Abstract
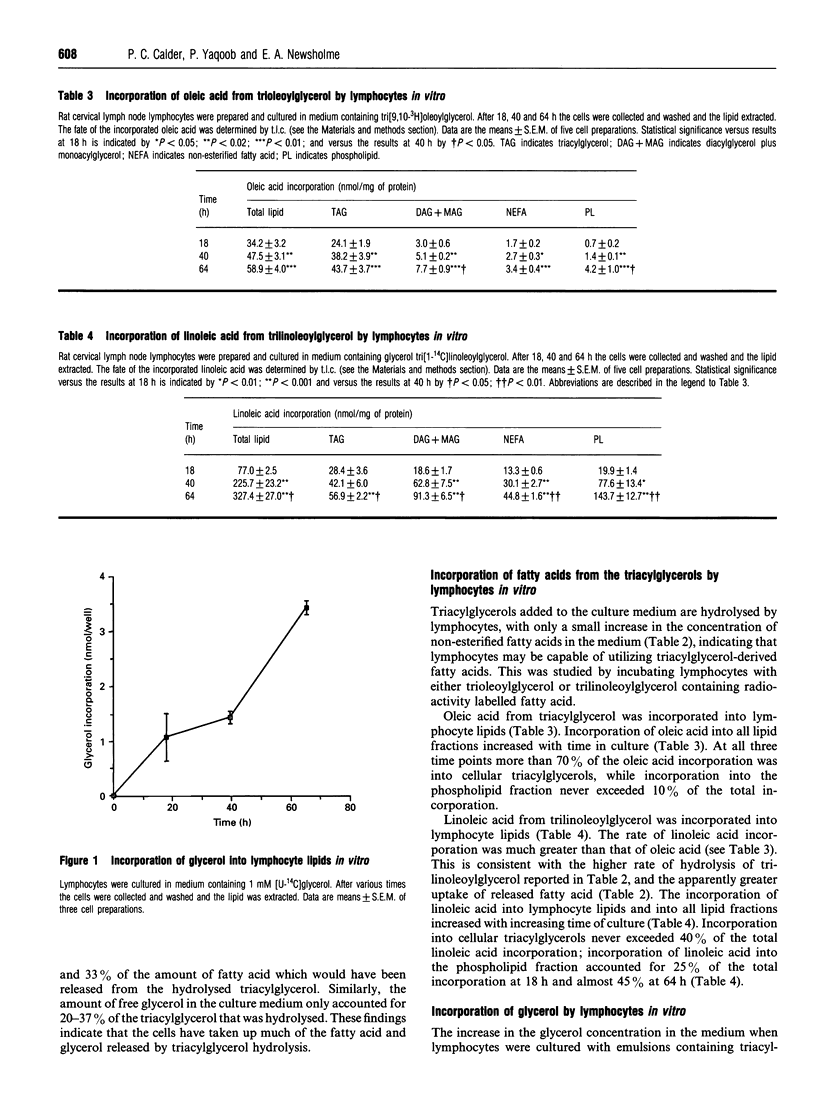
This study investigates the ability of lymphocytes to utilize fatty acids originating from triacylglycerols and the effect of triacylglycerols upon mitogen-stimulated lymphocyte proliferation. Lymphocytes isolated from rat lymph nodes, spleen, thymus and lymphatic duct had a lipoprotein lipase activity of approx. 10 units/mg of protein, indicating that the fatty acids of circulating triacylglycerols are accessible to these cells. In culture lymph node lymphocytes hydrolysed triacylglycerols added to the medium as emulsions. Both non-esterified fatty acids and free glycerol appeared in the cell culture medium, but their concentrations indicated that a high proportion of each (65-90% of fatty acids and 60-80% of glycerol) was taken up by the cells. The incorporation and fate of triacylglycerol-fatty acids was studied by culturing the cells in the presence of tri[3H]oleoylglycerol or tri[14C]inoleoylglycerol. Both fatty acids were incorporated into lymphocyte lipids in a time-dependent manner; linoleic acid was incorporated at a significantly greater rate than oleic acid. The majority of oleic acid (greater than 70%) was incorporated into cellular triacylglycerol, while less than 10% was incorporated into phospholipids. In contrast, linoleic acid incorporation into cellular triacylglycerol never exceeded 25%, while up to 45% was incorporated into phospholipids. Triacylglycerols containing polyunsaturated fatty acids inhibited concanavalin A-stimulated lymphocyte proliferation in a concentration- and time-dependent manner; triacylglycerols containing saturated fatty acids or oleic acid were not inhibitory. Such direct effects of certain triacylglycerols on lymphocyte function may explain why some clinical trials of polyunsaturated fatty acid-rich diets have been successful in improving the condition of patients suffering from inflammatory diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Howard B. V., Tillman S. F. Lipid metabolism in cultured cells. XI. Utilization of serum triglycerides. J Biol Chem. 1973 Feb 25;248(4):1240–1247. [PubMed] [Google Scholar]
- Baltzell J. K., Wooten J. T., Otto D. A. Lipoprotein lipase in rats fed fish oil: apparent relationship to plasma insulin levels. Lipids. 1991 Apr;26(4):289–294. doi: 10.1007/BF02537139. [DOI] [PubMed] [Google Scholar]
- Bates D., Cartlidge N. E., French J. M., Jackson M. J., Nightingale S., Shaw D. A., Smith S., Woo E., Hawkins S. A., Millar J. H. A double-blind controlled trial of long chain n-3 polyunsaturated fatty acids in the treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989 Jan;52(1):18–22. doi: 10.1136/jnnp.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A. Lipoprotein lipase. Annu Rev Nutr. 1991;11:217–237. doi: 10.1146/annurev.nu.11.070191.001245. [DOI] [PubMed] [Google Scholar]
- Bittiner S. B., Tucker W. F., Cartwright I., Bleehen S. S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988 Feb 20;1(8582):378–380. doi: 10.1016/s0140-6736(88)91181-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenner R. R. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog Lipid Res. 1984;23(2):69–96. doi: 10.1016/0163-7827(84)90008-0. [DOI] [PubMed] [Google Scholar]
