Abstract
Purpose
This study aims to determine the anti-inflammatory, antioxidant, and anti-apoptotic effects of valproic acid (VPA) on rat spinal cord tissue in ischemia-reperfusion (IR) injury model created by abdominal aorta occlusion.
Materials and Methods
Sprague Dawley rat (male sex) weighing 190–260 g divided into four experimental groups: control only underwent laparotomy, sham group, pre-IR injury (200 mg/kg dose), and post-IR injury (300 mg/kg) VPA. We measured serum levels of TNF-α, IL-6, IL-1β, IL-18, Total Oxidant Status (TOS) and Total Antioxidant Status (TAS), and serum Oxidative Stress Index (OSI) ratio, and tissue expression of Bax and Bcl2, Caspase3, and Bax/Bcl2 ratio.
Results
Serum IL-18 was higher in the sham than the control group(P = 0.001), and there were declines in the pre-IR treatment (P = 0.002) and the post-IR treatment when compared to sham (P = 0.001). Despite these reductions, IL-18 expression levels in both the pre- and post-IR treatment groups were higher than in the control group (P = 0.001 & P = 0.003). The favorable effects of pre-IR VPA administration on immunohistochemical biomarkers were superior to post-IR VPA administration.
Conclusions
Comparative analyses between prophylactic VPA administration and post-IR interventions revealed congruence in their anti-inflammatory and anti-apoptotic ramifications. VPA can reduce spinal cord IR injury in an aortic occlusion model of rats.
Keywords: Inflammation, Spinal cord, Ischemia-reperfusion injury, Valproic acid
Introduction
Spinal cord stroke is an infrequent health issue in emergency settings and accounts for 0.3–1% of all strokes, but it leads to increased fatality and disability (1). Ischemia of the spinal cord (ISC) can occur in various clinical conditions. However, the most common causes are aortic pathologies such as thoracoabdominal aortic aneurysms with or without dissection (2, 3). Ischemia-reperfusion (IR) injury is a momentous unfavorable outcome in aortic surgery due to cross-clamping and de-clamping, and it can cause postoperative sequelae like paraplegia (4).
Macrophages and neutrophils release oxygen free radicals (FRs) during IR injury. The FR formation, causing lipid peroxidation and calcium influx into neurons, can result in cell death (5, 6). FRs, which lead to the peroxidation of cell membrane lipids and create further FRs in a self-replicating cycle, can intercede with the destructive consequences of IR (7). Inflammation is a reasonable pathway in spinal cord IR injury. Upregulation of different proinflammatory cytokines probably contributes to spinal cord IR injury (8). This injury triggers such pathways including interleukins. They have neuroprotective and degenerative effects on cells following injuries. IL-6 and TNF-α are multifunctional cytokines mainly secreted by neurons and glial cells, and play a vital role in neuronal development and differentiation (9, 10). Analyzing main interleukins and oxidative stress markers in a combination can give physicians to understand efficiency of neuroprotective drugs.
Valproic Acid (VPA) suppresses the production of proinflammatory and pro-apoptotic substances in animal studies (11, 12). Although VPA’s neuro-protective effect on traumatic ISC was apparent, its protective effects on IR are unclear. Specifically, it remains uncertain if suppressing oxidative stress or neutrophil infiltration is the prime mechanism through which VPA acts (13). The absence of clinical or animal trials in current literature spotlighting VPA's potential therapeutic role in spinal cord IR injury leaves a gap in understanding. In this study, we designed a transient ISC model in rats to compare a prophylactic VPA administration and a post-injury single-dose treatment to determine the neuroprotective mechanisms related to decreased inflammation, oxidative stress, and apoptosis. Thus, we aimed to determine VPA's anti-inflammatory, antioxidant, and anti-apoptotic effects on rat spinal cord tissue in an IR-injury model created by abdominal aorta occlusion.
Materials and methods
Study design
This animal trial proceeded harmoniously to the Reporting of In-Vivo Experiments on Animal-Research (ARRIVE) guidelines at the Center of “Experimental Animal Research and Application”. The study has the approval of the University Animal Experiment Ethics (Approval date:24/06/2015, Approval number: 49533702/79). The study was conducted following the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies (14).
