Abstract
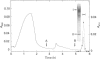
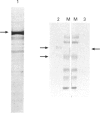
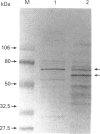
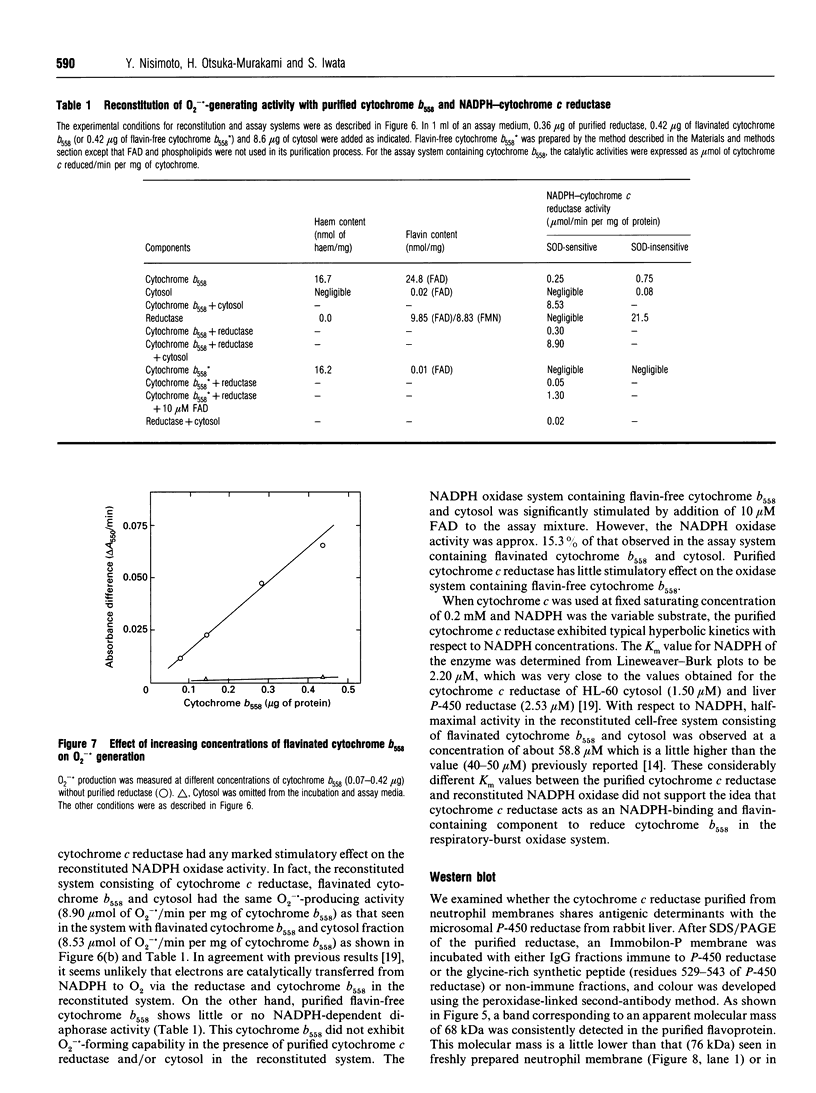
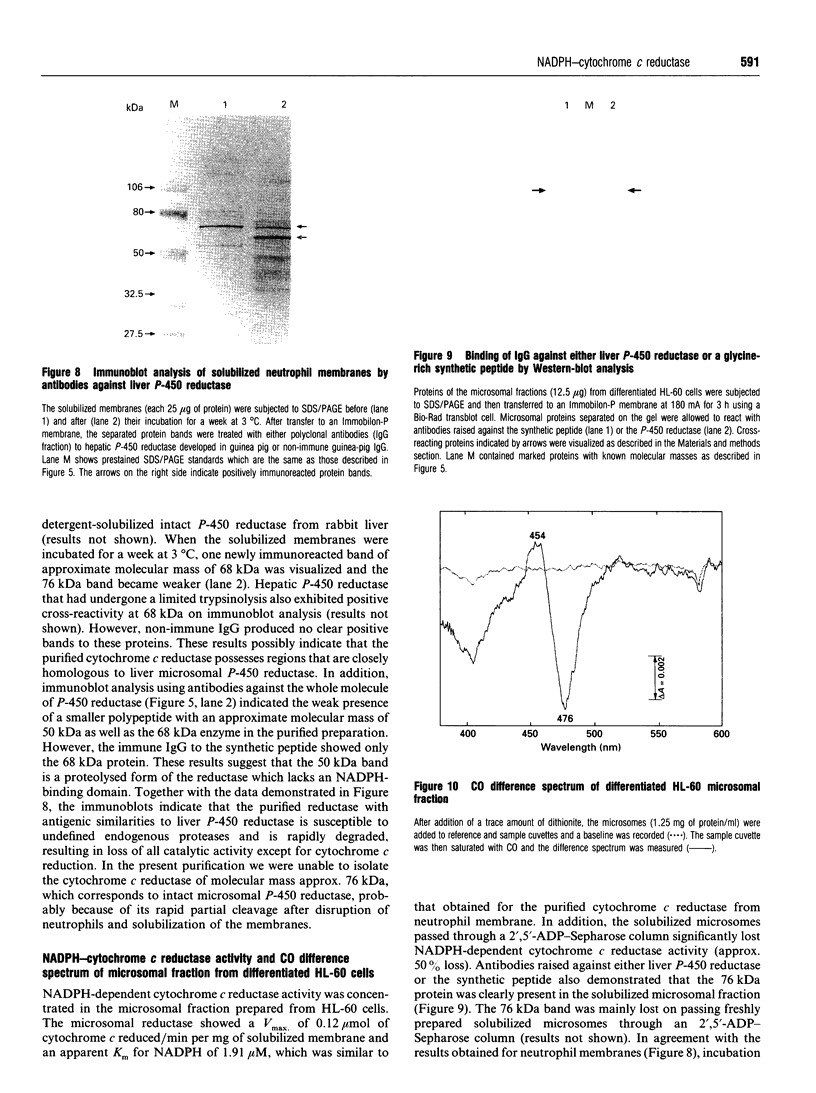
Neutrophil-membrane-associated NADPH-cytochrome c reductase and cytochrome b558 were separately eluted and highly purified by a combination of ion-exchange Sepharose, N-amino-octylagarose, 2',5'-ADP-Sepharose and heparin-Sepharose column chromatographies. The purified cytochrome c reductase with an apparent molecular mass of 68 kDa contained FMN and FAD (FMN/FAD approx. 1). Cytochrome b558 prepared in the presence of phospholipids and FAD showed marked O2-.-producing activity (Vmax., 8.53 mumol of O2-./min per mg of cytochrome; Km for NADPH 58.8 microM) in a cell-free assay system consisting of cytosol, arachidonate and GTP[S]. However, when it was obtained without FAD added to the purification process, it had negligible FAD and little or no O2-.-forming activity in the reconstituted system. The NADPH oxidase activity was not markedly stimulated on incubation of the purified reductase with either flavinated or flavin-depleted cytochrome b558 in the cell-free system, suggesting that the reductase is not likely to be involved in neutrophil O2-. generation. The purified reductase cross-reacted with polyclonal antibodies against both hepatic NADPH-cytochrome P-450 reductase and a synthetic peptide, ILVGPGTGIAPFRSF, which indicates residues 529-543 located in the glycine-rich NADPH-binding domain of the P-450 reductase, but cytochrome b558 did not produce any immunoreactive bands to these antibodies. These antibodies also produced a positive reaction with a 76 kDa protein from dimethyl sulphoxide-induced HL-60-cell microsomes. After solubilization of the microsomal membranes, the 76 kDa protein was readily converted into a partially proteolysed form (68 kDa) even in the presence of antiproteases. In addition, the microsomal fraction shows a CO difference spectrum with a peak at about 454 nm and a trough at 476 nm in the presence of dithionite, indicating the presence of a cytochrome P-450-like haemoprotein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991 Oct 17;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst oxidase. Adv Enzymol Relat Areas Mol Biol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- Black S. D., Coon M. J. Structural features of liver microsomal NADPH-cytochrome P-450 reductase. Hydrophobic domain, hydrophilic domain, and connecting region. J Biol Chem. 1982 May 25;257(10):5929–5938. [PubMed] [Google Scholar]
