Abstract
Objective
We aimed to evaluate the effect of light–dark cycle alteration and soft drink consumption on the acceleration of type 1 diabetes mellitus (T1DM) development among non-obese diabetic (NOD) mice model.
Methods
We exposed female NOD and C57BL/6 mice from the age of 5 weeks to either adlib soft drink consumption and/or T20 light–dark cycle alteration until the development of diabetes, or the mice reached the age of 30 weeks. Each group consisted of 7–15 mice. We monitored weight, length, blood glucose level, and insulin autoantibody (IAA) levels weekly.
Results
Out of 75 NOD and 22 C57BL/6 mice, 41 NOD mice developed diabetes, and 6 mice died between 7 and 8 weeks of age. The mean time to development of T1DM among NOD control mice was 20 weeks. The time to development of T1DM was accelerated by two weeks in the NOD mice exposed to light–dark cycle alteration, hazard ratio of 2.65,95th CI (0.70, 10.04) p = 0.15). The other groups developed T1DM, similar to the control group.
Conclusion
There was a trend toward earlier development of T1DM among NOD mice exposed to light–dark cycle alteration, but this difference was not statistically significant. Further studies are needed to confirm our findings using larger sample sizes and different animal species.
Keywords: type 1 diabetes mellitus, soft drink, sugar, light-dark cycle alteration, NOD/ShiLtJ
Introduction
Type 1 Diabetes Mellitus (T1DM) incidence has increased notably in the last 10 years worldwide.1,2 Although T1DM development is linked to genetic susceptibilities, especially with human leukocyte antigen (HLA class II), several studies have indicated that genetic susceptibility is insufficient to lead to autoimmunity.3 The exact T1DM pathogenesis is still not fully understood, but it is believed to be multifactorial, where both polygenic and environmental factors interact or contribute to the development of T1DM.4
One environmental factor that has gained attention in recent years is the alteration of the light–dark cycle, which can impact the immune system and glucose metabolism.5,6 Circadian rhythms, which are synchronized to the natural light–dark cycle, play a crucial role in regulating various physiological processes, including immune function and glucose homeostasis.5,7 Alteration of the light–dark cycle, as seen in shift work or exposure to artificial light at night, can lead to dysregulation of the immune system and alterations in glucose metabolism, potentially contributing to the development or acceleration of T1DM.8–10 Experimental research has shown that light–dark cycle alteration can affect the level of circulating lymphocytes, natural killer cells, antibodies, and cytokines, resulting in immune system dysregulation.5,11
Moreover, sleep alteration and circadian misalignment have been associated with β-cell dysfunction and a loss of β-cell mass.12 In addition to circadian rhythm alteration, excessive soft drink consumption has been linked to an increased risk of progression to T1DM among children.13,14 In light of the T1DM epidemic and the considerable healthcare costs associated with diabetes, policymakers, physicians, and the public need to understand the factors leading to the acceleration of T1DM to create new policies that offer primary disease prevention, reduction of the global disease burden, and plan for future healthcare resource distribution. By focusing on the factors influencing circadian rhythm alteration and the effects of soft drink consumption, this study will provide valuable insights into the potential role of environmental factors in the development and progression of T1DM and inform future research and interventions in this area. We hypothesized that light–dark cycle alteration and soft drink consumption accelerate the development of T1DM in NOD mice models by altering immune system function and glucose metabolism, leading to an increased risk of autoimmune destruction of pancreatic β-cells. Therefore, this study was conducted to investigate the impact of light–dark cycle alteration and soft drink consumption on the acceleration of T1DM development in NOD mice models, and to explore the potential underlying mechanisms that may contribute to this acceleration. The NOD mice model is the closest animal model that is similar to the T1DM in humans.
Methods
Animal Preparation and Housing Conditions
We purchased NOD/ShiLtJ mice aged 4 weeks post-weaning from Jackson Laboratory, Bar Harbor, ME, USA. Additionally, we obtained 22 C57BL/6 mice from the Experimental Surgery and Animal Laboratory at the College of Medicine, King Saud University in Riyadh, Saudi Arabia. We chose female NOD mice due to their well-established disease course. NOD females develop diabetes at a significantly higher rate (around 80% by week 25) compared to males, whose phenotype progresses slower and with lower overall incidence.15,16 This selection facilitated experimental feasibility and allowed for a more efficient study design.
The experiment started one week after the animals’ arrival from the USA to allow the mice to acclimate to the new laboratory environment. All experimental procedures were performed in line with the rules and approved by the Research Ethics Committee (REC) at King Saud University, ethical approval number: KSU-SE-21-32 on 22/04/2021.
Interventions
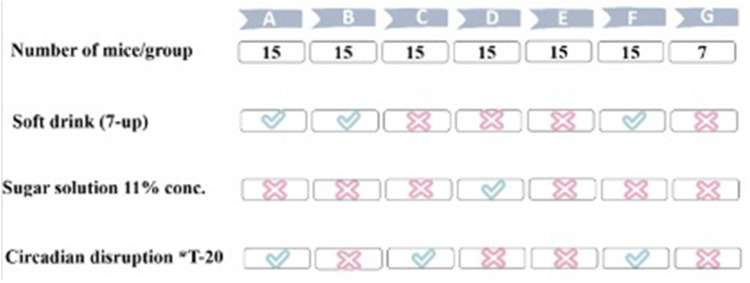
Female mice, aged five weeks, were randomly divided into seven groups labelled A through G. Groups A to F each consisted of 15 mice, while the control group (C57BL/6) had 7 mice (Figure 1). The interventions included an 11% ad libitum sugar solution, ad libitum decarbonated uncaffeinated regular 7-UP soft drink (PepsiCo®, Saudi Arabia), and/or an alteration in the light/dark (L/D) cycle.
