Abstract
Equilibrium fluorescence methods have been used to establish a model for Ca2+ binding to the (Ca(2+)-Mg2+)-ATPase of skeletal muscle sarcoplasmic reticulum and to define the effects of H+ and Mg2+ on Ca2+ binding. The basic scheme proposed is: E2 <--> E1 <--> E1Ca <--> El'Ca <--> E1'Ca2. The E1 conformation of the ATPase initially has one high-affinity binding site for Ca2+ exposed to the cytoplasmic side of the sarcoplasmic reticulum, but in the E2 conformation this site is unable to bind Ca2+; Ca2+ does not bind to luminal sites on E2. The second, outer, Ca(2+)-binding site on the ATPase is formed after binding of Ca2+ to the first, inner, site on E1 and the E1Ca <--> E1'Ca conformation change. The pH- and Mg(2+)-dependence of the E2 <--> E1 equilibrium has been established after changes in the fluorescence of the ATPase labelled with 4-nitrobenzo-2-oxa-1,3-diazole. It is proposed that Mg2+ from the cytoplasmic side of the sarcoplasmic reticulum can bind to the first Ca(2+)-binding site on both E1 and E2. It is proposed that the change in tryptophan fluorescence intensity after binding of Ca2+ follows from the E1Ca <--> E1'Ca change. The pH- and Mg(2+)-dependence of this change defines H(+)- and Mg(2+)-binding constants at the two Ca(2+)-binding sites. It is proposed that the change in tryptophan fluorescence observed on binding Mg2+ follows from binding at the second Ca(2+)-binding site. Effects of pH and Mg2+ on the fluorescence of the ATPase labelled with 4-(bromomethyl)-6,7-dimethoxycoumarin are proposed to follow from binding to a site on the ATPase, the 'gating' site, which affects the affinity of the first Ca(2+)-binding site for Ca2+ and affects the rate of dissociation of Ca2+ from the ATPase.
Full text
PDF

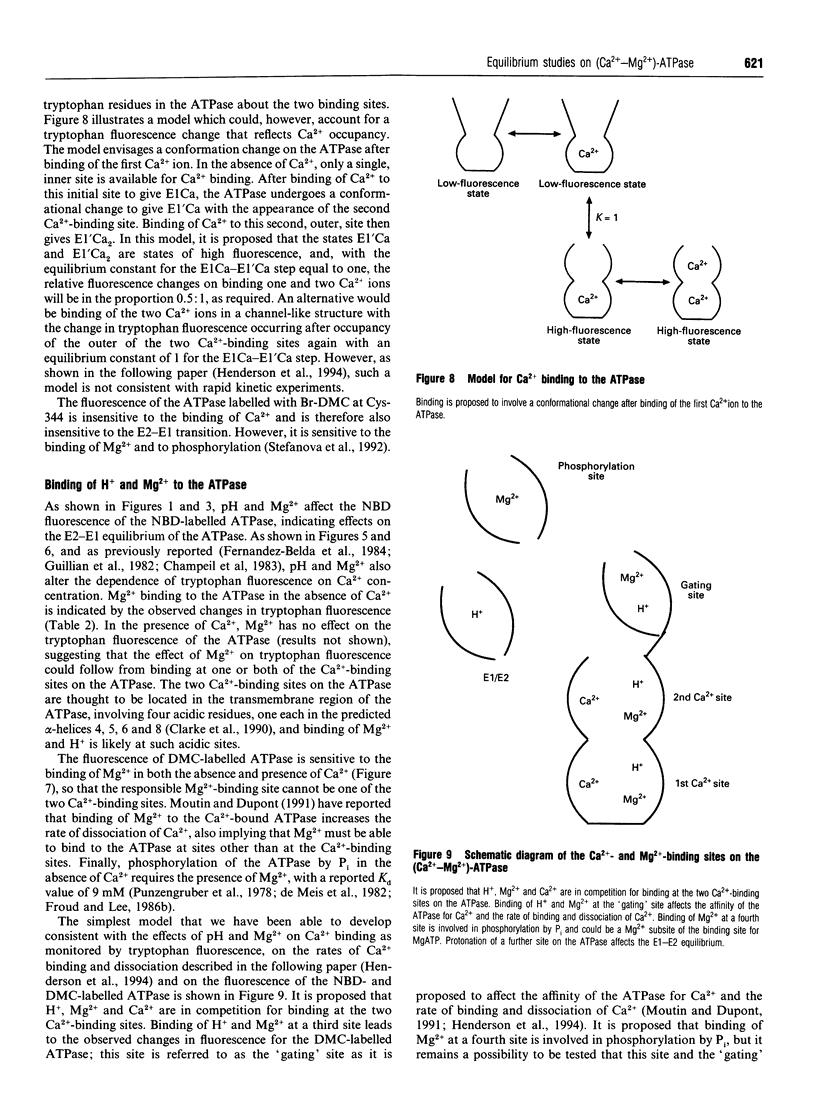
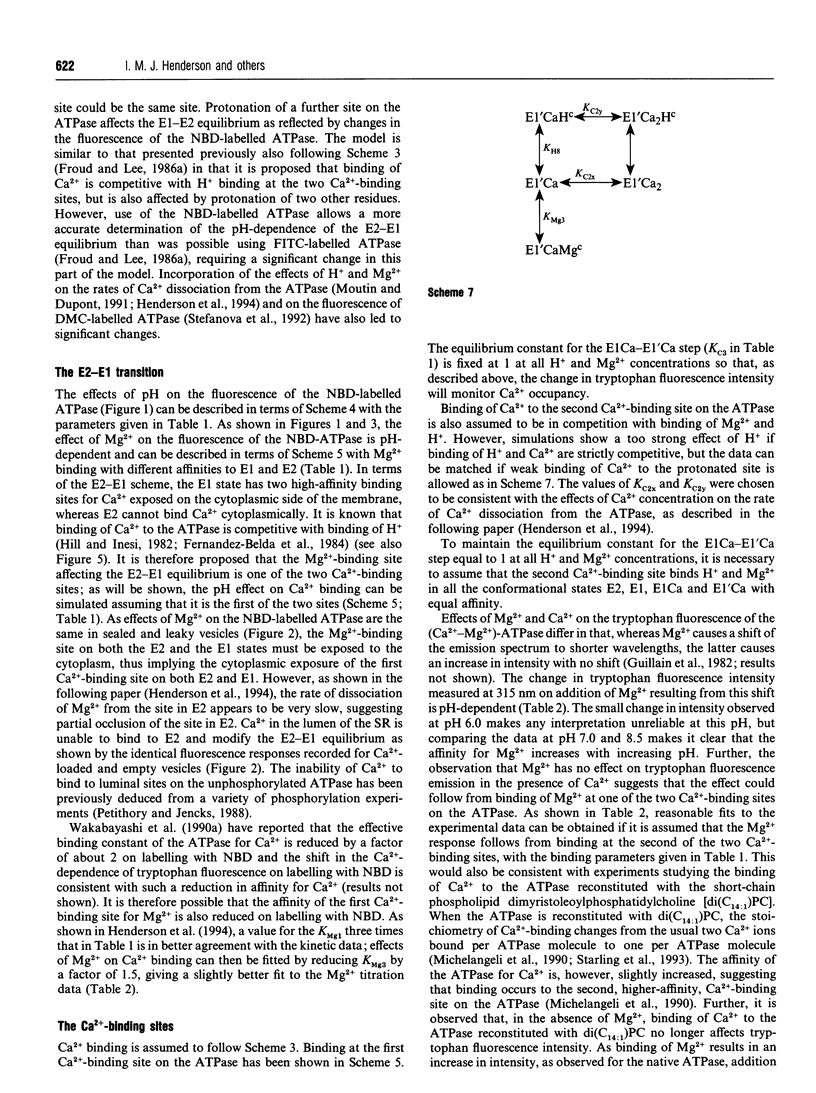


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Champeil P., Gingold M. P., Guillain F., Inesi G. Effect of magnesium on the calcium-dependent transient kinetics of sarcoplasmic reticulum ATPase, studied by stopped flow fluorescence and phosphorylation. J Biol Chem. 1983 Apr 10;258(7):4453–4458. [PubMed] [Google Scholar]
