Abstract
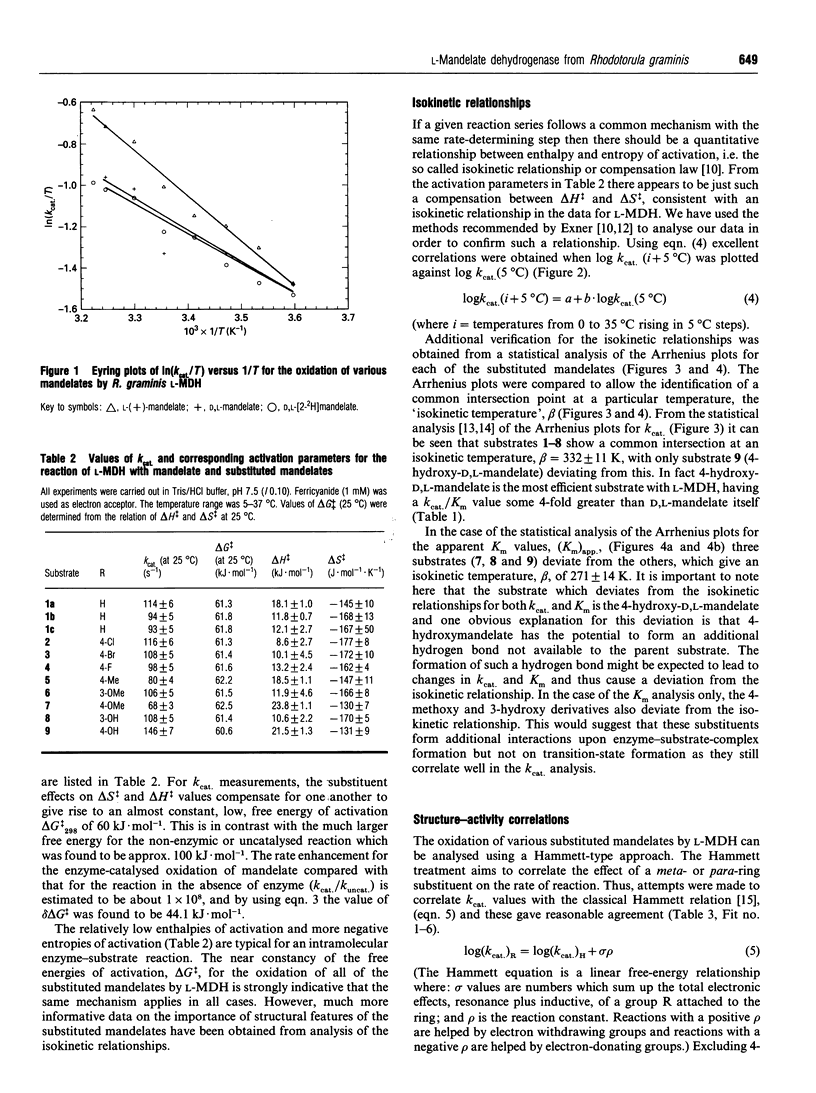
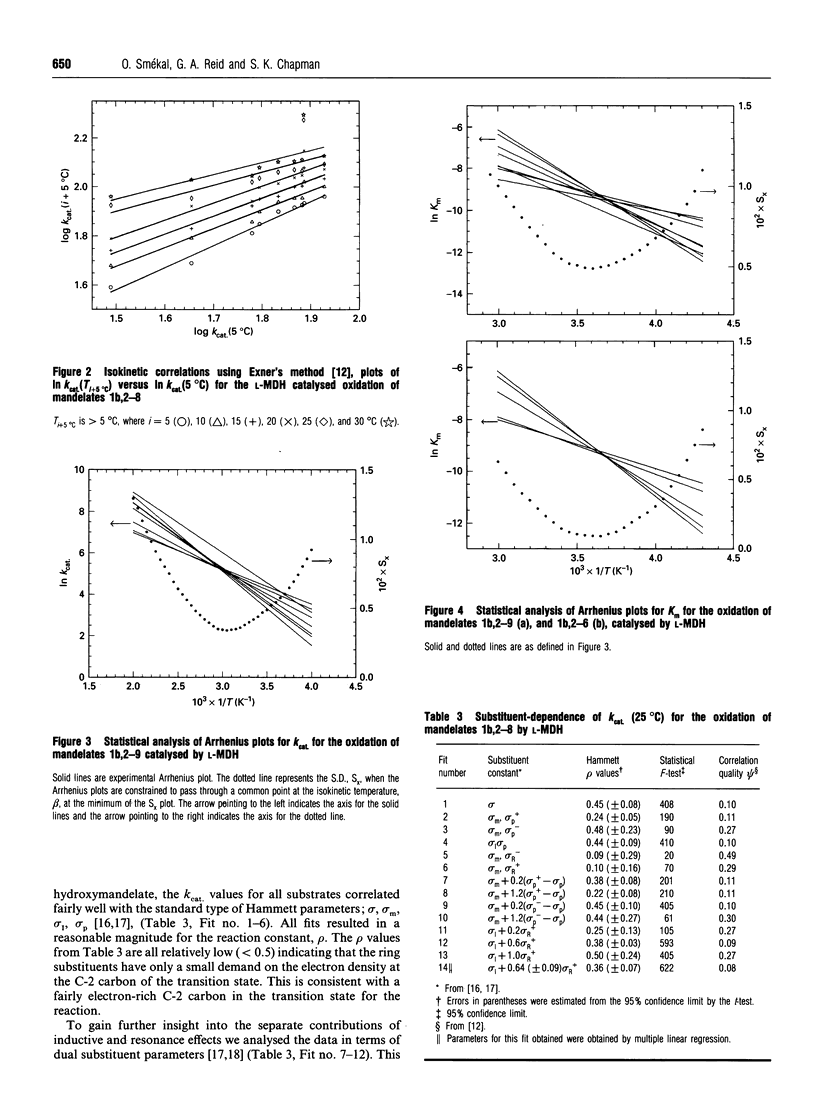
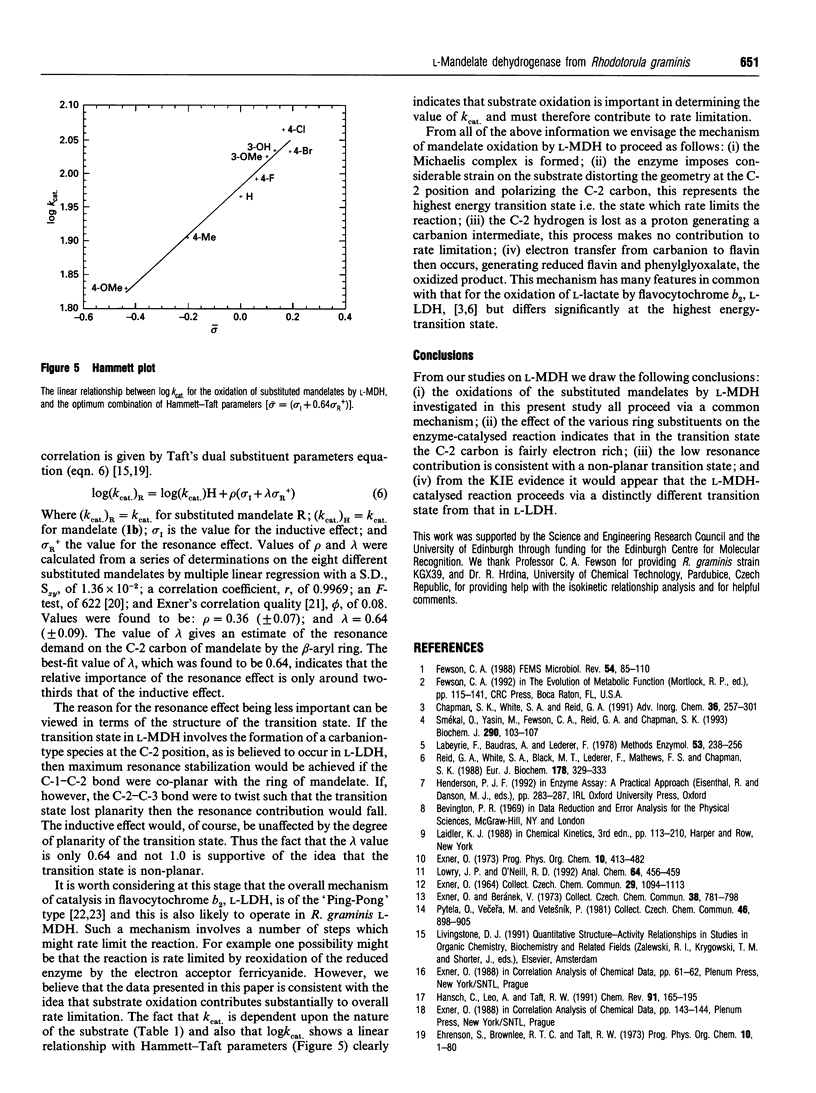
A detailed kinetic analysis of the oxidation of mono-substituted mandelates catalysed by L-(+)-mandelate dehydrogenase (L-MDH) from Rhodotorula graminis has been carried out to elucidate the role of the substrate in the catalytic mechanism. Values of Km and kcat. (25 degrees C, pH 7.5) were determined for mandelate and eight substrate analogues. Values of the activation parameters, delta H++ and delta S++ (determined over the range 5-37 degrees C), for mandelate and all substrate analogues were compensatory resulting in similar low values for free energies of activation delta G++ (approx. 60 kJ.mol-1 at 298.15 K) in all cases. A kinetic-isotope-effect value of 1.1 +/- 0.1 was observed using D,L-[2-2H]mandelate as substrate and was invariant over the temperature range studied. The logarithm of kcat. values for the enzymic oxidation of mandelate and all substrate analogues (except 4-hydroxymandelate) showed good correlation with Taft's dual substituent constant omega (where omega = omega I + 0.64 omega +R) and gave a positive