- Black S. D., French J. S., Williams C. H., Jr, Coon M. J. Role of a hydrophobic polypeptide in the N-terminal region of NADPH-cytochrome P-450 reductase in complex formation with P-450LM. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1528–1535. doi: 10.1016/0006-291x(79)91238-5. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Erickson R. W., Muhlebach T. J., Messner H., Orkin S. H., Seger R. A., Curnutte J. T. Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11231–11235. doi: 10.1073/pnas.88.24.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Strittmatter P. Cytochrome b5 reduction by NADPH-cytochrome P-450 reductase. J Biol Chem. 1979 Sep 25;254(18):8976–8981. [PubMed] [Google Scholar]
- Fujii H., Kakinuma K. Electron transfer reactions in the NADPH oxidase system of neutrophils--involvement of an NADPH-cytochrome c reductase in the oxidase system. Biochim Biophys Acta. 1991 Nov 12;1095(3):201–209. doi: 10.1016/0167-4889(91)90100-c. [DOI] [PubMed] [Google Scholar]
- Imai Y., Sato R. A gel-electrophoretically homogeneous preparation of cytochrome P-450 from liver microsomes of phenobarbital-pretreated rabbits. Biochem Biophys Res Commun. 1974 Sep 9;60(1):8–14. doi: 10.1016/0006-291x(74)90164-8. [DOI] [PubMed] [Google Scholar]
- Imajoh-Ohmi S., Tokita K., Ochiai H., Nakamura M., Kanegasaki S. Topology of cytochrome b558 in neutrophil membrane analyzed by anti-peptide antibodies and proteolysis. J Biol Chem. 1992 Jan 5;267(1):180–184. [PubMed] [Google Scholar]
- Isogai Y., Iizuka T., Makino R., Iyanagi T., Orii Y. Superoxide-producing cytochrome b. Enzymatic and electron paramagnetic resonance properties of cytochrome b558 purified from neutrophils. J Biol Chem. 1993 Feb 25;268(6):4025–4031. [PubMed] [Google Scholar]
- Isogai Y., Shiro Y., Nasuda-Kouyama A., Iizuka T. Superoxide production by cytochrome b558 purified from neutrophils in a reconstituted system with an exogenous reductase. J Biol Chem. 1991 Jul 25;266(21):13481–13484. [PubMed] [Google Scholar]
- Iyanagi T., Anan F. K., Imai Y., Mason H. S. Studies on the microsomal mixed function oxidase system: redox properties of detergent-solubilized NADPH-cytochrome P-450 reductase. Biochemistry. 1978 May 30;17(11):2224–2230. doi: 10.1021/bi00604a032. [DOI] [PubMed] [Google Scholar]