Figure 1.
Animal Experimental Groups.
The nutritional content per 100 mL of 7-UP, is as follows: total carbohydrates, 11 g; total sugars, 11 g (all added sugars); sodium, 23 mg. While the 11% sucrose solution was prepared by dissolving 11 g of table sugar (sucrose) in 100 mL of tap water. This concentration was chosen to match the sugar content in 7-UP, allowing us to isolate the effects of sugar content without the additional ingredients present in the beverage. Both drinks provide 44 kcal per 100 mL.
Mice were exposed to a light/dark (LD) cycle alteration using the T-20 method, which entails 10 hours of light followed by 10 hours of dark.17,18 Each chamber was illuminated using solid-state, electromagnetic fluorescent ballasts with rapid-start, cool-white lamps. These lamps were installed behind the mice cage racks and were connected to a separate 24-hour timer, set for a cycle of 10 hours light and 10 hours dark. To ensure isolation from the room’s ambient LD conditions, cages were covered with a black cover (Figure 2). The experimental groups were as follows:
Figure 2.
Light dark cycle Alteration setup. The cages at the bottom were covered with a black cover to ensure isolation from the room’s ambient L/D conditions. The lamps were installed behind the mice cage racks and were connected to a separate 24-hour timer, set for a cycle of 10 hours light and 10 hours dark.
- Group A: NOD mice (soft drink + T-20 LD alteration).
- Group B: NOD mice (Soft drink + T-24 standard/default diurnal LD (12-hr light: 12-hr dark (12L: 12D) with the lights being ON during the daytime (eg, lights are set to turn ON at 7:00 am and turn OFF at 7:00 pm).19
- Group C: NOD mice (T-20 LD alteration).
- Group D: NOD mice (11% sugar solution + T-24 standard/default diurnal LD).
- Group E: NOD mice (control) (T-24 standard/default diurnal LD).
- Group F: C57BL/6 mice (soft drink + T-20 LD alteration).
- Group G: C57BL/6 mice (control) (T-24 standard/default diurnal LD).
All mice underwent these interventions either until they reached 30 weeks of age or until they developed T1DM. The mice were housed in standard cages, three per cage, with ad libitum access to water and a standard rodent chow diet, which is a complete life cycle diet formulated using a managed formulation. This diet is recommended for rats, mice, hamsters, and gerbils, and is widely used in laboratory settings.20 Their environment was maintained in controlled rooms with a temperature of 23°C and a humidity ranging from 45% to 50%.
Measures
Weekly, we measured the mice’s weight, length, food consumption, and intake of either soft drinks or the sugar solution. Additionally, we withdrew 0.1 mL of blood from each mouse. Blood collection was done from the facial vein using the submandibular bleeding method while the mice were under anesthesia, achieved through inhalation of sevoflurane (provided by Tabuk Pharmaceuticals, KSA). The collected blood was used to measure glucose, insulin autoantibody (IAA), and corticosterone concentrations.
Glucose levels were determined weekly using the OneTouch Select Plus glucometer (LifeScan, UK). Readings exceeding 250 mg/dL were re-measured the following day to confirm hyperglycemia. After blood collection, the serum was separated by centrifugation at 3000 rpm for 10 minutes at 4°C. The circulating IAA concentration in the serum was assessed using the Mouse IAA ELISA Kit (Mybiosource, cat.no MBS763701). Measurements were taken at the following ages for all groups: 8, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, and 28 weeks. Corticosterone concentration in the serum was measured at 16 weeks of age for all groups using the Mouse Corticosterone ELISA Kit (Mybiosource, cat.no MBS2505570), adhering to the manufacturer’s instructions.
Outcomes
The primary outcome was time to development of stage 2 diabetes by finding glucose of >250 mg/dl on two consecutive days. Secondary outcomes included changes in the IAA concentration, and serum corticosterone.
Statistical Analysis
The sample size was calculated based on the primary outcome, which is the time to development of stage 2 T1DM. To detect a 3-week difference between the intervention and NOD control group, with an alpha level of 0.05 and a power of 0.80, and accounting for an estimated 15% loss to follow-up, we aimed to study 15 mice per group.
Continuous, normally distributed data were described using mean ± standard deviation (SD), while non-normally distributed data were described using median and interquartile range (IQR). Weekly changes in weight, length, food consumption, and soft drink/sugar solution intake across the groups were estimated using generalized equation estimation (GEE) modelling.
Survival analysis was performed using Cox proportional hazard models to assess the time to diabetes development among the experimental and NOD control groups. The hazard ratio of diabetes development up to age 20 weeks was estimated, as the risk of further development is expected to be similar based on NOD biology.