reaction constant value, rho, of 0.36 +/- 0.07. This linear free-energy relationship was verified by analysing the data using isokinetic methods. These findings support the hypothesis that the enzyme-catalysed reaction proceeds via the same transition state for each substrate and indicates that this transition state is relatively nonpolar but has an electron-rich centre at the alpha-carbon position.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig P. N., Hansch C. H., McFarland J. W., Martin Y. C., Purcell W. P., Zahradník R. Minimal statistical data for structure-function correlations. J Med Chem. 1971 May;14(5):447–447. doi: 10.1021/jm00287a018. [DOI] [PubMed] [Google Scholar]
- Fewson C. A. Microbial metabolism of mandelate: a microcosm of diversity. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):85–110. doi: 10.1111/j.1574-6968.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Lowry J. P., O'Neill R. D. Homogeneous mechanism of ascorbic acid interference in hydrogen peroxide detection at enzyme-modified electrodes. Anal Chem. 1992 Feb 15;64(4):453–456. doi: 10.1021/ac00028a022. [DOI] [PubMed] [Google Scholar]
- Miles C. S., Rouvière-Fourmy N., Lederer F., Mathews F. S., Reid G. A., Black M. T., Chapman S. K. Tyr-143 facilitates interdomain electron transfer in flavocytochrome b2. Biochem J. 1992 Jul 1;285(Pt 1):187–192. doi: 10.1042/bj2850187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. A., White S., Black M. T., Lederer F., Mathews F. S., Chapman S. K. Probing the active site of flavocytochrome b2 by site-directed mutagenesis. Eur J Biochem. 1988 Dec 15;178(2):329–333. doi: 10.1111/j.1432-1033.1988.tb14454.x. [DOI] [PubMed] [Google Scholar]
- Smékal O., Yasin M., Fewson C. A., Reid G. A., Chapman S. K. L-mandelate dehydrogenase from Rhodotorula graminis: comparisons with the L-lactate dehydrogenase (flavocytochrome b2) from Saccharomyces cerevisiae. Biochem J. 1993 Feb 15;290(Pt 1):103–107. doi: 10.1042/bj2900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegoni M., Janot J. M., Labeyrie F. Inhibition of L-lactate: cytochrome-c reductase (flavocytochrome b2) by product binding to the semiquinone transient. Loss of reactivity towards monoelectronic acceptors. Eur J Biochem. 1990 Jun 20;190(2):329–342. doi: 10.1111/j.1432-1033.1990.tb15580.x. [DOI] [PubMed] [Google Scholar]