- Kakinuma K., Kaneda M., Chiba T., Ohnishi T. Electron spin resonance studies on a flavoprotein in neutrophil plasma membranes. Redox potentials of the flavin and its participation in NADPH oxidase. J Biol Chem. 1986 Jul 15;261(20):9426–9432. [PubMed] [Google Scholar]
- Kennedy R. C., Henkel R. D., Pauletti D., Allan J. S., Lee T. H., Essex M., Dreesman G. R. Antiserum to a synthetic peptide recognizes the HTLV-III envelope glycoprotein. Science. 1986 Mar 28;231(4745):1556–1559. doi: 10.1126/science.3006246. [DOI] [PubMed] [Google Scholar]
- Kikuta Y., Kusunose E., Endo K., Yamamoto S., Sogawa K., Fujii-Kuriyama Y., Kusunose M. A novel form of cytochrome P-450 family 4 in human polymorphonuclear leukocytes. cDNA cloning and expression of leukotriene B4 omega-hydroxylase. J Biol Chem. 1993 May 5;268(13):9376–9380. [PubMed] [Google Scholar]
- Koizumi K., Shimizu S., Koizumi K. T., Nishida K., Sato C., Ota K., Yamanaka N. Rapid isolation and lipid characterization of plasma membranes from normal and malignant lymphoid cells of mouse. Biochim Biophys Acta. 1981 Dec 7;649(2):393–403. doi: 10.1016/0005-2736(81)90429-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laporte F., Doussiere J., Mechin V., Vignais P. V. NADPH-cytochrome c reductase from rabbit peritoneal neutrophils. Purification, properties and function in the respiratory burst. Eur J Biochem. 1991 Feb 26;196(1):59–66. doi: 10.1111/j.1432-1033.1991.tb15785.x. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Garrett M. C., Fujii H., Nunoi H. Characterization of neutrophil NADPH oxidase factors p47-phox and p67-phox from recombinant baculoviruses. J Biol Chem. 1991 Oct 15;266(29):19812–19818. [PubMed] [Google Scholar]
- Lomax K. J., Leto T. L., Nunoi H., Gallin J. I., Malech H. L. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989 Jul 28;245(4916):409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Junk K. W., Coon M. J. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969 Jul 10;244(13):3714–3721. [PubMed] [Google Scholar]
- Lutter R., van Schaik M. L., van Zwieten R., Wever R., Roos D., Hamers M. N. Purification and partial characterization of the b-type cytochrome from human polymorphonuclear leukocytes. J Biol Chem. 1985 Feb 25;260(4):2237–2244. [PubMed] [Google Scholar]
