Abstract
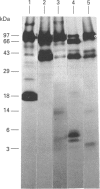
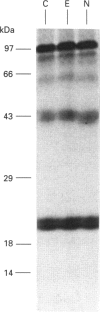
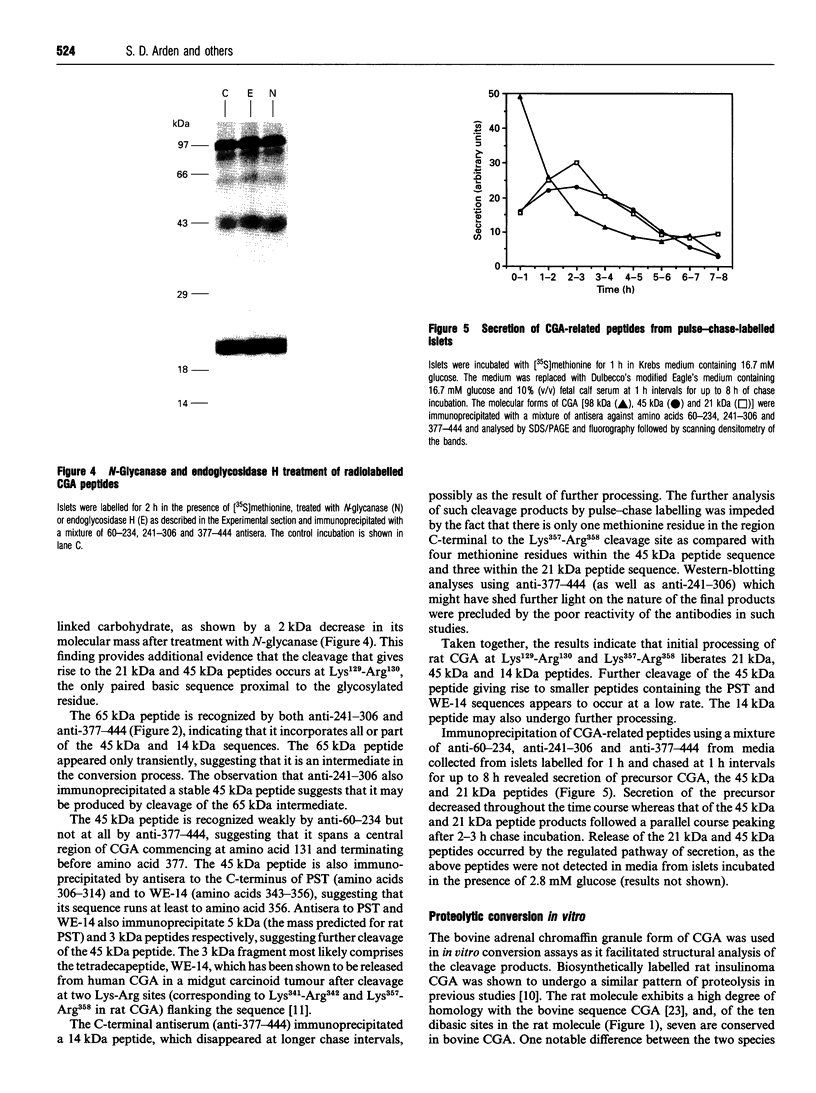
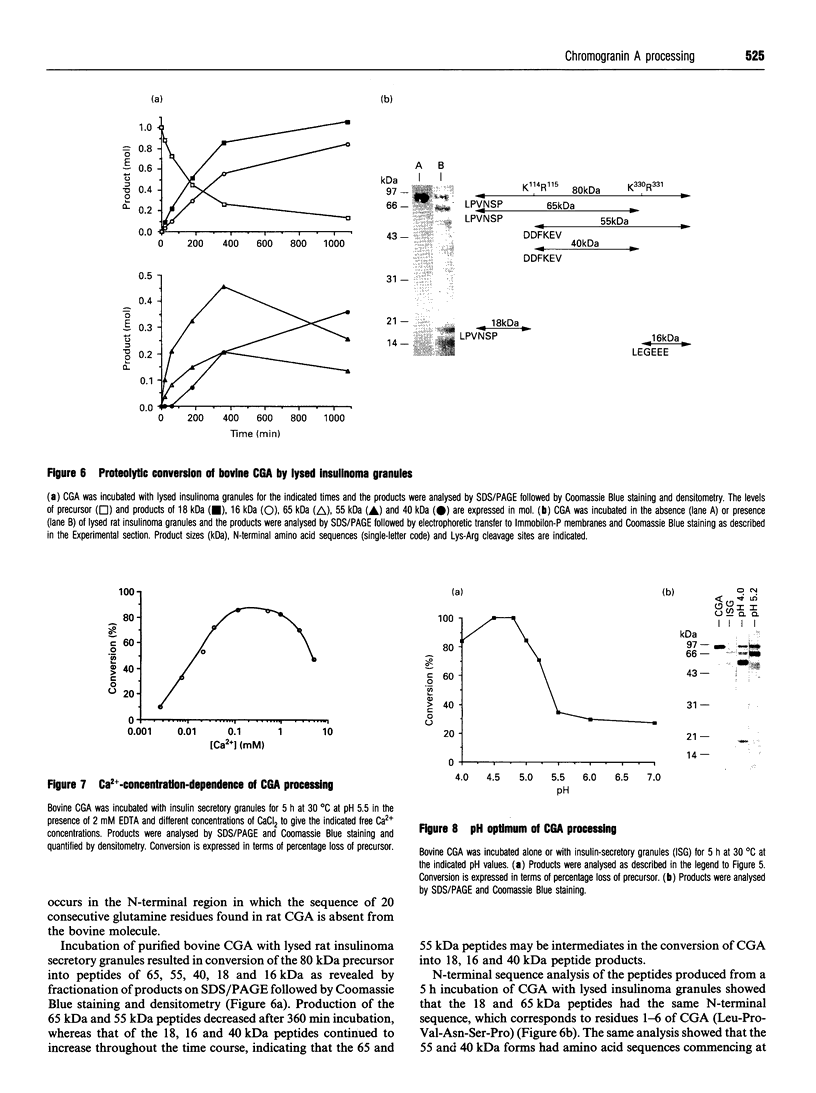
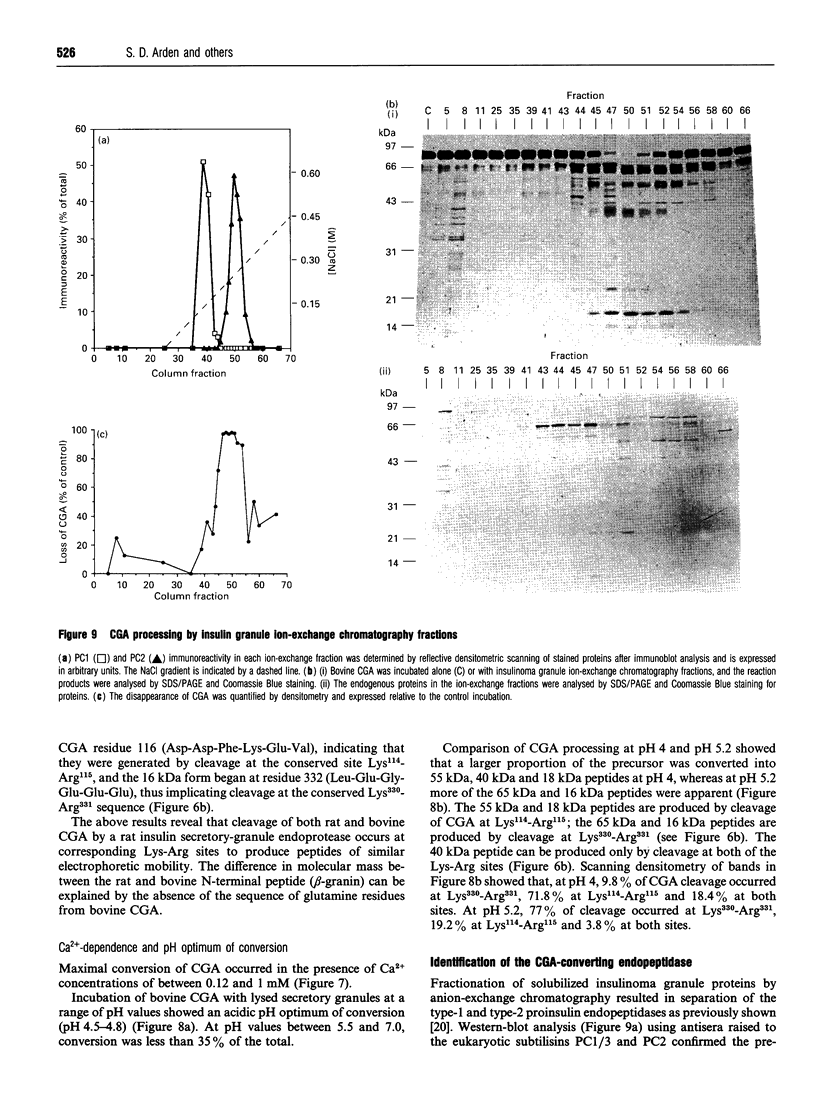
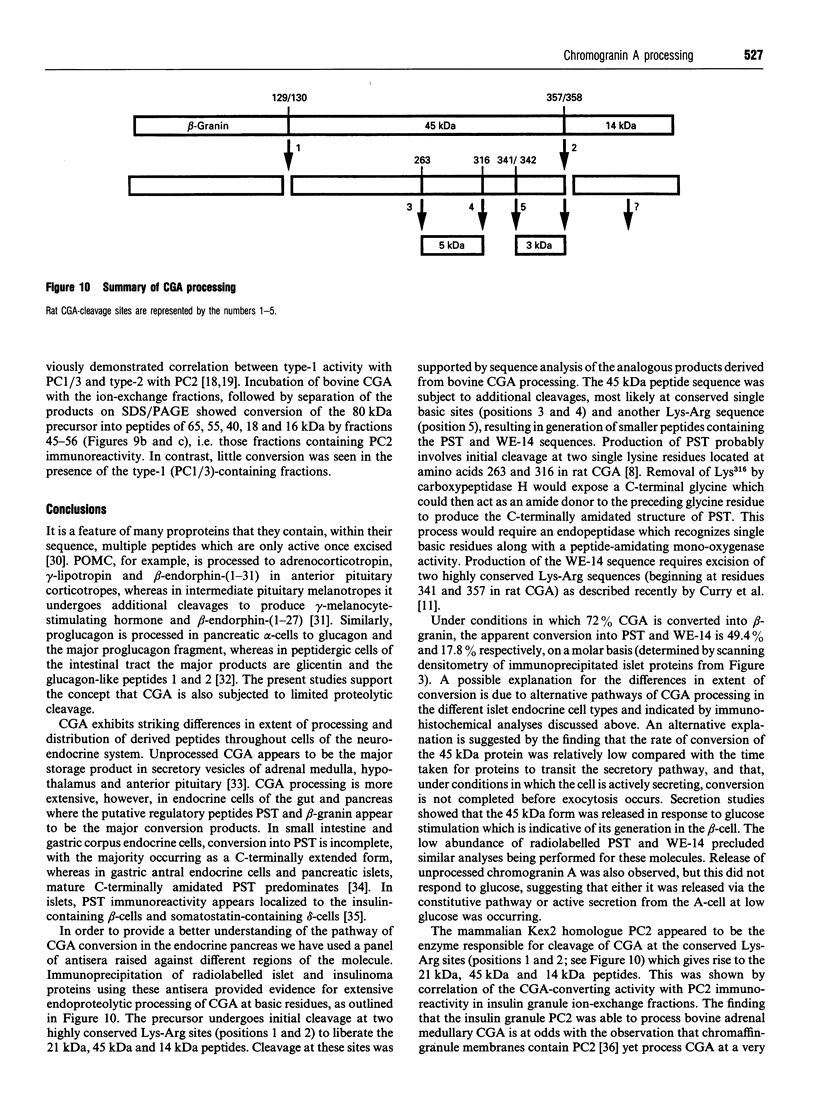
The post-translational processing of chromogranin A (CGA) and the nature of the enzyme(s) involved were investigated in rat pancreatic islet and insulinoma tissue. Pulse-chase radiolabelling experiments using sequence-specific antisera showed that the 98 kDa (determined by SDS/PAGE) precursor was processed to an N-terminal 21 kDa peptide, a C-terminal 14 kDa peptide and a 45 kDa centrally located peptide with a rapid time course (t1/2 approx. 30 min) after an initial delay of 30-60 min. The 45 kDa peptide was, in turn, converted partially into a 5 kDa peptide with pancreastatin immunoreactivity and a 3 kDa peptide with WE-14 immunoreactivity over a longer time period. Incubation of bovine CGA with rat insulinoma secretory-granule lysate produced peptides of 18, 16 and 40 kDa via intermediates of 65 and 55 kDa. N-terminal sequence analysis indicated that cleavage occurred at the conserved paired basic sites Lys114-Arg115 and Lys330-Arg331, suggesting that cleavage of the equivalent sites (Lys129-Arg130 and Lys357-Arg358) in the rat molecule produced the initial post-translational products observed in intact pancreatic beta-cells. The enzyme activity responsible for the cleavage of bovine CGA co-chromatographed on DEAE-cellulose with the type-2 proinsulin endopeptidase and with PC2 immunoreactivity. The type-1 enzyme (PC1/3) appeared inactive towards CGA. The requirement for Ca2+ ions and an acidic pH for conversion was consistent with the involvement of a member of the eukaryote subtilisin family, and the composition of the released peptides in