- Chiesi M., Inesi G. Adenosine 5'-triphosphate dependent fluxes of manganese and and hydrogen ions in sarcoplasmic reticulum vesicles. Biochemistry. 1980 Jun 24;19(13):2912–2918. doi: 10.1021/bi00554a015. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., MacLennan D. H. Functional consequences of alterations to polar amino acids located in the transmembrane domain of the Ca2(+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1990 Apr 15;265(11):6262–6267. [PubMed] [Google Scholar]
- Dupont Y. Fluorescence studies of the sarcoplasmic reticulum calcium pump. Biochem Biophys Res Commun. 1976 Jul 26;71(2):544–550. doi: 10.1016/0006-291x(76)90821-4. [DOI] [PubMed] [Google Scholar]
- Dupont Y., Leigh J. B. Transient kinetics of sarcoplasmic reticulum CA2+ + Mg2+ ATPase studied by fluorescence. Nature. 1978 Jun 1;273(5661):396–398. doi: 10.1038/273396a0. [DOI] [PubMed] [Google Scholar]
- Dupont Y. Low-temperature studies of the sarcoplasmic reticulum calcium pump. Mechanisms of calcium binding. Biochim Biophys Acta. 1982 May 21;688(1):75–87. doi: 10.1016/0005-2736(82)90580-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Belda F., Kurzmack M., Inesi G. A comparative study of calcium transients by isotopic tracer, metallochromic indicator, and intrinsic fluorescence in sarcoplasmic reticulum ATPase. J Biol Chem. 1984 Aug 10;259(15):9687–9698. [PubMed] [Google Scholar]
- Froud R. J., Lee A. G. A model for the phosphorylation of the Ca2+ + Mg2+-activated ATPase by phosphate. Biochem J. 1986 Jul 1;237(1):207–215. doi: 10.1042/bj2370207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froud R. J., Lee A. G. Conformational transitions in the Ca2+ + Mg2+-activated ATPase and the binding of Ca2+ ions. Biochem J. 1986 Jul 1;237(1):197–206. doi: 10.1042/bj2370197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., East J. M., Froud R. J., McWhirter J. M., Stefanova H. I., Lee A. G. A kinetic model for the Ca2+ + Mg2+-activated ATPase of sarcoplasmic reticulum. Biochem J. 1986 Jul 1;237(1):217–227. doi: 10.1042/bj2370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillain F., Champeil P., Lacapère J. J., Gingold M. P. Stopped flow and rapid quenching measurement of the transient steps induced by calcium binding to sarcoplasmic reticulum adenosine triphosphatase. Competition with Ca2+-independent phosphorylation. J Biol Chem. 1981 Jun 25;256(12):6140–6147. [PubMed] [Google Scholar]
- Guillain F., Gingold M. P., Büschlen S., Champeil P. A direct fluorescence study of the transient steps induced by calcium binding to sarcoplasmic reticulum ATPase. J Biol Chem. 1980 Mar 10;255(5):2072–2076. [PubMed] [Google Scholar]