The current research used thirty-two male sex rats weighing 190–260 g (Sprague–Dawley). The animals were acclimated in environmentally controlled cages and were fed on an ad libitum basis. They were randomized equally into four experimental groups. We defined the first experimental group as the control, and the animals in this group underwent laparotomy without being exposed to ischemia. The second group was the sham group, in which the animals were left untreated after experimental ISC. We treated the third group with a 200 mg/kg dose of VPA (Depakin, Sanofi, Turkey) by intraperitoneal injection twice a day for one week before exposure to ischemia. The fourth group was administered a single dose of 300 mg/kg VPA by intraperitoneal injection 90 min after ischemia.
While three animals died after exposure to ischemia (22, 34, and 54 min after) and before intraperitoneal injection in the fourth group, we did not experience any animal loss in the other groups. Autopsies were performed on these three dead animals, but the cause of death could not be determined. As a result, eight animals in the first three groups and five in the fourth group could be included in the analysis.
Experimental procedure and rat characteristics
The subjects were observed in spontaneous respiration without needing respiratory support during anesthesia. All the rats fasted 12 h before the surgery and were anesthetized with 40 mg/kg of ketamine-intramuscular (Ketalar-Eczacibasi, Turkey). Five minutes after the premedication with a 5 mg/kg dose of xylazine (Rompun-Bayer, Turkey). The amount was repeated as a cocktail of 25 mg/kg of ketamine and 5 mg/kg of xylazine when necessary. Following anesthesia, rats were tied to the operating table by their extremities to prevent the contamination of the surgical area with possible sudden movements.
Afterward, intravenous 100U/kg of heparin (Nevparin, Mustafa Nevzat, Turkey) was administered for anticoagulation. Three minutes after anticoagulation, aortic vascular clamps were placed first on the distal and proximal abdominal aorta of the subjects in groups 2, 3, and 4. After 30 min of clamping, reperfusion was applied for 24 h. Clamping was not used for group one (control group). Following the bleeding control, the abdominal muscles and skin were closed in two layers with 3/0 silk suture material. The rats were fed typically after the recovery period from anesthesia, which lasted approximately two hours following surgery. After 24 h following the surgical procedure, tissue and blood samples were collected for histopathological and biochemical examinations under second ketamine anesthesia. Consequently, the spinal cords of the subjects were obliterated, and the rats were euthanized by intracardiac injection of potassium chloride (KCI).
Biochemical analysis
The automatic colorimetric measurement method measured the Total Oxidant Status (TOS) and Total Antioxidant Status (TAS) (15). Hydrogen peroxide calibrated TOS measurement, given as µmol H2O2 Equiv/L. Serum TAS value was expressed as mmol Trolox Equiv/L. Oxidative Stress Index (OSI) is calculated by the ratio of TOS to TAS [OSI = (TOS/TAS)] (16). To determine cytokines, we saved the serum samples at −80°C. Cytokine levels were determined by a Microplate Spectrophotometer (Epoch, Biotek, Agilent Tech., USA) using specific ELISA kits (E-Bioscience, Vienna, Austria). We followed the manufacturer's instructions for ELISA protocols.
Histopathological evaluations
Samples were fixed in 10% formalin and sectioned at 5 μm thickness. These sections were placed on polylysine slides. Immunohistochemical analysis utilized Bcl2, caspase3, and Bax primary antibodies. Deparaffinization involved heating, xylene immersion, and progressive rehydration. Antigen retrieval was achieved by microwave boiling in Citrate Buffer. After washing with phosphate buffer, sections underwent peroxidase removal, staining blockage, and overnight incubation with primary antibodies. The following day, sections were washed and exposed to the UltraVision-HRP kit as a secondary antibody and later stained using AEC chromogen. Counterstaining was done with Mayer's hematoxylin. A light microscope (Nikon E-600 Eclipse) assessed motor neuron staining in the spinal cord. Immunopositive cells were counted in five fields under 20x magnification using image analysis software.
