Abstract
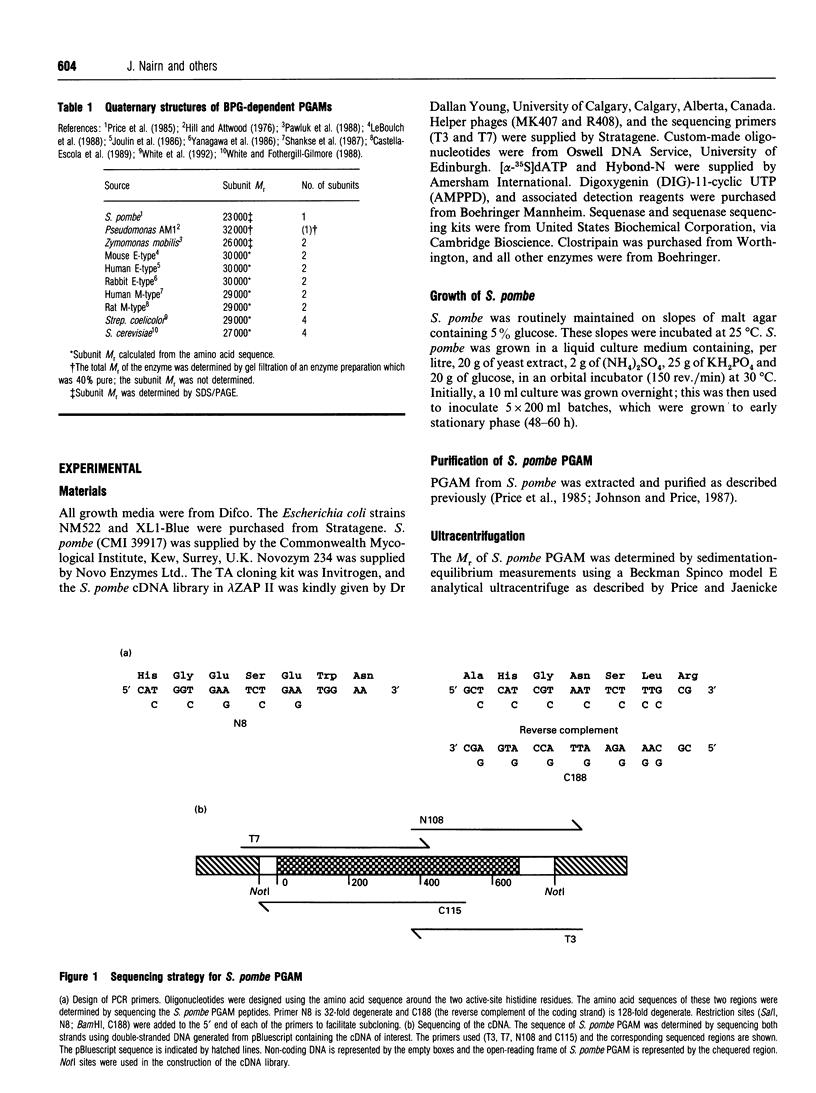
The amino acid sequence of the monomeric 2,3-bisphosphoglycerate (BPG)-dependent phosphoglycerate mutase (PGAM) from the fission yeast Schizosaccharomyces pombe has been determined. Amino acid sequencing of proteolytic fragments of the enzyme showed the S. pombe mutase to be similar in sequence to the tetrameric enzyme of baker's yeast (Saccharomyces cerevisiae). An S. pombe cDNA library was screened using a PCR fragment generated from two oligonucleotides complementary to sequences encoding the regions at the two active-site histidine residues. The 0.63 kb cDNA encoded an open reading frame of 210 amino acids. This sequence agreed completely with sequences of peptides derived from the purified protein. The amino acid sequence of S. pombe PGAM is 43% identical with that of S. cerevisiae PGAM and shows an equally high degree of identity with BPG-dependent PGAMs from other sources. However, the sequence of the S. pombe enzyme differs from other BPG-dependent enzymes in three important ways: (i) it does not contain the alanine- and lysine-rich sequence of amino acids at the C-terminus which have been proposed to constitute a flexible tail involved in catalysis; (ii) the sequence spanning residues 122-146 (S. cerevisiae PGAM numbering) is not present in the S. pombe PGAM sequence; in the S. cerevisiae PGAM crystal structure this stretch of sequence has been shown to occur as an extended loop, part of which is involved in inter-subunit interactions; (iii) the amino acid sequence in the region of a second S. cerevisiae inter-subunit contact (residues 74-78) shows radical mutations in the S. pombe enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carreras J., Mezquita J., Bosch J., Bartrons R., Pons G. Phylogeny and ontogeny of the phosphoglycerate mutases--IV. Distribution of glycerate-2,3-P2 dependent and independent phosphoglycerate mutases in algae, fungi, plants and animals. Comp Biochem Physiol B. 1982;71(4):591–597. doi: 10.1016/0305-0491(82)90467-9. [DOI] [PubMed] [Google Scholar]
- Carter P. E., Dunbar B., Fothergill J. E. The serine proteinase chain of human complement component C1s. Cyanogen bromide cleavage and N-terminal sequences of the fragments. Biochem J. 1983 Dec 1;215(3):565–571. doi: 10.1042/bj2150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellà-Escolà J., Montoliu L., Pons G., Puigdomènech P., Cohen-Solal M., Carreras J., Rigau J., Climent F. Sequence of rat skeletal muscle phosphoglycerate mutase cDNA. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1345–1351. doi: 10.1016/0006-291x(89)92751-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill-Gilmore L. A., Watson H. C. The phosphoglycerate mutases. Adv Enzymol Relat Areas Mol Biol. 1989;62:227–313. doi: 10.1002/9780470123089.ch6. [DOI] [PubMed] [Google Scholar]
- Fothergill L. A., Harkins R. N. The amino acid sequence of yeast phosphoglycerate mutase. Proc R Soc Lond B Biol Sci. 1982 Apr 22;215(1198):19–44. doi: 10.1098/rspb.1982.0026. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Carreras J. Phosphoglycerate mutase from yeast, chicken breast muscle, and kidney (2, 3-PGA-dependent). Methods Enzymol. 1975;42:435–450. doi: 10.1016/0076-6879(75)42149-8. [DOI] [PubMed] [Google Scholar]
- Hill B., Attwood M. M. Purification and characterization of phosphoglycerate mutase from methanol-grown Hyphomicrobium X and Pseudomonas AM1. J Gen Microbiol. 1976 Sep;96(1):185–193. doi: 10.1099/00221287-96-1-185. [DOI] [PubMed] [Google Scholar]