For skewed data, we performed log transformation. We then conducted a one-way analysis of variance (ANOVA) tests with Bonferroni adjustment to test differences in IAA, and corticosterone levels among the experimental groups and the control NOD mice. Additionally, we assessed the difference in IAA and corticosterone levels between the light–dark (LD) alteration and control groups, adjusted for diabetes status, using a linear regression model with bootstrapping of 1000 samples to improve the statistical power to detect a difference.
Results
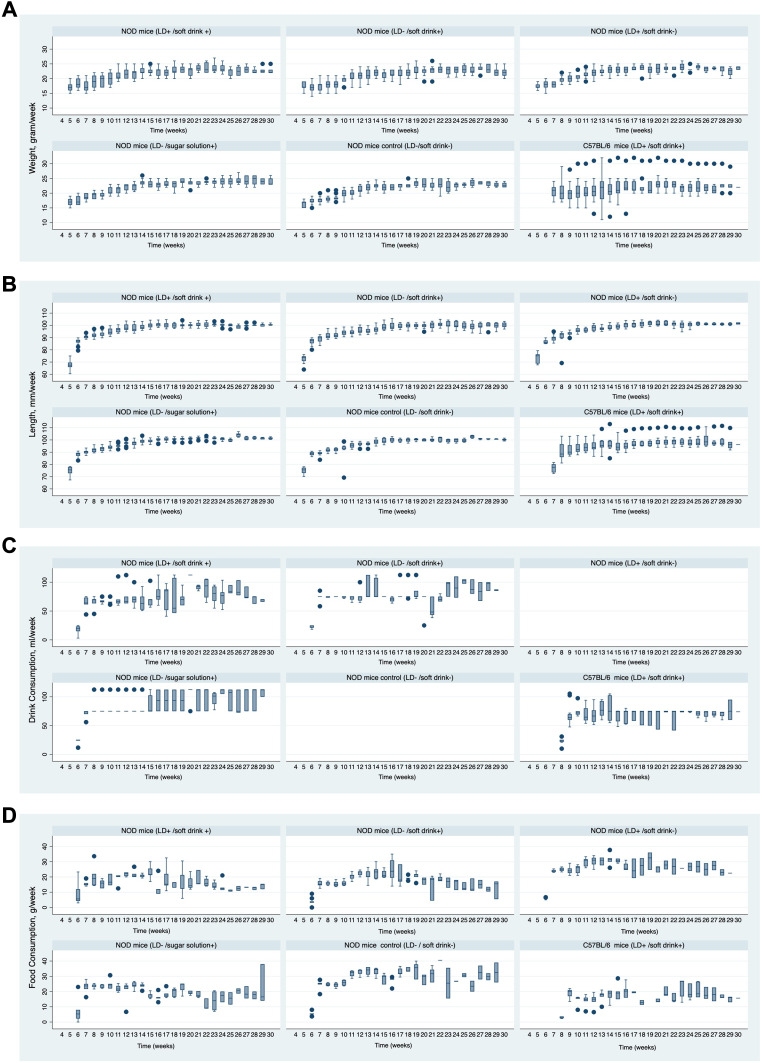
A total of 75 NOD and 22 C57BL/6 mice were exposed to the interventions for up to 30 weeks. Out of these, 41 NOD mice developed diabetes and were euthanized, while six mice were found dead in the cage (one from the 11% sugar solution group, three from the control NOD group, and three from the soft drink + LD alteration C57BL/6 group). Those mice passed away at 7–8 weeks of age and had euglycemia in the days prior to death. The baseline measurements of weight, length, food consumption, and drink consumption are provided in the appendix Table 1. The mean incremental increases in weight and length were similar between the intervention groups and the control group, with the exception of the group that received the 11% sugar solution (Table 1, Figure 3). In this group, the sugar solution significantly induced an average weekly body weight gain of 1.34 grams and an average weekly length increase of 1.75 mm compared to the NOD control group.
Table 1.