Variables and outcomes
There are three primary outcomes of the study. The first one is the difference in the distributions of TNF-α, IL-1β, IL-6, and IL-18. The second is the change in oxidative stress status, which we evaluated using TAS, TOS, and OSI, and the last is the difference in immunohistochemical parameters (Bax, Caspase3, Bcl2, and Bax/Bcl2 ratio) among the groups.
Statistical analysis
The SPSS software v22 performed the statistical analysis, and GraphPADv9.2 software was used to draw detailed graphs showing group comparisons. Descriptive statistics were presented as mean and standard deviation (SD). All data were subjected to tests for normality. Data that did not exhibit a normal/Gaussian distribution were analyzed via a non-parametric equivalent. Before the difference analysis of the measurement data, the Kolmogorov–Smirnov test was performed to test the homogeneity of the data distribution. According to the test results, the Independent Sample T-test was used in the double-group difference analysis of the normally distributed data, and the Mann–Whitney U test was used for comparing numeric variables between groups. Box-plot graphs were used for visual comparisons of biomarkers. A value of p < 0.05 was set as a statistically significant level.
Results
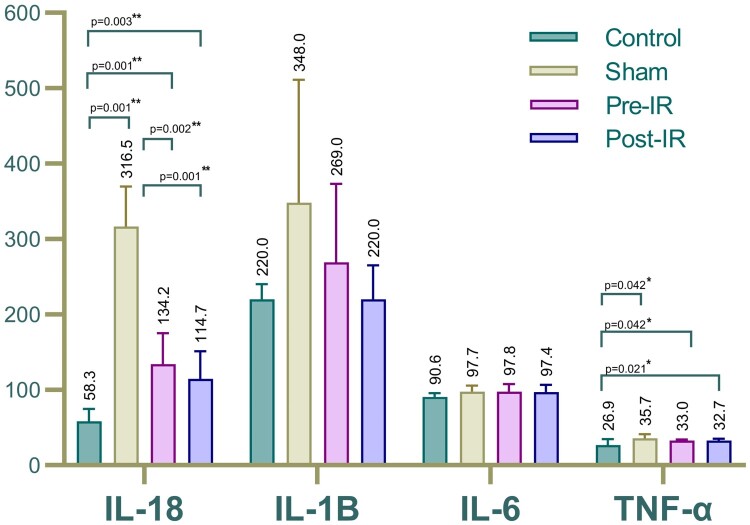
Comparisons of serum cytokine levels between the study groups are shown in Table 1 and Fig. 1. The IL-18 level in the sham group was significantly higher than the control (P = 0.001). Similarly, IL-18 expression levels in the pre- and post-IR treatment groups differed notably from the control (P = 0.001 & P = 0.003). IL-1B and IL-6 expression levels showed no significant variations between the sham, treatment groups, and control. However, TNF-α levels in the sham and treatment groups showed significant changes compared to the control, but no marked difference was observed between the treatment and the sham groups.
Table 1.
Distribution of serum cytokine levels.
| Cytokines | Control | Sham | Pre-IR treatment |
Post-IR treatment |
|---|---|---|---|---|
| IL-18, pg/ml | 58.31 ± 16.4 | 316.59 ± 53 | 134.17 ± 41 | 114.7 ± 36.6 |
| IL-1B, pg/ml | 220 ± 20.3 | 348 ± 163.4 | 269 ± 104 | 220 ± 45.48 |
| IL-6, pg/ml | 90.64 ± 5.25 | 97.74 ± 7.89 | 97.83 ± 9.68 | 97.42 ± 9.56 |
| TNF-α, pg/ml | 26.89 ± 7.92 | 35.73 ± 5.67 | 32.97 ± 1.49 | 32.74 ± 2.37 |
Abbreviations. IL: Interleukin, TNF: Tumor Necrose Factor, IR: Ischemia reperfusion. Mann-Whitney U test was used for comparing numeric variables between groups.
Figure 1.
Comparison of serum (a) IL-18, (b) IL-1B, (c) IL-6 and (d) TNF-α.