- Guillain F., Gingold M. P., Champeil P. Direct fluorescence measurements of Mg2+ binding to sarcoplasmic reticulum ATPase. J Biol Chem. 1982 Jul 10;257(13):7366–7371. [PubMed] [Google Scholar]
- Henao F., Orlowski S., Merah Z., Champeil P. The metal sites on sarcoplasmic reticulum membranes that bind lanthanide ions with the highest affinity are not the ATPase Ca2+ transport sites. J Biol Chem. 1992 May 25;267(15):10302–10312. [PubMed] [Google Scholar]
- Henderson I. M., Starling A. P., Wictome M., East J. M., Lee A. G. Binding of Ca2+ to the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum: kinetic studies. Biochem J. 1994 Feb 1;297(Pt 3):625–636. doi: 10.1042/bj2970625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Inesi G. Equilibrium cooperative binding of calcium and protons by sarcoplasmic reticulum ATPase. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3978–3982. doi: 10.1073/pnas.79.13.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N., Garcia A. M., Kurobe Y., Scott T. L. Nonequivalent subunits in the calcium pump of sarcoplasmic reticulum. J Biol Chem. 1981 Aug 25;256(16):8593–8601. [PubMed] [Google Scholar]
- Ikemoto N., Morgan J. F., Yamada S. Ca2+-controlled conformational states of the Ca2+ transport enzyme of sarcoplasmic reticulum. J Biol Chem. 1978 Nov 25;253(22):8027–8033. [PubMed] [Google Scholar]
- Inesi G., Kurzmack M., Coan C., Lewis D. E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Apr 10;255(7):3025–3031. [PubMed] [Google Scholar]
- Inesi G. Sequential mechanism of calcium binding and translocation in sarcoplasmic reticulum adenosine triphosphatase. J Biol Chem. 1987 Dec 5;262(34):16338–16342. [PubMed] [Google Scholar]
- Lee A. G., East J. M., Jones O. T., McWhirter J., Rooney E. K., Simmonds A. C. Binding of dansyl propranolol to the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1983 Jul 27;732(2):441–454. doi: 10.1016/0005-2736(83)90061-5. [DOI] [PubMed] [Google Scholar]
- Mata A. M., Stefanova H. I., Gore M. G., Khan Y. M., East J. M., Lee A. G. Localization of Cys-344 on the (Ca(2+)-Mg(2+)-ATPase of sarcoplasmic reticulum using resonance energy transfer. Biochim Biophys Acta. 1993 Apr 8;1147(1):6–12. doi: 10.1016/0005-2736(93)90309-n. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Orlowski S., Champeil P., Grimes E. A., East J. M., Lee A. G. Effects of phospholipids on binding of calcium to (Ca2(+)-Mg2(+)-ATPase. Biochemistry. 1990 Sep 11;29(36):8307–8312. doi: 10.1021/bi00488a015. [DOI] [PubMed] [Google Scholar]