Weekly Changes in Anthropometric Measurements, Food Intake, and Drink Consumption Compared to NOD Control
| Variable | Groups | Mean Difference | Std. Err. | P-value | 95% Confidence Interval |
|---|---|---|---|---|---|
| Weight, gram/week | A.NOD mice (LD+ /soft drink +) | 0.74 | 0.47 | 0.11 | −0.18, 1.65 |
| B.NOD mice (LD- /soft drink+) | 0.08 | 0.57 | 0.89 | −1.04, 1.20 | |
| C.NOD mice (LD+ /soft drink-) | 0.75 | 0.50 | 0.13 | −0.23, 1.73 | |
| D.NOD mice (LD- /sugar solution+) | 1.34 | 0.45 | 0.00* | 0.47, 2.21 | |
| F. C57BL/6 mice (LD+ /soft drink+) | 1.30 | 1.06 | 0.22 | −0.78, 3.38 | |
| Length, mm/week | A.NOD mice (LD+ /soft drink +) | 0.83 | 0.96 | 0.39 | −1.05, 2.70 |
| B.NOD mice (LD- /soft drink+) | 0.39 | 1.20 | 0.75 | −1.96, 2.73 | |
| C.NOD mice (LD+ /soft drink-) | 0.72 | 1.20 | 0.55 | −1.63, 3.08 | |
| D.NOD mice (LD- /sugar solution+) | 1.76 | 0.89 | 0.05 * | 0.02, 3.50 | |
| F. C57BL/6 mice (LD+ /soft drink+) | 1.86 | 1.78 | 0.30 | −1.62, 5.33 | |
| Food Consumption, g/week | A.NOD mice (LD+ /soft drink +) | −9.64 | 0.94 | < 0.00* | −11.49, −7.79 |
| B.NOD mice (LD- /soft drink+) | −9.60 | 0.97 | < 0.00* | −11.51, −7.70 | |
| C.NOD mice (LD+ /soft drink-) | −1.62 | 0.94 | 0.09 | −3.46, 0.23 | |
| D.NOD mice (LD- /sugar solution+) | −7.73 | 0.79 | < 0.00* | −9.28, −6.18 | |
| F. C57BL/6 mice (LD+ /soft drink+) | −11.03 | 0.84 | < 0.00* | −12.68, −9.38 | |
| Drink Consumption, mL/week | NOD mice (LD- /soft drink+) vs NOD mice (LD- /sugar solution+) | −9.65 | 2.59 | < 0.00* | −14.72, −4.57 |
| NOD mice (LD- /soft drink+) vs C57BL/6 mice (LD+ /soft drink+) | 6.12 | 2.85 | 0.03* | 0.53, 11.70 | |
| NOD mice (LD+ /soft drink +) vs NOD mice (LD- /soft drink+) | −4.63 | 2.80 | 0.10 | −10.12, 0.85 | |
| NOD mice (LD+ /soft drink +) vs C57BL/6 mice (LD+ /soft drink+) | 1.26 | 3.57 | 0.72 | −5.73, 8.25 |
Notes: All variables are compared against the NOD control group except drink consumption. Comparison is done via generalized estimating equation (GEE) population-averaged model. The asterisk (*) represents the statistical difference (p < 0.05).
Figure 3.
Panel (A–D) shows the weekly changes in weight, length, food intake, drink intake in light dark cycle alteration compared to NOD control group.
In all mice exposed to soft drinks or sugar solution, food consumption was reduced by an average of 7 to 11g/week/mouse, except for the group with LD cycle alteration. The average weekly food consumption for the control NOD mice was significantly higher by a mean of 27.64 g/week/mouse compared to all intervention groups, except for the LD alteration group, where the average intake was similar to the control group by an average of 25.71 g/week/mouse. Although both soft drink and sugar solution groups showed a significant reduction in food intake, the amount of soft drink consumed (mean of 74.88 mL/week/mouse) was significantly less than the sugar solution (mean of 84.74 mL/week/mouse), with a mean difference of −9.65 mL/week (p < 0.00).
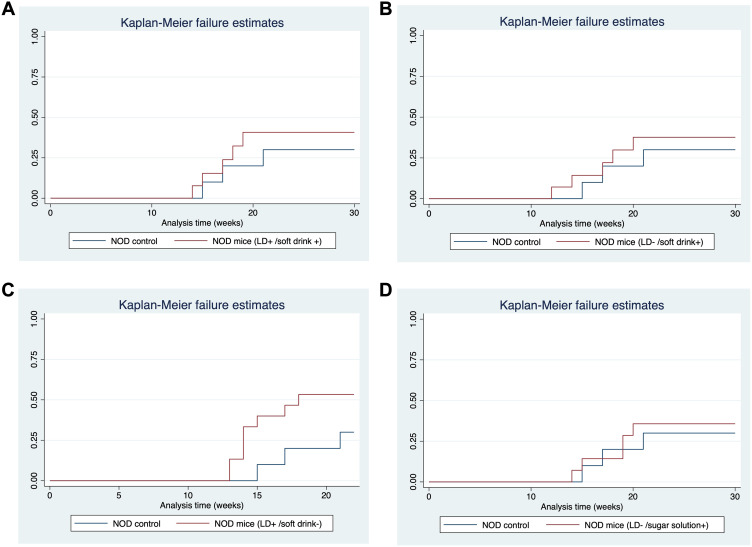
The time to T1DM development was accelerated by LD cycle alteration in the NOD mice compared to the control NOD mice. The mean age of T1DM development in the control NOD group was 20 weeks, while the mean age of T1DM development in the LD alteration group was 18 weeks, with the earliest mice developing diabetes at the age of 12 weeks. The hazard ratio (HR) was 2.65, with a 95% confidence interval (CI) of (0.70, 10.04) and a p-value of 0.15 (Table 2, Figure 4). Although the difference was not statistically significant, the incidence rate had doubled compared to the control group. Moreover, none of the C57BL/6 mice developed diabetes.
Table 2.
Time of Type 1 Diabetes Development
| Groups | Hazard Ratio | Std. Err. | P-value | Incidence Rate | Mean Age at Diabetes (weeks) |
95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| A | NOD mice LD+ /soft drink + |
1.54 (0.37, 6.45) | 1.12 | 0.56 | 0.02 | 24 | 20.80, 28.28 |
| B | NOD mice LD- /soft drink+ |
1.44 (0.34, 6.03) | 1.05 | 0.62 | 0.02 | 24.80 | 21.00, 28.5 |
| C | NOD mice LD+ /soft drink- |
2.65 (0.70, 10.04) | 1.8 | 0.15 | 0.03 | 18 | 16.20, 20.07 |
| D | NOD mice LD- /sugar solution+ |
1.29 (0.31, 5.42) | 0.94 | 0.73 | 0.01 | 25 | 22.24, 28.75 |
| E | Control NOD mice | N/A | N/A | N/A | 0.01 | 20.70 | 19.21, 22.19 |
| F | C57BL/6 LD+ /soft drink+ |
None developed diabetes | |||||
Notes: Comparisons are against NOD control via survival analysis using cox proportional hazard.