- Johnson C. M., Price N. C. Do metal ions promote the re-activation of the 2,3-bisphosphoglycerate-independent phosphoglycerate mutases? Biochem J. 1988 May 15;252(1):111–117. doi: 10.1042/bj2520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joulin V., Peduzzi J., Roméo P. H., Rosa R., Valentin C., Dubart A., Lapeyre B., Blouquit Y., Garel M. C., Goossens M. Molecular cloning and sequencing of the human erythrocyte 2,3-bisphosphoglycerate mutase cDNA: revised amino acid sequence. EMBO J. 1986 Sep;5(9):2275–2283. doi: 10.1002/j.1460-2075.1986.tb04495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J., Keppi E., Dimarcq J. L., Wicker C., Reichhart J. M., Dunbar B., Lepage P., Van Dorsselaer A., Hoffmann J., Fothergill J. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(1):262–266. doi: 10.1073/pnas.86.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boulch P., Joulin V., Garel M. C., Rosa J., Cohen-Solal M. Molecular cloning and nucleotide sequence of murine 2,3-bisphosphoglycerate mutase cDNA. Biochem Biophys Res Commun. 1988 Oct 31;156(2):874–881. doi: 10.1016/s0006-291x(88)80925-2. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Pawluk A., Scopes R. K., Griffiths-Smith K. Isolation and properties of the glycolytic enzymes from Zymomonas mobilis. The five enzymes from glyceraldehyde-3-phosphate dehydrogenase through to pyruvate kinase. Biochem J. 1986 Aug 15;238(1):275–281. doi: 10.1042/bj2380275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. C., Duncan D., Ogg D. J. Purification and preliminary characterization of phosphoglycerate mutase from Schizosaccharomyces pombe. Int J Biochem. 1985;17(7):843–846. doi: 10.1016/0020-711x(85)90275-7. [DOI] [PubMed] [Google Scholar]
- Price N. C., Jaenicke R. The quaternary structure of phosphoglycerate mutase from yeast: evidence against dissociation of the tetrameric enzyme at low concentrations. FEBS Lett. 1982 Jul 5;143(2):283–286. doi: 10.1016/0014-5793(82)80117-8. [DOI] [PubMed] [Google Scholar]
- Price N. C., Stevens E. Distinction between cofactor-dependent and -independent phosphoglycerate mutases by chromatography on Cibacron Blue-Sepharose. Biosci Rep. 1983 Sep;3(9):857–861. doi: 10.1007/BF01133784. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki R., Sugimoto R., Chiba H. Yeast phosphoglyceric acid mutase-modifying enzyme. Arch Biochem Biophys. 1966 Jul;115(1):53–61. doi: 10.1016/s0003-9861(66)81037-8. [DOI] [PubMed] [Google Scholar]
- Shanske S., Sakoda S., Hermodson M. A., DiMauro S., Schon E. A. Isolation of a cDNA encoding the muscle-specific subunit of human phosphoglycerate mutase. J Biol Chem. 1987 Oct 25;262(30):14612–14617. [PubMed] [Google Scholar]
- Sharp P. M., Cowe E., Higgins D. G., Shields D. C., Wolfe K. H., Wright F. Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res. 1988 Sep 12;16(17):8207–8211. doi: 10.1093/nar/16.17.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Fothergill-Gilmore L. A. Development of a mutagenesis, expression and purification system for yeast phosphoglycerate mutase. Investigation of the role of active-site His181. Eur J Biochem. 1992 Jul 15;207(2):709–714. doi: 10.1111/j.1432-1033.1992.tb17099.x. [DOI] [PubMed] [Google Scholar]
- White M. F., Fothergill-Gilmore L. A. Sequence of the gene encoding phosphoglycerate mutase from Saccharomyces cerevisiae. FEBS Lett. 1988 Mar 14;229(2):383–387. doi: 10.1016/0014-5793(88)81161-x. [DOI] [PubMed] [Google Scholar]
- White P. J., Nairn J., Price N. C., Nimmo H. G., Coggins J. R., Hunter I. S. Phosphoglycerate mutase from Streptomyces coelicolor A3(2): purification and characterization of the enzyme and cloning and sequence analysis of the gene. J Bacteriol. 1992 Jan;174(2):434–440. doi: 10.1128/jb.174.2.434-440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn S. I., Watson H. C., Harkins R. N., Fothergill L. A. Structure and activity of phosphoglycerate mutase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):121–130. doi: 10.1098/rstb.1981.0066. [DOI] [PubMed] [Google Scholar]
- Yanagawa S., Hitomi K., Sasaki R., Chiba H. Isolation and characterization of cDNA encoding rabbit reticulocyte 2,3-bisphosphoglycerate synthase. Gene. 1986;44(2-3):185–191. doi: 10.1016/0378-1119(86)90181-2. [DOI] [PubMed] [Google Scholar]