- Moutin M. J., Dupont Y. Interaction of potassium and magnesium with the high affinity calcium-binding sites of the sarcoplasmic reticulum calcium-ATPase. J Biol Chem. 1991 Mar 25;266(9):5580–5586. [PubMed] [Google Scholar]
- Orlowski S., Champeil P. Kinetics of calcium dissociation from its high-affinity transport sites on sarcoplasmic reticulum ATPase. Biochemistry. 1991 Jan 15;30(2):352–361. doi: 10.1021/bi00216a007. [DOI] [PubMed] [Google Scholar]
- Petithory J. R., Jencks W. P. Binding of Ca2+ to the calcium adenosinetriphosphatase of sarcoplasmic reticulum. Biochemistry. 1988 Nov 15;27(23):8626–8635. doi: 10.1021/bi00423a018. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Regulation of the conformation transition in the Ca-ATPase from sarcoplasmic reticulum by pH, temperature, and calcium ions. J Biol Chem. 1982 Jun 10;257(11):6120–6126. [PubMed] [Google Scholar]
- Pick U. The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J Biol Chem. 1982 Jun 10;257(11):6111–6119. [PubMed] [Google Scholar]
- Punzengruber C., Prager R., Kolassa N., Winkler F., Suko J. Calcium gradient-dependent and calcium gradient-independent phosphorylation of sarcoplasmic reticulum by orthophosphate. The role of magnesium. Eur J Biochem. 1978 Dec;92(2):349–359. doi: 10.1111/j.1432-1033.1978.tb12754.x. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Wade J. B., Inesi G. A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem. 1992 Jan 15;267(2):1286–1292. [PubMed] [Google Scholar]
- Scofano H., Barrabin H., Inesi G., Cohen J. A. Stoichiometric and electrostatic characterization of calcium binding to native and lipid-substituted adenosinetriphosphatase of sarcoplasmic reticulum. Biochim Biophys Acta. 1985 Sep 25;819(1):93–104. doi: 10.1016/0005-2736(85)90199-3. [DOI] [PubMed] [Google Scholar]
- Squier T. C., Bigelow D. J., Fernandez-Belda F. J., deMeis L., Inesi G. Calcium and lanthanide binding in the sarcoplasmic reticulum ATPase. J Biol Chem. 1990 Aug 15;265(23):13713–13720. [PubMed] [Google Scholar]
- Stahl N., Jencks W. P. Reactions of the sarcoplasmic reticulum calcium adenosinetriphosphatase with adenosine 5'-triphosphate and Ca2+ that are not satisfactorily described by an E1-E2 model. Biochemistry. 1987 Dec 1;26(24):7654–7667. doi: 10.1021/bi00398a019. [DOI] [PubMed] [Google Scholar]
