand. 1953;145(6):419–423. doi: 10.1111/j.0954-6820.1953.tb07038.x. [DOI] [PubMed] [Google Scholar]
- Krieger M., Brown M. S., Faust J. R., Goldstein J. L. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. Reconstitution of a biologically active lipoprotein particle. J Biol Chem. 1978 Jun 25;253(12):4093–4101. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nestler J. E., Bamberger M., Rothblat G. H., Strauss J. F., 3rd Metabolism of high density lipoproteins reconstituted with [3H]cholesteryl ester and [14C]cholesterol in the rat, with special reference to the ovary. Endocrinology. 1985 Aug;117(2):502–510. doi: 10.1210/endo-117-2-502. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 1991 Mar 1;5(3):301–308. doi: 10.1096/fasebj.5.3.2001789. [DOI] [PubMed] [Google Scholar]
- Oram J. F., Brinton E. A., Bierman E. L. Regulation of high density lipoprotein receptor activity in cultured human skin fibroblasts and human arterial smooth muscle cells. J Clin Invest. 1983 Nov;72(5):1611–1621. doi: 10.1172/JCI111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. F., Johnson C. J., Brown T. A. Interaction of high density lipoprotein with its receptor on cultured fibroblasts and macrophages. Evidence for reversible binding at the cell surface without internalization. J Biol Chem. 1987 Feb 15;262(5):2405–2410. [PubMed] [Google Scholar]
- Patrick A. D., Lake B. D. Deficiency of an acid lipase in Wolman's disease. Nature. 1969 Jun 14;222(5198):1067–1068. doi: 10.1038/2221067a0. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Knecht T. P., Rosenbaum M. S., Taylor C. A., Jr A nonendocytotic mechanism for the selective uptake of high density lipoprotein-associated cholesterol esters. J Biol Chem. 1987 Feb 25;262(6):2443–2450. [PubMed] [Google Scholar]
- Price P., McMillan T. J. Use of the tetrazolium assay in measuring the response of human tumor cells to ionizing radiation. Cancer Res. 1990 Mar 1;50(5):1392–1396. [PubMed] [Google Scholar]
- Radom J., Salvayre R., Levade T., Douste-Blazy L. Influence of chain length of pyrene fatty acids on their uptake and metabolism by Epstein-Barr-virus-transformed lymphoid cell lines from a patient with multisystemic lipid storage myopathy and from control subjects. Biochem J. 1990 Jul 1;269(1):107–113. doi: 10.1042/bj2690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radom J., Salvayre R., Maret A., Nègre A., Douste-Blazy L. Metabolism of 1-pyrenedecanoic acid and accumulation of neutral fluorescent lipids in cultured fibroblasts of multisystemic lipid storage myopathy. Biochim Biophys Acta. 1987 Jul 31;920(2):131–139. doi: 10.1016/0005-2760(87)90252-9. [DOI] [PubMed] [Google Scholar]
- Radom J., Salvayre R., Mussini J. M., De Lisle B., Negre A., Maret A., Billaudel S., Douste-Blazy L. Biochemical and ultrastructural features of human fibroblasts cultured from a new variant of type 3 lipid storage myopathy. Biol Cell. 1988;62(1):39–45. [PubMed] [Google Scholar]
- Radom J., Salvayre R., Negre A., Douste-Blazy L. Metabolism of pyrenedecanoic acid in Epstein-Barr virus-transformed lymphoid cell lines from normal subjects and from a patient with multisystemic lipid storage myopathy. Biochim Biophys Acta. 1989 Sep 25;1005(2):130–136. doi: 10.1016/0005-2760(89)90178-1. [DOI] [PubMed] [Google Scholar]
- Radom J., Salvayre R., Negre A., Maret A., Douste-Blazy L. Metabolism of neutral lipids in cultured fibroblasts from multisystemic (or type 3) lipid storage myopathy. Eur J Biochem. 1987 May 4;164(3):703–708. doi: 10.1111/j.1432-1033.1987.tb11183.x. [DOI] [PubMed] [Google Scholar]