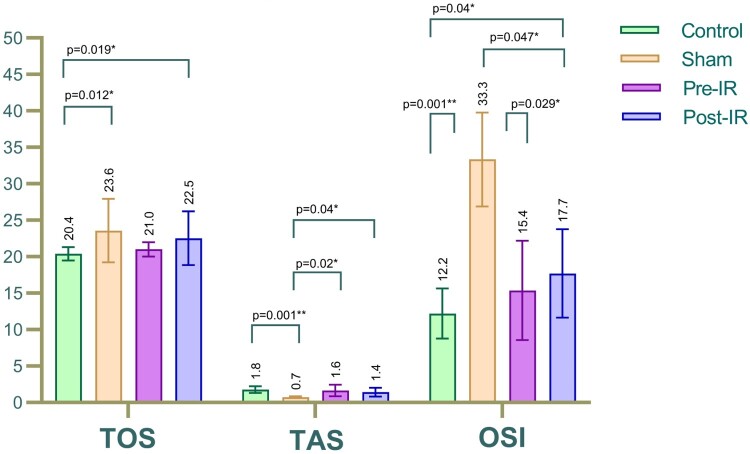
The serum TOS in the sham group was higher than in the control group (P = 0.012). Noteworthy differences were observed in the TOS levels of the pre-and post-IR treatment groups compared to the sham, although these weren't significant. The pre-IR treatment group's TOS was similar to the control, reflecting the effectiveness of prophylactic VPA. The serum TAS in the sham was lower than the control (P = 0.001). The pre-and post-IR treatment groups showed an increased TAS level compared to the sham, with significant variations (P = 0.020 & P = 0.040). The oxidative stress markers are detailed in Table 2 and Fig. 2. The OSI levels, another oxidative stress marker, significantly increased in the sham group compared to the control (P = 0.001). The pre- and post-IR treatment groups exhibited a marked decline in OSI ratio compared to the sham, with significant reductions (P = 0.029 & P = 0.047). The OSI ratio in the pre-IR treatment group was in line with the control, suggesting the efficacy of the prophylactic VPA administration.
Table 2.
Distribution of oxidative stress markers.
| Markers | Control | Sham | Pre-IR treatment |
Post-IR treatment |
|---|---|---|---|---|
| TOS, μmol Trolox Eq/L | 20,39 ± 0,91 | 23,57 ± 4,35 | 21,01 ± 0,98 | 22,54 ± 3,69 |
| TAS, μmol H2O2 Eq/L | 1,78 ± 0,45 | 0,72 ± 0,12 | 1,64 ± 0,79 | 1,43 ± 0,60 |
| OSI | 12.19 ± 3.44 | 33.34 ± 6.43 | 15.36 ± 6.81 | 17.7 ± 6.05 |
Abbreviations. OSI: Oxidative Stress Index, TOS: Total Oxidant Status, TAS: Total Antioxidant Status, IR: Ischemia reperfusion. Mann-Whitney U test was used for comparing numeric variables between groups.
Figure 2.
Comparison of serum (a) TOS, (b) TAS, and (c) OSI between the study groups.
