Abstract
An IMP-hydrolysing enzyme was purified to homogeneity from yeast extract. It was a soluble protein with an apparent molecular mass of 220 kDa, with a subunit molecular mass of 55 kDa. It was highly specific for IMP, and there was virtually no detectable activity with the other purine and pyrimidine nucleotides tested, including AMP and dIMP. The enzyme had a pH optimum of 6.0-6.5. Its activity was absolutely dependent on bivalent metal salts: Mg2+ was most potent, followed by Co2+ and Mn2+. The velocity/substrate-concentration plot of the enzyme was slightly sigmoidal (h = 1.7) and the s0.5 was 0.4 mM. ATP stimulated the enzyme by decreasing both h and s0.5. Diadenosine tetraphosphate stimulated the enzyme as effectively as ATP. Although the properties of the enzyme are similar to those of the IMP/GMP 5'-nucleotidase identified in various animals [Itoh (1993) Comp. Biochem. Physiol. 105B, 13-19], the substrate specificity of the former was much more strict than the latter.
Full text
PDF

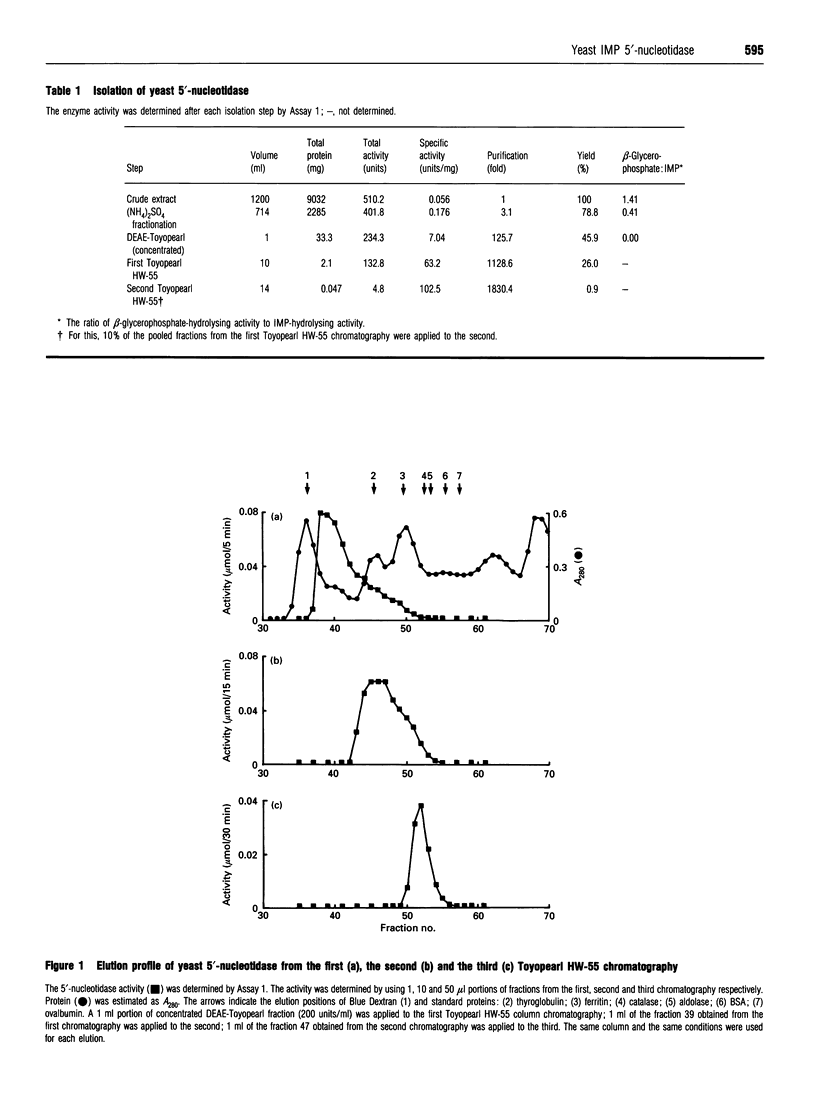
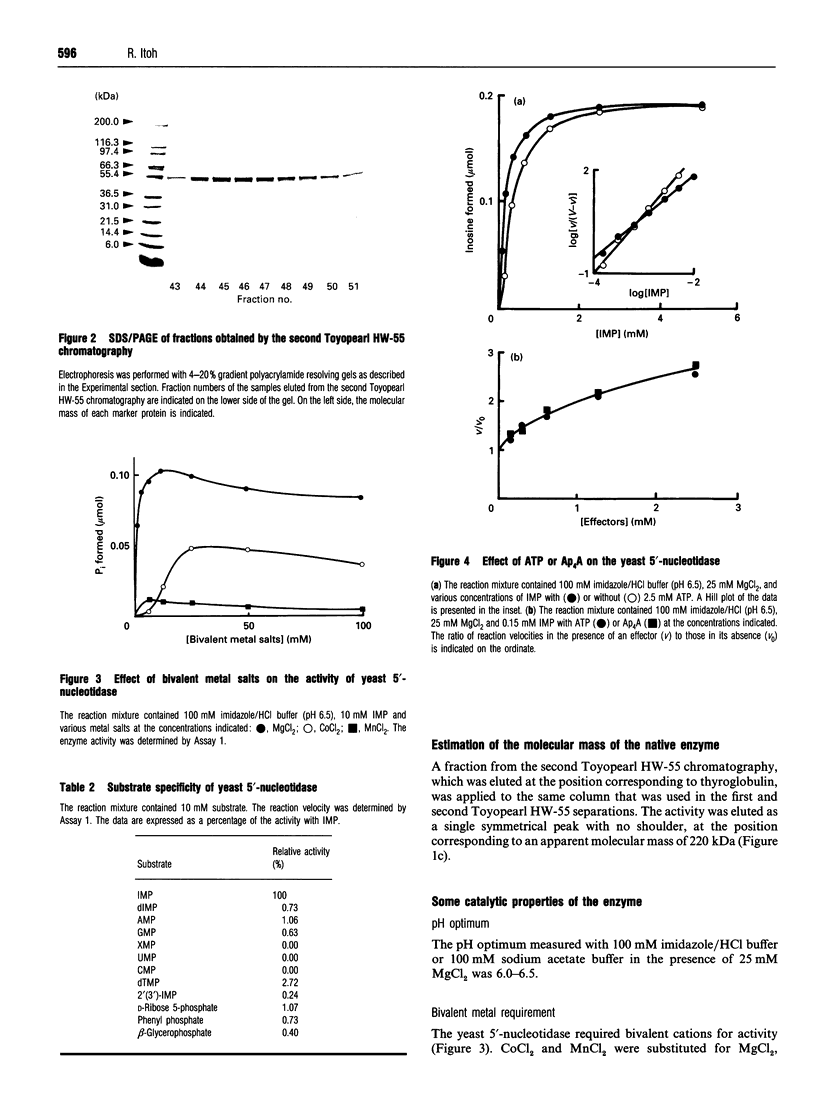


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aposhian H. V., Tremblay G. Y. Deoxythymidylate 5'-nucleotidase. Purification and properties of an enzyme found after infection of Bacillus subtilis with phage SP5C. J Biol Chem. 1966 Nov 10;241(21):5095–5101. [PubMed] [Google Scholar]
- Darvish A., Metting P. J. Purification and regulation of an AMP-specific cytosolic 5'-nucleotidase from dog heart. Am J Physiol. 1993 May;264(5 Pt 2):H1528–H1534. doi: 10.1152/ajpheart.1993.264.5.H1528. [DOI] [PubMed] [Google Scholar]