- Starling A. P., East J. M., Lee A. G. Effects of phosphatidylcholine fatty acyl chain length on calcium binding and other functions of the (Ca(2+)-Mg2+)-ATPase. Biochemistry. 1993 Feb 16;32(6):1593–1600. doi: 10.1021/bi00057a025. [DOI] [PubMed] [Google Scholar]
- Stefanova H. I., East J. M., Gore M. G., Lee A. G. Labeling the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum with 4-(bromomethyl)-6,7-dimethoxycoumarin: detection of conformational changes. Biochemistry. 1992 Jul 7;31(26):6023–6031. doi: 10.1021/bi00141a010. [DOI] [PubMed] [Google Scholar]
- Verjovski-Almeida S., Silva J. L. Different degrees of cooperativity of the Ca2+-induced changes in fluorescence intensity of solubilized sarcoplasmic reticulum ATPase. J Biol Chem. 1981 Mar 25;256(6):2940–2944. [PubMed] [Google Scholar]
- Wakabayashi S., Imagawa T., Shigekawa M. Does fluorescence of 4-nitrobenzo-2-oxa-1,3-diazole incorporated into sarcoplasmic reticulum ATPase monitor putative E1-E2 conformational transition? J Biochem. 1990 Apr;107(4):563–571. doi: 10.1093/oxfordjournals.jbchem.a123087. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Ogurusu T., Shigekawa M. Participation of H+ in the Ca2(+)-induced conformational transition of 4-nitro-2,1,3-benzoxadiazole-labeled sarcoplasmic reticulum ATPase. Biochemistry. 1990 Nov 27;29(47):10613–10620. doi: 10.1021/bi00499a006. [DOI] [PubMed] [Google Scholar]
- Wictome M., Henderson I., Lee A. G., East J. M. Mechanism of inhibition of the calcium pump of sarcoplasmic reticulum by thapsigargin. Biochem J. 1992 Apr 15;283(Pt 2):525–529. doi: 10.1042/bj2830525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wictome M., Michelangeli F., Lee A. G., East J. M. The inhibitors thapsigargin and 2,5-di(tert-butyl)-1,4-benzohydroquinone favour the E2 form of the Ca2+,Mg(2+)-ATPase. FEBS Lett. 1992 Jun 15;304(2-3):109–113. doi: 10.1016/0014-5793(92)80599-c. [DOI] [PubMed] [Google Scholar]
- de Meis L., de Souza Otero A., Martins O. B., Alves E. W., Inesi G., Nakamoto R. Phosphorylation of sarcoplasmic reticulum ATPase by orthophosphate in the absence of Ca2+ gradient. Contribution of water activity to the enthalpy and the entropy changes. J Biol Chem. 1982 May 10;257(9):4993–4998. [PubMed] [Google Scholar]