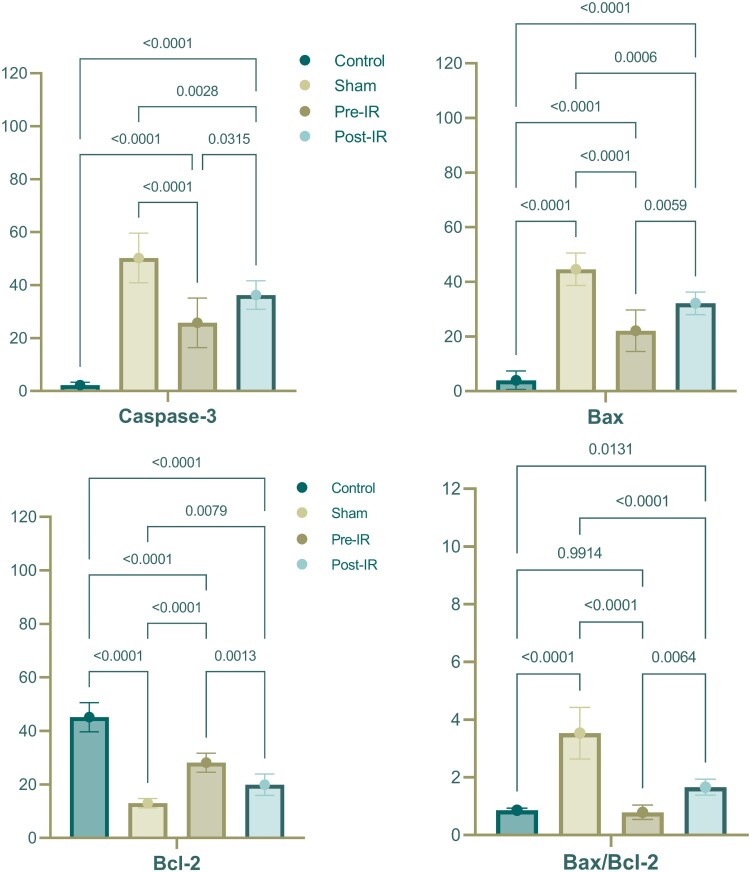
Immunohistochemical markers, as presented in Table 3 and Fig. 3, demonstrated an increase in the numbers of Caspase3(+) and Bax(+) neurons and the Bax/Bcl-2 ratio in the sham group compared to the control (p < 0.001). The Bcl-2(+) neuron number in the sham was notably lower than the control (p < 0.001). The pre-IR and post-IR treatment groups showed variations in these markers compared to the sham and control, with the pre-IR VPA administration displaying more favorable results than the post-IR VPA administration.
Table 3.
Distribution of immunohistochemical marker expression levels.
| Markers | Control | Sham | Pre-IR treatment |
Post-IR treatment |
|---|---|---|---|---|
| Caspase-3, npn | 2.25 ± 1.04 | 50.25 ± 9.35 | 25.75 ± 9.35 | 36.25 ± 5.37 |
| Bax, npn | 4 ± 3.38 | 44.63 ± 5.95 | 22.13 ± 7.62 | 32.13 ± 4.12 |
| Bcl-2, npn | 45.13 ± 5.44 | 13 ± 1.77 | 28.13 ± 3.56 | 19.88 ± 4.02 |
| Bax/Bcl-2 ratio | 0.86 ± 0.07 | 3.53 ± 0.89 | 0.79 ± 0.25 | 1.66 ± 0.28 |
Abbreviations. IR: Ischemia reperfusion, npn: number of positive neurons. Mann-Whitney U test was used for comparing numeric variables between groups.
Figure 3.
Comparison of expression of (a) Caspase-3, (b) Bax and (c) Bcl-2, and (d) Bax/Bcl-2 ratio between the groups.
Discussion
The main focus of the study research was the potential role of VPA in aortic surgeries. Our findings suggest that VPA should be considered for prophylactic use before aortic surgery to mitigate the risk of IR injuries. The drug seems to have multifaceted effects, attributed to its anti-inflammatory, antioxidant, and anti-apoptotic properties. Furthermore, the outcome variables were chosen based on their relevance to the IR injury mechanism.
Interleukins have neuroprotective and degenerative effects on cells following injuries. IL-6 is a multifunctional cytokine mainly secreted by neurons and glial cells, and it plays a vital role in neuronal development and differentiation (17). It triggers neuronal survival after injury and causes neuronal death in neurodegenerative diseases (9). Another strong interleukin, TNF-α, is a proinflammatory cytokine that plays a crucial role in host defense (18). It activates microglia to induce the progressive loss of neurons (19). If so, we demonstrated a transient ISC model in rats to compare a prophylactic VPA administration and a post-injury single-dose treatment to determine the neuroprotective mechanisms related to decreased inflammation, oxidative stress, and apoptosis. We created an experimental IR injury in a rat spinal cord using a model of occlusion of the abdominal aorta. We found that it significantly increased serum IL-18 and TNF-α levels, and VPA administration before or after IR reduced this IL-18 level but not to pre-ischemic levels. While the decrease in TNF-α was insignificant, there was no increase in IL-6 and IL-1B levels of the sham and the treatment groups compared to the control. While serum TOS level and OSI ratio increased and TAS level decreased in the sham group, pre- and post-IR VPA treatments significantly improved TAS and OSI levels but not TOS levels compared to the sham. Pre-IR VPA brought these three parameters to similar levels with the pre-ischemia period. Finally, there were increases in apoptosis markers in the sham compared to the control. Both pre and post-IR VPA administration improved compared to the sham group but did not reach the control.