- Itoh R. IMP-GMP 5'-nucleotidase. Comp Biochem Physiol B. 1993 May;105(1):13–19. doi: 10.1016/0305-0491(93)90163-y. [DOI] [PubMed] [Google Scholar]
- Itoh R. Purification and some properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Itoh R., Yamada K. Pig lung 5'-nucleotidase: effect of diadenosine 5',5'''-P1, P4-tetraphosphate and its related compounds. Int J Biochem. 1990;22(3):231–238. doi: 10.1016/0020-711x(90)90334-y. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomax C. A., Henderson J. F. Adenosine formation and metabolism during adenosine triphosphate catabolism in Ehrlich ascites tumor cells. Cancer Res. 1973 Nov;33(11):2825–2829. [PubMed] [Google Scholar]
- Merkler D. J., Wali A. S., Taylor J., Schramm V. L. AMP deaminase from yeast. Role in AMP degradation, large scale purification, and properties of the native and proteolyzed enzyme. J Biol Chem. 1989 Dec 15;264(35):21422–21430. [PubMed] [Google Scholar]
- Skladanowski A. C., Newby A. C. Partial purification and properties of an AMP-specific soluble 5'-nucleotidase from pigeon heart. Biochem J. 1990 May 15;268(1):117–122. doi: 10.1042/bj2680117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Hers H. G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980 Jun 15;188(3):913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992 Jul 15;285(Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]



