Abstract
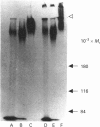
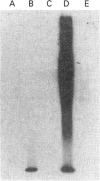
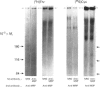
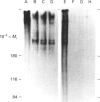
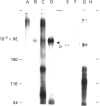
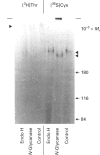
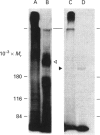
Mucins, high-M(r) glycoproteins with a large amount of O-glycosidically linked carbohydrate, protect the colonic epithelial surface and are altered in ulcerative colitis and colon cancer. At least two mucin genes, MUC2 and MUC3, are expressed at high levels in the human intestine. As an experimental model for studying the biosynthesis of human intestinal mucins, we used HM3 colon cancer cells. When mature mucins labelled with [3H]glucosamine or [3H]threonine were analysed by gel filtration, it was found that secreted mucins (M(r) > 10(8) were larger than soluble cellular mucins (M(r) approx. 5 x 10(6)). Only secreted mucin was sensitive to reduction. Both MUC2 and MUC3 proteins, identified by labelling with [3H]threonine or [35S]cysteine and immunoprecipitation with antibodies to synthetic mucin peptides, were already of large size (M(r) > 180,000) by the earliest labelling time (5 min). The MUC3 precursor was completely degraded by trypsin, but the MUC2 precursor had a trypsin-resistant fragment of M(r) approx. 240,000 containing threonine and cysteine. The trypsin-resistant MUC2 fragment contained N-linked carbohydrate, as indicated by a decrease in size as a result of peptidyl N-glycosidase digestion or tunicamycin treatment of HM3 cells. These results show that HM3 colon cancer cells produce at least two distinct human intestinal mucins. They also indicate that the mechanisms of biosynthesis of intestinal mucins differ from those of other mucin-like glycoproteins that have been studied.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bresalier R. S., Niv Y., Byrd J. C., Duh Q. Y., Toribara N. W., Rockwell R. W., Dahiya R., Kim Y. S. Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J Clin Invest. 1991 Mar;87(3):1037–1045. doi: 10.1172/JCI115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C., Lamport D. T., Siddiqui B., Kuan S. F., Erickson R., Itzkowitz S. H., Kim Y. S. Deglycosylation of mucin from LS174T colon cancer cells by hydrogen fluoride treatment. Biochem J. 1989 Jul 15;261(2):617–625. doi: 10.1042/bj2610617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C., Nardelli J., Siddiqui B., Kim Y. S. Isolation and characterization of colon cancer mucin from xenografts of LS174T cells. Cancer Res. 1988 Dec 1;48(23):6678–6685. [PubMed] [Google Scholar]
- Dekker J., van der Ende A., Aelmans P. H., Strous G. J. Rat gastric mucin is synthesized and secreted exclusively as filamentous oligomers. Biochem J. 1991 Oct 1;279(Pt 1):251–256. doi: 10.1042/bj2790251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Burchell J. M., Duhig T., Lamport D., White R., Parker M., Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. V., Miller F. Comparison of human colonic mucoprotein antigen from normal and neoplastic mucosa. Cancer Res. 1978 Oct;38(10):3204–3211. [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Rothe E. M., Lagace R. E., Kim Y. S. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992 Oct 25;267(30):21375–21383. [PubMed] [Google Scholar]
- Hilkens J., Buijs F. Biosynthesis of MAM-6, an epithelial sialomucin. Evidence for involvement of a rare proteolytic cleavage step in the endoplasmic reticulum. J Biol Chem. 1988 Mar 25;263(9):4215–4222. [PubMed] [Google Scholar]
- Ho S. B., Niehans G. A., Lyftogt C., Yan P. S., Cherwitz D. L., Gum E. T., Dahiya R., Kim Y. S. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993 Feb 1;53(3):641–651. [PubMed] [Google Scholar]
- Hull S. R., Sugarman E. D., Spielman J., Carraway K. L. Biosynthetic maturation of an ascites tumor cell surface sialomucin. Evidence for O-glycosylation of cell surface glycoprotein by the addition of new oligosaccharides during recycling. J Biol Chem. 1991 Jul 25;266(21):13580–13586. [PubMed] [Google Scholar]
- Kuan S. F., Byrd J. C., Basbaum C., Kim Y. S. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989 Nov 15;264(32):19271–19277. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Kruijshaar L., Buijs F., van Meijer M., Litvinov S. V., Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem. 1992 Mar 25;267(9):6171–6177. [PubMed] [Google Scholar]
- Linsley P. S., Kallestad J. C., Horn D. Biosynthesis of high molecular weight breast carcinoma associated mucin glycoproteins. J Biol Chem. 1988 Jun 15;263(17):8390–8397. [PubMed] [Google Scholar]
- Nguyen V. C., Aubert J. P., Gross M. S., Porchet N., Degand P., Frézal J. Assignment of human tracheobronchial mucin gene(s) to 11p15 and a tracheobronchial mucin-related sequence to chromosome 13. Hum Genet. 1990 Dec;86(2):167–172. doi: 10.1007/BF00197699. [DOI] [PubMed] [Google Scholar]
- Niv Y., Byrd J. C., Ho S. B., Dahiya R., Kim Y. S. Mucin synthesis and secretion in relation to spontaneous differentiation of colon cancer cells in vitro. Int J Cancer. 1992 Jan 2;50(1):147–152. doi: 10.1002/ijc.2910500129. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B., Bresalier R. S., Kim Y. S. The role of mucin in colon-cancer metastasis. Int J Cancer. 1992 Aug 19;52(1):60–65. doi: 10.1002/ijc.2910520113. [DOI] [PubMed] [Google Scholar]
- Siddiqui B., Byrd J. C., Fearney F. J., Kim Y. S. Comparison of metabolically labeled mucins of LS174T human colon cancer cells in tissue culture and xenograft. Tumour Biol. 1989;10(2):83–94. doi: 10.1159/000217600. [DOI] [PubMed] [Google Scholar]
- Toribara N. W., Gum J. R., Jr, Culhane P. J., Lagace R. E., Hicks J. W., Petersen G. M., Kim Y. S. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991 Sep;88(3):1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Roberton A. M., Ho S. B., Kuo W. L., Gum E., Hicks J. W., Gum J. R., Jr, Byrd J. C., Siddiki B., Kim Y. S. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993 Mar 15;268(8):5879–5885. [PubMed] [Google Scholar]
- Xing P. X., Prenzoska J., Layton G. T., Devine P. L., McKenzie I. F. Second-generation monoclonal antibodies to intestinal MUC2 peptide reactive with colon cancer. J Natl Cancer Inst. 1992 May 6;84(9):699–703. doi: 10.1093/jnci/84.9.699. [DOI] [PubMed] [Google Scholar]
- Yan P. S., Ho S. B., Itzkowitz S. H., Byrd J. C., Siddiqui B., Kim Y. S. Expression of native and deglycosylated colon cancer mucin antigens in normal and malignant epithelial tissues. Lab Invest. 1990 Nov;63(5):698–706. [PubMed] [Google Scholar]
- Yonezawa S., Byrd J. C., Dahiya R., Ho J. J., Gum J. R., Griffiths B., Swallow D. M., Kim Y. S. Differential mucin gene expression in human pancreatic and colon cancer cells. Biochem J. 1991 Jun 15;276(Pt 3):599–605. doi: 10.1042/bj2760599. [DOI] [PMC free article] [PubMed] [Google Scholar]