- Miki T., Yoshida L. S., Kakinuma K. Reconstitution of superoxide-forming NADPH oxidase activity with cytochrome b558 purified from porcine neutrophils. Requirement of a membrane-bound flavin enzyme for reconstitution of activity. J Biol Chem. 1992 Sep 15;267(26):18695–18701. [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y., Otsuka-Murakami H., Iwata S., Isogai Y., Iizuka T. Characterization of superoxide dismutase-insensitive cytochrome c reductase activity in HL-60 cytosol as NADPH-cytochrome P450 reductase. Arch Biochem Biophys. 1993 May;302(2):315–321. doi: 10.1006/abbi.1993.1217. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y., Otsuka-Murakami H. NADPH: nitroblue tetrazolium reductase found in plasma membrane of human neutrophil. Biochim Biophys Acta. 1990 Sep 3;1040(2):260–266. doi: 10.1016/0167-4838(90)90085-t. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Walker L. E., Allen R. A., Jesaitis A. J., Orkin S. H. Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci U S A. 1988 May;85(10):3319–3323. doi: 10.1073/pnas.85.10.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Barnes K. C., Brandt S. J., Kinkade J. M., Jr Density heterogeneity of neutrophilic polymorphonuclear leukocytes: gradient fractionation and relationship to chemotactic stimulation. Blood. 1983 Jun;61(6):1105–1115. [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Leto T. L., Malech H. L., Kwong C. H. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992 Jun 5;256(5062):1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Rudolph S. A., Krueger B. K. Endogenous protein phosphorylation and dephosphorylation. Adv Cyclic Nucleotide Res. 1979;10:107–133. [PubMed] [Google Scholar]
- Segal A. W., West I., Wientjes F., Nugent J. H., Chavan A. J., Haley B., Garcia R. C., Rosen H., Scrace G. Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J. 1992 Jun 15;284(Pt 3):781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Leukotriene B4 omega-hydroxylase in human polymorphonuclear leukocytes. Partial purification and identification as a cytochrome P-450. J Clin Invest. 1985 Sep;76(3):1218–1228. doi: 10.1172/JCI112077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Donelson J. E., Moser D. R., Clark R. A. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7195–7199. doi: 10.1073/pnas.86.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]