ISC with subsequent paraplegia is still fatal following aortic aneurysm or dissection repair. Disruption of arterial blood flow, trauma to the spinal cord collaterals during surgery, hypoperfusion of the distal aorta, prolonged aortic cross-clamp time, elevated cerebrospinal fluid (CSF) pressure, and postop-hypotension are all considered antecedent causes of the injury (20). Although methods such as intraperitoneal, intrathecal, or intravenous administered drugs and CSF drainage have been used for many years to raise distal aortic flow-perfusion and to minimize spinal cord injury following aortic surgery, none has been able to prevent the damage and neurological complications, completely (21).
Antioxidant and anti-inflammatory drugs have been studied to prevent aortic ischemia-related spinal cord injury in animal models (8). In vitro, VPA therapies reduce glutamate-induced-excitotoxicity and inhibit lipopolysaccharide-induced IL6-TNFα production (10, 12). Additionally, VPA shows protective effects in vivo against various IR injury models and inflammatory disease models (6, 22–24). Regrettably, clinical trials utilizing a variety of preclinical neuroprotective medicines for stroke have been ineffective (25). Although numerous variables may contribute to the inability to create new stroke treatments, one of the primary reasons is the treatment-limiting side effects of current medications (26). Most of VPA's adverse effects (i.e. thrombocytopenia, hyperammonemia, and parkinsonism) are dose-dependent; a single-dose treatment should not result in severe adverse effects (27).
Being a broad-spectrum inhibitor of histone deacetylase, VPA provides that it directly inhibits histone deacetylases (HDACs), reducing the interaction of histone with DNA (28). Thus, loosening the nucleosome structure facilitates the binding of the transcription factor to the DNA. At therapeutic doses, VPA exhibits neuroprotective and neuroregenerative properties (29). VPA increases the expression of Hsp70 (heat-shock protein)in cortical neurons during ischemia, followed by histone hyperacetylation (30). VPA inhibits caspase3 activation following ischemia and optic nerve crush injury (29, 31). VPA prevented disruption of the blood–brain transition by inhibiting HDAC and MMP-9 in the ischemic model (32).
VPA has been researched in IR injury studies in other tissues outside the nervous system. It may have antioxidant and anti-apoptotic properties. Antioxidant, anti-apoptotic, and anti-inflammatory impacts of VPA have been shown in acute lung injury induced by the IR model by Kim et al. (6). According to Zhang et al. (23), the efficacy of VPA in the rat retina was investigated in the IR model. In this model created with an increase in ocular pressure, VPA’s protective effect on the retina in IR damage was demonstrated, and this activity was mediated by anti-apoptotic properties (23). In IR injury, FRs are the most important cause of neuronal loss. Overproduction of FRs will further aggravate IR injury, inevitably resulting in apoptotic cascade activation (33).
The anti-apoptotic effect of VPA was investigated in different trials. The IR model that Zhang et al. created noticed that VPA adjusted bax and bcl-2 mRNA expression (34). Our study indicated that bax and bcl2 expression decreased in the group given VPA. Our findings on the anti-apoptotic activity of VPA were compatible with the literature. In the IR model made by Wang et al., created with 60 min cerebral artery occlusion, VPA was applied for 14 days (35). They demonstrated the positive effects of VPA on angiogenesis and functional improvement. The retinal IR injury model created by Zhang et al. reported that VPA application decreased malondialdehyde levels and increased SOD and GSH-Px activities (36). Our study aligns with the literature and supports the claim that increased serum TAS levels and decreased OSI VPA have antioxidant properties.
The study presented some limitations due to the nature of animal research. It examined the values of biomarkers at the 24th hour to examine the effects of VPA in the emergency period, but there may be differences in the long-term effects. The study enrolled the biochemical and immunohistochemical parameters that we thought might be crucial in the pathophysiology. Still, other biochemical and immunohistochemical factors that play a role in spinal cord IR injury may also be associated with the VPA. The last limitation of the study is the death of three animals in the fourth group.