Figure 4.
Comparison of time to T1DM development against NOD control. (A) NOD mice subjected to soft drink. (B) NOD mice subjected to sugar solution. (C) NOD mice subjected to LD alteration. (D) NOD mice subjected to both soft drink and light dark cycle alteration.
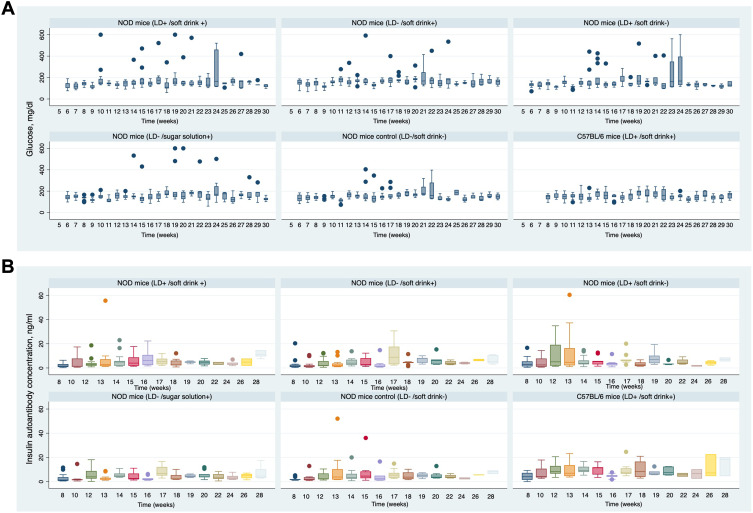
The changes in IAA concentration at ages 8, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, and 28 weeks are shown in Figure 5. Both the NOD LD alteration group and the NOD control group showed a rapid increase in IAA concentration, with the peak reached at 12 weeks (median IAA concentration = 5.71 ng/mL, interquartile range (IQR) = 1.32 to 25.49). The increase in IAA concentration from 8 to 12 weeks in the LD alteration group was 8.5 ng/mL, compared to 3.5 ng/mL in the control NOD group P-value 0.04. Overall, the IAA level at 12 weeks in the LD alteration group was similar to the control group, except when adjusted for diabetes status. Mice that developed diabetes had significantly higher serum IAA levels at 12 weeks, with an increase of 1.37 optical density (OD) units (p = 0.00) (Table 3).
Figure 5.
Panel (A–B) shows the weekly changes in blood glucose and Insulin autoantibody (IAA) development in LD alteration compared to NOD control group.
Table 3.
Association Between IAA and Corticosterone Level in NOD Mice Subjected to LD Alteration Compared to Control NOD at Age of 12 and 16 Weeks Respectively
| Variable | Coefficient | Std. Err. | P-value | 95% Confidence Interval | |
|---|---|---|---|---|---|
| IAA level at 12 weeks | NOD (LD+ / soft drink -) vs NOD mice (LD- / soft drink -) | 0.27 | 0.45 | 0.54 | −0.60, 1.15 |
| Diabetes vs non-diabetes | 1.37 | 0.47 | 0.00* | 0.44, 2.30 | |
| Corticosterone Level at 16 weeks | NOD (LD+ / soft drink -) vs NOD mice (LD- / soft drink -) | −0.08 | 0.36 | 0.83 | −0.78, 0.62 |
| Diabetes vs no non-diabetes | 0.57 | 0.28 | 0.05* | 0.01, 1.12 | |
Notes: Linear regression model to assess the comparison against NOD control with bootstrap of 1000 samples, variables in the model have been log-transformed. The asterisk (*) represents the statistical difference (p < 0.05).
Serum corticosterone concentrations were generally similar across all groups in the study. However, a significant reduction in corticosterone levels was observed in C57BL/6 mice exposed to both LD alteration and soft drinks, compared to the non-obese diabetic (NOD) control group (log-transformed mean = 2.55 ng/mL, p < 0.00). At 16-weeks, corticosterone concentration in the LD alteration group was significantly higher than the NOD control group by log-transformed mean = 0.57 ng/mL, p = 0.05 when considering diabetes status (Table 3).
Discussion
Our study is the first to assess the impact of early life chronic exposure to LD cycle alteration and soft drink consumption as modern life environmental factors on the acceleration of time T1DM development in genetically predisposed NOD mice. The mice that were exposed to LD alteration developed diabetes earlier by two weeks compared to the NOD control group, with the earliest mice seroconverting very early at 12 weeks. Although statistically, it was not a significant difference, the observed acceleration is considered huge when translated into human age. This observation can partly explain the increased incidence of T1DM among children in the last 10 years.
The chronic exposure to LD rhythm alteration leading to β-cells stress is supported by a higher IAA concentration at 12 weeks just before the appearance of hyperglycemia and higher corticosterone level when adjusted to the diabetes status of the mice. While, there was a similar risk of diabetes development in other interventions compared to the NOD control mice. At the same time, diabetes did not develop in any of the C57BL/6 mice as expected.