pulse-chase and secretion studies suggested that conversion occurred in the secretory-granule compartment. The overall catalytic rate as well as the relative susceptibilities of the Lys114-Arg115 and Lys330-Arg331 sites to cleavage were affected by pH, suggesting that the ionic environment of the processing compartment may play a role in the differential processing of CGA which is evident in various neuroendocrine cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailyes E. M., Bennett D. L., Hutton J. C. Proprotein-processing endopeptidases of the insulin secretory granule. Enzyme. 1991;45(5-6):301–313. doi: 10.1159/000468903. [DOI] [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. L., Bailyes E. M., Nielsen E., Guest P. C., Rutherford N. G., Arden S. D., Hutton J. C. Identification of the type 2 proinsulin processing endopeptidase as PC2, a member of the eukaryote subtilisin family. J Biol Chem. 1992 Jul 25;267(21):15229–15236. [PubMed] [Google Scholar]
- Büller H. A., Rings E. H., Montgomery R. K., Sasak W. V., Grand R. J. Further studies of glycosylation and intracellular transport of lactase-phlorizin hydrolase in rat small intestine. Biochem J. 1989 Oct 1;263(1):249–254. doi: 10.1042/bj2630249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry W. J., Johnston C. F., Hutton J. C., Arden S. D., Rutherford N. G., Shaw C., Buchanan K. D. The tissue distribution of rat chromogranin A-derived peptides: evidence for differential tissue processing from sequence specific antisera. Histochemistry. 1991;96(6):531–538. doi: 10.1007/BF00267079. [DOI] [PubMed] [Google Scholar]