Conclusion
The research elucidated VPA's anti-inflammatory, antioxidant, and anti-apoptotic attributes in the spinal cord IR process, employing biochemical and immunohistochemical methodologies. Comparative analyses between prophylactic VPA administration and post-IR interventions revealed congruence in their anti-inflammatory and anti-apoptotic ramifications. However, the pre-IR treatment manifested superior antioxidant efficacy. Based on our empirical findings, VPA exhibited therapeutic potential. Advocating its routine administration before aortic surgical procedures, especially in emergencies, could portend a promising strategy for facilitating spinal cord IR injuries.
Disclaimer statements
Acknowledgement The present study is based on a scientific research thesis.
Author contributions All authors, literature review, study design, data acquisition, analysis, and interpretation approved the version to be published and participated in the study's processes.
Availability of data and materials Not available.
Conflict of Interest The authors declare no conflict of interest.
Ethical statement This study was approved by University Animal Experiments Ethics Committee (24/06/2015 – 49533702/79).
Financial support None
Funding This trial was supported by the Afyon Kocatepe University Scientific Research Projects Unit supported the present study (project no: 15.TIP.12).
Presentation at a national or international medical society None.
Type of Study and Level of Evidence Level-II, Prospective Rat Trial.
References
- 1.Romi F, Naess H.. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76(3–4):95–98. [DOI] [PubMed] [Google Scholar]
- 2.Zalewski NL, Rabinstein AA, Krecke KN, Brown RD, Wijdicks EFM, Weinshenker BG, Kaufmann TJ, Morris JM, Aksamit AJ, Bartleson JD, et al. . Characteristics of spontaneous spinal cord infarction and proposed diagnostic criteria. JAMA Neurol. 2019;76(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge L, Arul K, Stoner M, Mesfin A.. Etiology and outcomes of spinal cord infarct: a case series from a level 1 trauma center. Global Spine J. 2020;10(6):735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katseni K, Chalkias A, Kotsis T, Dafnios N, Arapoglou V, Kaparos G, Logothetis E, Iacovidou N, Karvouni E, Katsenis K.. The effect of perioperative ischemia and reperfusion on multiorgan dysfunction following abdominal aortic aneurysm repair. Biomed Res Int. 2015;2015:598980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halici Z, Karaca M, Keles ON, Borekci B, Odabasoglu F, Suleyman H, Cadirci E, Bayir Y, Unal B.. Protective effects of amlodipine on ischemia-reperfusion injury of rat ovary: biochemical and histopathologic evaluation. Fertil Steril. 2008;90(6):2408–2415. [DOI] [PubMed] [Google Scholar]
- 6.Kim K, Li Y, Jin G, Chong W, Liu B, Lu J, Lee K, deMoya M, Velmahos GC, Alam HB.. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation. 2012;83(2):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazzaa SM, Abdou AG, Ibraheim EO, Salem EA, Hassan MHA, Abdel-Razek HAD.. Effect of L-carnitine and atorvastatin on a rat model of ischemia-reperfusion injury of spinal cord. J Immunoassay Immunochem. 2021;42(6):596–619. [DOI] [PubMed] [Google Scholar]
- 8.Zhu P, Li J-x, Fujino M, Zhuang J, Li X-K.. Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediat Inflamm. 2013;2013:701970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruol DL, Nelson TE.. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol. 1997;15(3):307–339. [DOI] [PubMed] [Google Scholar]
- 10.Ichiyama T, Okada K, Lipton JM, Matsubara T, Hayashi T, Furukawa S.. Sodium valproate inhibits production of TNF-α and IL-6 and activation of NF-κB. Brain Res. 2000;857(1–2):246–251. [DOI] [PubMed] [Google Scholar]
- 11.Chen J-Y, Chu L-W, Cheng K-I, Hsieh S-L, Juan Y-S, Wu B-N.. Valproate reduces neuroinflammation and neuronal death in a rat chronic constriction injury model. Sci Rep. 2018;8(1):16457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng G-S, Li G, Tzeng N-S, Chen P-S, Chuang D-M, Hsu Y-D, Yang S, Hong J-S, Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: role of microglia. Mol Brain Res. 2005;134(1):162–169. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, Li Y, Fu H, Li S.. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J Neuroinflammation. 2018;15(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tveden-Nyborg P, Bergmann TK, Jessen N, Simonsen U, Lykkesfeldt J.. BCPT policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2021;128(1):4–8. [DOI] [PubMed] [Google Scholar]