The effect of sleep alteration is not only associated with immune system dysfunction but also with β-cells dysfunction.21 Rodent studies showed that LD alteration leads to a decline in insulin secretion, accelerates the incidence of β-cells dysfunction, and contributes to changes within the pancreatic structure in rodent models.6,22 All these observations support the hypothesis that β-cells stress might accelerate the autoimmunity with genetic T1DM susceptibility.
Moreover, the alteration in melatonin circadian rhythm secretion has been linked to various autoimmune diseases, including T1DM, where pinealectomy in NOD mice accelerates the development of autoimmune diabetes, which emphasizes the importance of steady melatonin secretion.23,24 In line with the observed acceleration of T1DM incidence in the LD alteration group, many countries have observed an increase in T1DM incidence during the COVID lockdown period and the following years.25–28
While T1DM development was similar among soft drink (7-up) sugar solution of 11% concentration and NOD control groups, our study supports that sugar-induced stress on mice β-cells follows a gradient-dependent response compared to human β-cell. In a previous study where β-cells were exposed to glucose, the mice β-cells showed a response to increased insulin demand via the remarkable ability to proliferate sufficient β-cell mass responding to rising glucose compared to human β-cells.29–31 Therefore, we speculate that NOD β-cells might exhibit a U-shaped effect in proliferation and ER stress, where under lower concentration it leads to β-cells proliferation and under higher glucose concentration it leads to accelerated ER stress and apoptosis. This was seen in the recent work by Li et al they allowed NOD/Ltj mice ad libitum intake of strictly 20% glucose water starting at 4 weeks of age (about 1 gram daily). NOD mice exposed to the high glucose intake developed diabetes early at 12 weeks with a mean difference of 2 weeks compared to the control group. There was upregulation of endoplasmic reticulum (ER) stress markers and higher IAA concentration. Similar to our study, they noticed a reduction in the consumed food by 50% compared to control NOD mice. Therefore, we assume that the compensatory reduction in food intake in our study, combined with the lower glucose concentration of 11%, had led to a reduction in total consumed carbohydrates weekly. In humans, previous cohort studies observed faster diabetes progression among children who consumed higher glycemic index beverages the year before T1DM clinical onset.14,32,33 Therefore, future studies are needed to test different concentrations of glucose in the induction of T1DM.
The similarity in corticosterone among groups could be explained by the impact of stress during handling and sampling techniques during blood withdrawal. LD alteration in NOD mice led to significantly increased corticosterone levels only among mice who developed diabetes. Disrupted LD cycles can directly impact the circadian regulation of corticosterone secretion. This process is centrally regulated by the suprachiasmatic nucleus in the hypothalamus, which synchronizes with exposure to light and stress.34,35 Chronic LD alteration may lead to elevated corticosterone levels, a potential contributor to T1DM development through several mechanisms.36–44 Chronic ER stress is one of the leading mechanisms to β-cell stress and autophagy initiating the T-cell mediated β-cell destruction. Furthermore, chronic stress, potentially induced by elevated corticosterone, is known to induce insulin resistance, which in turn increases insulin demand on β-cells.45–47 This prolonged insulin demand can lead to ER stress and adaptive unfolded protein response (UPR) leading to the activation of pro-apoptotic signalling pathways, ultimately resulting in β-cell death.47–49 Future studies should investigate the specific mechanisms by which LD alteration interacts with the diabetic state to elevate corticosterone and explore its potential role in different pathways leading to T1DM development in NOD mice.
Our study limitations include that the mice were exposed to travel and a long shipment process, which might be considered a stressful factor that affected our primary outcome, T1DM development. The time of T1DM development was delayed in our control NOD mice by 2 weeks compared to the Jackson lab NOD mice colony and other labs15,50 However, there was no other way to conduct the experiment on mice breaded locally because this strain is very difficult to breed in regular animal laboratories. Second, the weekly handling blood extraction might have impacted the corticosterone level and the mice growth. Third, the early mortality of six mice during the study warrants further investigation. While the cause is uncertain, the stress of international travel and acclimatization to the new environment could be a contributing factor.51–54 In conclusion, the early chronic effect of LD alteration as modern environmental factors led to earlier T1DM development in NOD mice. The observed acceleration by two weeks in mice is large and could explain the recent increase in diabetes incidence in Saudi Arabia and worldwide. Confirmatory study with a larger sample size and or different animal species is needed to inform the policymakers. The introduction of sugar-containing beverages in all interventions led to reduced chow consumption, and overgrowth only in the sugar solution group. This observation is important for those dealing with childhood obesity policymakers to put forth recommendations regarding sugar caloric drinks consumption in children.
Acknowledgments
We would like to thank the University Diabetes Centre for funding this work, as well OneTouch for providing the glucose strips to measure the glucose level. Special thanks for support by the College of Medicine Research Centre, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia. In addition, we would like to acknowledge the following colleagues for their contribution during the experimental work (Dr. Mohammad Shamsul Ola, Mr. Ahmed Almenayzel, Mr. Hisham Aloudah).
Abbreviations
NOD, non-obese diabetic; T1DM, Type 1 diabetes mellitus; IAA, insulin autoantibody; LD, light-dark.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.IDF. IDF diabetes atlas 10 TH edition. International Diabetes Federation (2021).