- Curry W. J., Shaw C., Johnston C. F., Thim L., Buchanan K. D. Isolation and primary structure of a novel chromogranin A-derived peptide, WE-14, from a human midgut carcinoid tumour. FEBS Lett. 1992 Apr 27;301(3):319–321. doi: 10.1016/0014-5793(92)80266-j. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988 May 5;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Falkensammer G., Fischer-Colbrie R., Richter K., Winkler H. Cell-free and cellular synthesis of chromogranin A and B of bovine adrenal medulla. Neuroscience. 1985 Feb;14(2):735–746. doi: 10.1016/0306-4522(85)90323-9. [DOI] [PubMed] [Google Scholar]
- Fasciotto B. H., Gorr S. U., DeFranco D. J., Levine M. A., Cohn D. V. Pancreastatin, a presumed product of chromogranin-A (secretory protein-I) processing, inhibits secretion from porcine parathyroid cells in culture. Endocrinology. 1989 Sep;125(3):1617–1622. doi: 10.1210/endo-125-3-1617. [DOI] [PubMed] [Google Scholar]
- Galindo E., Rill A., Bader M. F., Aunis D. Chromostatin, a 20-amino acid peptide derived from chromogranin A, inhibits chromaffin cell secretion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1426–1430. doi: 10.1073/pnas.88.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest P. C., Ravazzola M., Davidson H. W., Orci L., Hutton J. C. Molecular heterogeneity and cellular localization of carboxypeptidase H in the islets of Langerhans. Endocrinology. 1991 Aug;129(2):734–740. doi: 10.1210/endo-129-2-734. [DOI] [PubMed] [Google Scholar]
- Guest P. C., Rhodes C. J., Hutton J. C. Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem J. 1989 Jan 15;257(2):431–437. doi: 10.1042/bj2570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle K. B., Reed R. K., Pihl K. E., Serck-Hanssen G. Osmotic properties of the chromogranins and relation to osmotic pressure in catecholamine storage granules. Acta Physiol Scand. 1985 Jan;123(1):21–33. doi: 10.1111/j.1748-1716.1985.tb07556.x. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Gerdes H. H., Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991 Jan;16(1):27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Peshavaria M. Proteolytic processing of chromogranin A in purified insulin granules. Formation of a 20 kDa N-terminal fragment (betagranin) by the concerted action of a Ca2+-dependent endopeptidase and carboxypeptidase H (EC 3.4.17.10). Biochem J. 1987 Jun 1;244(2):457–464. doi: 10.1042/bj2440457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Hansen F., Peshavaria M. beta-Granins: 21 kDa co-secreted peptides of the insulin granule closely related to adrenal medullary chromogranin A. FEBS Lett. 1985 Sep 2;188(2):336–340. doi: 10.1016/0014-5793(85)80398-7. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Nielsen E., Kastern W. The molecular cloning of the chromogranin A-like precursor of beta-granin and pancreastatin from the endocrine pancreas. FEBS Lett. 1988 Aug 29;236(2):269–274. doi: 10.1016/0014-5793(88)80036-x. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Peshavaria M., Johnston C. F., Ravazzola M., Orci L. Immunolocalization of betagranin: a chromogranin A-related protein of the pancreatic B-cell. Endocrinology. 1988 Mar;122(3):1014–1020. doi: 10.1210/endo-122-3-1014. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Kar S., Bretherton-Watt D., Gibson S. J., Steel J. H., Gentleman S. M., Roberts G. W., Valentino K., Tatemoto K., Ghatei M. A., Bloom S. R. Novel peptide pancreastatin: its occurrence and codistribution with chromogranin A in the central nervous system of the pig. J Comp Neurol. 1989 Oct 22;288(4):627–639. doi: 10.1002/cne.902880409. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. J., Goldenring J. R., Asher V. A., Modlin I. M. Pancreastatin: a novel peptide inhibitor of parietal cell signal transduction. Biochem Biophys Res Commun. 1989 Sep 15;163(2):667–673. doi: 10.1016/0006-291x(89)92275-4. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Brownstein M. J., Gainer H. Proteolysis in neuropeptide processing and other neural functions. Annu Rev Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Ravazzola M., Efendic S., Ostenson C. G., Tatemoto K., Hutton J. C., Orci L. Localization of pancreastatin immunoreactivity in porcine endocrine cells. Endocrinology. 1988 Jul;123(1):227–229. doi: 10.1210/endo-123-1-227. [DOI] [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Ca2+ binding to chromaffin vesicle matrix proteins: effect of pH, Mg2+, and ionic strength. Biochemistry. 1986 Jul 29;25(15):4402–4406. doi: 10.1021/bi00363a034. [DOI] [PubMed] [Google Scholar]
- Rhodes C. J., Thorne B. A., Lincoln B., Nielsen E., Hutton J. C., Thomas G. Processing of proopiomelanocortin by insulin secretory granule proinsulin processing endopeptidases. J Biol Chem. 1993 Feb 25;268(6):4267–4275. [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Sharp R. R., Richards E. P. Molecular mobilities of soluble components in the aqueous phase of chromaffin granules. Biochim Biophys Acta. 1977 Mar 29;497(1):260–271. doi: 10.1016/0304-4165(77)90159-3. [DOI] [PubMed] [Google Scholar]
- Simon J. P., Aunis D. Biochemistry of the chromogranin A protein family. Biochem J. 1989 Aug 15;262(1):1–13. doi: 10.1042/bj2620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Watkinson A., Jönsson A. C., Davison M., Young J., Lee C. M., Moore S., Dockray G. J. Heterogeneity of chromogranin A-derived peptides in bovine gut, pancreas and adrenal medulla. Biochem J. 1991 Jun 1;276(Pt 2):471–479. doi: 10.1042/bj2760471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- Zhou A., Bloomquist B. T., Mains R. E. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993 Jan 25;268(3):1763–1769. [PubMed] [Google Scholar]