- Roberts D. C., Miller N. E., Price S. G., Crook D., Cortese C., La Ville A., Masana L., Lewis B. An alternative procedure for incorporating radiolabelled cholesteryl ester into human plasma lipoproteins in vitro. Biochem J. 1985 Feb 15;226(1):319–322. doi: 10.1042/bj2260319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvayre R., Negre A., Maret A., Radom J., Douste-Blazy L. Extracellular origin of the lipid lysosomal storage in cultured fibroblasts from Wolman's disease. Eur J Biochem. 1987 Dec 30;170(1-2):453–458. doi: 10.1111/j.1432-1033.1987.tb13721.x. [DOI] [PubMed] [Google Scholar]
- Salvayre R., Nègre A., Radom J., Douste-Blazy L. Independence of triacylglycerol-containing compartments in cultured fibroblasts from Wolman disease and multisystemic lipid storage myopathy. FEBS Lett. 1989 Jun 19;250(1):35–39. doi: 10.1016/0014-5793(89)80679-9. [DOI] [PubMed] [Google Scholar]
- Schmitz G., Robenek H., Lohmann U., Assmann G. Interaction of high density lipoproteins with cholesteryl ester-laden macrophages: biochemical and morphological characterization of cell surface receptor binding, endocytosis and resecretion of high density lipoproteins by macrophages. EMBO J. 1985 Mar;4(3):613–622. doi: 10.1002/j.1460-2075.1985.tb03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow C. P., Pittman R. C. Cholesterol esters selectively taken up from high-density lipoproteins are hydrolyzed extralysosomally. Biochim Biophys Acta. 1990 Apr 2;1043(2):203–210. doi: 10.1016/0005-2760(90)90297-b. [DOI] [PubMed] [Google Scholar]
- Srebrnik A., Tur E., Perluk C., Elman M., Messer G., Ilie B., Krakowski A. Dorfman-Chanarin syndrome. A case report and a review. J Am Acad Dermatol. 1987 Nov;17(5 Pt 1):801–808. doi: 10.1016/s0190-9622(87)70266-7. [DOI] [PubMed] [Google Scholar]
- Stagsted J., Ziebe S., Satoh S., Holman G. D., Cushman S. W., Olsson L. Insulinomimetic effect on glucose transport by epidermal growth factor when combined with a major histocompatibility complex class I-derived peptide. J Biol Chem. 1993 Jan 25;268(3):1770–1774. [PubMed] [Google Scholar]
- Stein Y., Dabach Y., Hollander G., Halperin G., Stein O. Metabolism of HDL-cholesteryl ester in the rat, studied with a nonhydrolyzable analog, cholesteryl linoleyl ether. Biochim Biophys Acta. 1983 Jun 16;752(1):98–105. doi: 10.1016/0005-2760(83)90237-0. [DOI] [PubMed] [Google Scholar]
- Tabas I., Tall A. R. Mechanism of the association of HDL3 with endothelial cells, smooth muscle cells, and fibroblasts. Evidence against the role of specific ligand and receptor proteins. J Biol Chem. 1984 Nov 25;259(22):13897–13905. [PubMed] [Google Scholar]
- Takahashi K., Fukuda S., Naito M., Horiuchi S., Takata K., Morino Y. Endocytic pathway of high density lipoprotein via trans-Golgi system in rat resident peritoneal macrophages. Lab Invest. 1989 Sep;61(3):270–277. [PubMed] [Google Scholar]
- Tóth I. E., Szabó D., Bácsy E., Szalay K. S., Hesz A., Szollár L. G. Morphological evidence of lysosomal uptake of high-density lipoproteins by rat adrenocortical cells in vitro. Mol Cell Endocrinol. 1986 Feb;44(2):185–194. doi: 10.1016/0303-7207(86)90062-6. [DOI] [PubMed] [Google Scholar]
- WOLMAN M., STERK V. V., GATT S., FRENKEL M. Primary familial xanthomatosis with involvement and calcification of the adrenals. Report of two more cases in siblings of a previously described infant. Pediatrics. 1961 Nov;28:742–757. [PubMed] [Google Scholar]
- Weech P. K., McTaggart F., Mills G. L. Comparison of guinea-pig serum lipoproteins after iodination by two different methods. Biochem J. 1978 Mar 1;169(3):687–695. doi: 10.1042/bj1690687. [DOI] [PMC free article] [PubMed] [Google Scholar]