- 2.Ogle GD, Wang F, Gregory GA, Maniam J IDF ATLAS REPORTS. (2022).
- 3.McDevitt HO. Characteristics of autoimmunity in type 1 diabetes and type 1.5 overlap with type 2 diabetes. Diabetes. 2005;54(Suppl 2):S4–10. doi: 10.2337/diabetes.54.suppl_2.S4 [DOI] [PubMed] [Google Scholar]
- 4.Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–2339. doi: 10.1016/S0140-6736(16)30582-7 [DOI] [PubMed] [Google Scholar]
- 5.Jerigova V, Zeman M, Okuliarova M. Circadian disruption and consequences on innate immunity and inflammatory response. Int J Mol Sci. 2022;23(22):13722. doi: 10.3390/ijms232213722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale J, Cox HI, Qian J, et al. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26(5):423–433. doi: 10.1177/0748730411416341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan X, Chen D, Wang Y, et al. Light intensity alters the effects of light-induced circadian disruption on glucose and lipid metabolism in mice. Am J Physiol Endocrinol Metab. 2022;322(1):E1–E9. doi: 10.1152/ajpendo.00025.2021 [DOI] [PubMed] [Google Scholar]
- 8.Mason IC, Qian J, Adler GK, Scheer FAJL. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020;63(3):462–472. doi: 10.1007/s00125-019-05059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3(4):372–383. doi: 10.1016/j.molmet.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young J, Waclawski E, Young JA, Spencer J. Control of type 1 diabetes mellitus and shift work. Occup Med. 2013;63(1):70–72. doi: 10.1093/occmed/kqs176 [DOI] [PubMed] [Google Scholar]
- 11.Abo SMC, Layton AT. Modeling the circadian regulation of the immune system: sexually dimorphic effects of shift work. PLoS Comput Biol. 2021;17(3):e1008514. doi: 10.1371/journal.pcbi.1008514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–34. doi: 10.1073/pnas.1418955112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen M, Jarvis SE, Tinajero MG, et al. Sugar-sweetened beverage consumption and weight gain in children and adults: a systematic review and meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2023;117(1):160–174. doi: 10.1016/j.ajcnut.2022.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Lamb MM, Frederiksen B, Seifert JA, et al. Sugar intake is associated with progression from islet autoimmunity to type 1 diabetes: the diabetes autoimmunity study in the young. Diabetologia. 2015;58(9):2027–2034. doi: 10.1007/s00125-015-3657-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.In’t Veld P. Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin Immunopathol. 2014;36(5):569–579. doi: 10.1007/s00281-014-0438-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen Y. Development of the nonobese diabetic mouse and contribution of animal models for understanding type 1 diabetes. Pancreas. 2017;46(4):455–466. doi: 10.1097/MPA.0000000000000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dauchy RT, Dauchy EM, Tirrell RP, et al. Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp Med. 2010;60(5):348–356. [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan S, Foster RG, Vyazovskiy VV, Peirson SN. Effects of circadian misalignment on sleep in mice. Sci Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-33480-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IACUC Guidelines. Kansas State University. Available from: https://www.k-state.edu/comply/committees/iacuc/aop-assurances/guidelines/. Accessed June 29, 2024. [Google Scholar]
- 20.Standard Rodent Diets. LabDiet. Available from: https://www.labdiet.com/diets/diet-detail/RODENT. Accessed June 29, 2024.
- 21.Anderson G. Type I diabetes pathoetiology and pathophysiology: roles of the gut microbiome, pancreatic cellular interactions, and the “bystander” activation of memory CD8(+) T cells. Int J Mol Sci. 2023;24(4):3300. doi: 10.3390/ijms24043300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian B, Ramakrishnan G, Tamilmaran P, Sundarakrishnan B, Seshadri K. 1606-P: effect of changes in dark/light cycle on physiological homeostasis as a confounding prelude to diabetes in Fischer 334 animal model. Diabetes. 2020;69(Supplement_1):1606–P. doi: 10.2337/db20-1606-P [DOI] [Google Scholar]
- 23.Lin G-J, Huang S-H, Chen S-J, et al. Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int J Mol Sci. 2013;14(6):11742–11766. doi: 10.3390/ijms140611742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti A, Maestroni GJM. Role of the pineal gland and melatonin in the development of autoimmune diabetes in non-obese diabetic mice. J Pineal Res. 1996;20(3):164–172. doi: 10.1111/j.1600-079X.1996.tb00253.x [DOI] [PubMed] [Google Scholar]
- 25.Birkebaek NH, Kamrath C, Grimsmann JM, et al. Impact of the COVID-19 pandemic on long-term trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes: an international multicentre study based on data from 13 national diabetes registries. Lancet Diabetes Endocrinol. 2022;10(11):786–794. doi: 10.1016/S2213-8587(22)00246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahmati M, Keshvari M, Mirnasuri S, et al. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: a systematic review and meta-analysis. J Med Virol. 2022;94(11):5112–5127. doi: 10.1002/jmv.27996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Qahtani MH, Bukhamseen FM, Al-Qassab AT, et al. The impact of covid-19 lockdown on the incidence of type 1 dm and the glycemic control of diabetic children: findings from a teaching hospital, Saudi Arabia. Rev Diabet Stud. 2022;18(3):152–156. doi: 10.1900/RDS.2022.18.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra S, DiMeglio LA. COVID-19 and incident type 1 diabetes: deciphering the associations. Diabetes. 2022;71(12):2480–2482. doi: 10.2337/dbi22-0009 [DOI] [PubMed] [Google Scholar]