- 15.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. [DOI] [PubMed] [Google Scholar]
- 16.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–1111. [DOI] [PubMed] [Google Scholar]
- 17.Erta M, Quintana A, Hidalgo J.. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tracey KJ, Cerami A.. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. [DOI] [PubMed] [Google Scholar]
- 19.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7(3):231–240. [DOI] [PubMed] [Google Scholar]
- 20.Koçogullari CU, Becit N, Erkut B, Keleş MS, Ceviz M, Ates A, Gündoğdu C, Kaygin MA, Koçak H.. Prevention of reperfusion injury of the spinal cord in aortic surgery: an experimental study. Surg Today. 2008;38(3):237–244. [DOI] [PubMed] [Google Scholar]
- 21.Parotto M, Ouzounian M, Djaiani G.. Spinal cord protection in elective thoracoabdominal aortic procedures. J Cardiothorac Vasc Anesth. 2019;33(1):200–208. [DOI] [PubMed] [Google Scholar]
- 22.Bhavsar P, Ahmad T, Adcock IM.. The role of histone deacetylases in asthma and allergic diseases. J Allergy Clin Immunol. 2008;121(3):580–584. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv X, Wu XW, Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia–reperfusion injury. Neurosci Lett. 2011;504(2):88–92. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhang Z-Y, Wu Y, Schluesener H.. Valproic acid ameliorates inflammation in experimental autoimmune encephalomyelitis rats. Neuroscience. 2012;221:140–150. [DOI] [PubMed] [Google Scholar]
- 25.Broderick JP, Hacke W.. Treatment of acute ischemic stroke: part II: neuroprotection and medical management. Circulation. 2002;106(13):1736–1740. [DOI] [PubMed] [Google Scholar]
- 26.Dirnagl U, Macleod MR.. Stroke research at a road block: the streets from adversity should be paved with meta-analysis and good laboratory practice. Br J Pharmacol. 2009;157(7):1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chateauvieux S, Morceau F, Dicato M, Diederich M.. Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol. 2010;2010:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You D, Wen X, Gorczyca L, Morris A, Richardson JR, Aleksunes LM.. Increased MDR1 transporter expression in human brain endothelial cells through enhanced histone acetylation and activation of aryl hydrocarbon receptor signaling. Mol Neurobiol. 2019;56(10):6986–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM.. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89(6):1358–1367. [DOI] [PubMed] [Google Scholar]
- 30.Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, Leeds P, Chuang D-M.. Valproic acid induces functional heat-shock protein 70 via class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem. 2009;111(4):976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagrèze WA.. Valproic acid–mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Visual Sci. 2010;51(1):526–534. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Leng Y, Tsai L-K, Leeds P, Chuang D-M.. Valproic acid attenuates blood–brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31(1):52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S-D, Yang D-I, Lin T-K, Shaw F-Z, Liou C-W, Chuang Y-C.. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12(10):7199–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang ZZ, Wu XW, Gong YY, Zhang W, Yin LL.. Protective effect of valproic acid on ischemia-reperfusion induced injury in retina of rat. [Zhonghua yan ke za zhi] Chin J Ophthalmol. 2012;48(8):739–743. [PubMed] [Google Scholar]
- 35.Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, Leeds P, Chuang D-M.. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke. 2012;43(9):2430–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Qin X, Zhao X, Tong N, Gong Y, Zhang W, Wu XW.. Valproic acid regulates antioxidant enzymes and prevents ischemia/reperfusion injury in the rat retina. Curr Eye Res. 2012;37(5):429–437. [DOI] [PubMed] [Google Scholar]