- 29.Pechhold K, Koczwara K, Zhu X, et al. Blood glucose levels regulate pancreatic β-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS One. 2009;4(3):e4827. doi: 10.1371/journal.pone.0004827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56(7):1792–1801. doi: 10.2337/db06-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54(3):572–582. doi: 10.1007/s00125-010-1919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamb MM, Yin X, Barriga K, et al. Dietary glycemic index, development of islet autoimmunity, and subsequent progression to type 1 diabetes in young children. J Clin Endocrinol Metab. 2008;93(10):3936–3942. doi: 10.1210/jc.2008-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benson VS, Vanleeuwen JA, Taylor J, McKinney PA, Van Til L. Food consumption and the risk of type 1 diabetes in children and youth: a population-based, case-control study in prince edward Island, Canada. J Am Coll Nutr. 2008;27(3):414–420. doi: 10.1080/07315724.2008.10719719 [DOI] [PubMed] [Google Scholar]
- 34.Robertson-Dixon I, Murphy MJ, Crewther SG, Riddell N. The influence of light wavelength on human hpa axis rhythms: a systematic review. Life. 2023;13(10):1968. doi: 10.3390/life13101968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oster H, Challet E, Ott V, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38(1):3–45. doi: 10.1210/er.2015-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erickson RL, Browne CA, Lucki I. Hair corticosterone measurement in mouse models of type 1 and type 2 diabetes mellitus. Physiol Behav. 2017;178:166–171. doi: 10.1016/j.physbeh.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elahi-Moghaddam Z, Behnam-Rassouli M, Mahdavi-Shahri N, Hajinejad-Boshroue R, Khajouee E. Comparative study on the effects of type 1 and type 2 diabetes on structural changes and hormonal output of the adrenal cortex in male Wistar rats. J Diabetes Metab Disord. 2013;12(9). doi: 10.1186/2251-6581-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest. 2021;131(19). doi: 10.1172/JCI148286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi: 10.2147/NSS.S134864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartar JL, Ward CP, Cordeira JW, et al. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197(2):450–453. doi: 10.1016/j.bbr.2008.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgoifo A, Buwalda B, Roos M, et al. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31(2):197–208. doi: 10.1016/j.psyneuen.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 42.Meerlo P, Koehl M, van der Borght K, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14(5):397–402. doi: 10.1046/j.0007-1331.2002.00790.x [DOI] [PubMed] [Google Scholar]
- 43.van Dalfsen JH, Markus CR. The influence of sleep on human hypothalamic–pituitary–adrenal (HPA) axis reactivity: a systematic review. Sleep Med Rev. 2018;39:187–194. doi: 10.1016/j.smrv.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 44.El Mlili N, Ahabrach H, Cauli O. Hair cortisol concentration as a biomarker of sleep quality and related disorders. Life. 2021;11(2):81. doi: 10.3390/life11020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Y-X, Xiao H-B, Wang -S-S, et al. Investigation of the relationship between chronic stress and insulin resistance in a Chinese population. J Epidemiol. 2016;26(7):355–360. doi: 10.2188/jea.JE20150183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huybrechts I, De Vriendt T, Breidenassel C, et al. Mechanisms of stress, energy homeostasis and insulin resistance in European adolescents--the HELENA study. Nutr, Metab Cardiovasc Dis. 2014;24(10):1082–1089. doi: 10.1016/j.numecd.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 47.Cao Z-H, Wu Z, Hu C, et al. Endoplasmic reticulum stress and destruction of pancreatic β cells in type 1 diabetes. Chin Med J. 2020;133(1):68–73. doi: 10.1097/CM9.0000000000000583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270 [DOI] [PubMed] [Google Scholar]
- 49.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–995. doi: 10.1101/gad.12.7.982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hillhouse EE, Collin R, Chabot-Roy G, et al. Nearby construction impedes the progression to overt autoimmune diabetes in NOD mice. J Diabetes Res. 2013;2013:1–7. doi: 10.1155/2013/620313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marx JO, Brice AK, Boston RC, Smith AL. Incidence rates of spontaneous disease in laboratory mice used at a large biomedical research institution. J Am Assoc Lab Anim Sci. 2013;52(6):782–791. [PMC free article] [PubMed] [Google Scholar]
- 52.Snyder JM, Ward JM, Treuting PM. Cause-of-death analysis in rodent aging studies. Vet Pathol. 2016;53(2):233–243. doi: 10.1177/0300985815610391 [DOI] [PubMed] [Google Scholar]
- 53.Apreutese A, Levi M, Taylor I, et al. Causes of mortality and profile of spontaneous tumors in young CD-1 mice. Toxicol Pathol. 2022;50(6):776–786. doi: 10.1177/01926233221105391 [DOI] [PubMed] [Google Scholar]
- 54.Montonye DR, Ericsson AC, Busi SB, et al. Acclimation and institutionalization of the mouse microbiota following transportation. Front Microbiol. 2018;9:1085. doi: 10.3389/fmicb.2018.01085 [DOI] [PMC free article] [PubMed] [Google Scholar]