- Calder P. C., Bevan S. J., Newsholme E. A. The inhibition of T-lymphocyte proliferation by fatty acids is via an eicosanoid-independent mechanism. Immunology. 1992 Jan;75(1):108–115. [PMC free article] [PubMed] [Google Scholar]
- Calder P. C., Bond J. A., Bevan S. J., Hunt S. V., Newsholme E. A. Effect of fatty acids on the proliferation of concanavalin A-stimulated rat lymph node lymphocytes. Int J Biochem. 1991;23(5-6):579–588. doi: 10.1016/0020-711x(87)90052-8. [DOI] [PubMed] [Google Scholar]
- Calder P. C., Bond J. A., Harvey D. J., Gordon S., Newsholme E. A. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990 Aug 1;269(3):807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C., Newsholme E. A. Polyunsaturated fatty acids suppress human peripheral blood lymphocyte proliferation and interleukin-2 production. Clin Sci (Lond) 1992 Jun;82(6):695–700. doi: 10.1042/cs0820695. [DOI] [PubMed] [Google Scholar]
- Camps L., Reina M., Llobera M., Bengtsson-Olivecrona G., Olivecrona T., Vilaró S. Lipoprotein lipase in lungs, spleen, and liver: synthesis and distribution. J Lipid Res. 1991 Dec;32(12):1877–1888. [PubMed] [Google Scholar]
- Camps L., Reina M., Llobera M., Vilaró S., Olivecrona T. Lipoprotein lipase: cellular origin and functional distribution. Am J Physiol. 1990 Apr;258(4 Pt 1):C673–C681. doi: 10.1152/ajpcell.1990.258.4.C673. [DOI] [PubMed] [Google Scholar]
- Chait A., Iverius P. H., Brunzell J. D. Lipoprotein lipase secretion by human monocyte-derived macrophages. J Clin Invest. 1982 Feb;69(2):490–493. doi: 10.1172/JCI110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa M., Yamashita N., Yamazaki K., Sugiyama E., Suzuki H., Hamazaki T. Eicosapentaenoic acid inhibits antigen-presenting cell function of murine splenocytes. Immunology. 1992 Feb;75(2):330–335. [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L. The effect of the continuous re-infusion of lymph and lymphocytes on the output of lymphocytes from the thoracic duct of unanaesthetized rats. Br J Exp Pathol. 1957 Feb;38(1):67–78. [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. J., Handley D. A., Vanni T., Paterniti J. R., Jr, Cornicelli J. A. Membrane-bound lipoprotein lipase on human monocyte-derived macrophages: localization by immunocolloidal gold technique. Biochim Biophys Acta. 1988 Apr 15;959(3):220–228. doi: 10.1016/0005-2760(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Goldberg I. J., Soprano D. R., Wyatt M. L., Vanni T. M., Kirchgessner T. G., Schotz M. C. Localization of lipoprotein lipase mRNA in selected rat tissues. J Lipid Res. 1989 Oct;30(10):1569–1577. [PubMed] [Google Scholar]
- KORN E. D. The fatty acid and positional specificities of lipoprotein lipase. J Biol Chem. 1961 Jun;236:1638–1642. [PubMed] [Google Scholar]
- Kremer J. M., Jubiz W., Michalek A., Rynes R. I., Bartholomew L. E., Bigaouette J., Timchalk M., Beeler D., Lininger L. Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987 Apr;106(4):497–503. doi: 10.7326/0003-4819-106-4-497. [DOI] [PubMed] [Google Scholar]
- Mahoney E. M., Khoo J. C., Steinberg D. Lipoprotein lipase secretion by human monocytes and rabbit alveolar macrophages in culture. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1639–1642. doi: 10.1073/pnas.79.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan M. W., Artiss J. D., Strandbergh D. R., Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983 Mar;29(3):538–542. [PubMed] [Google Scholar]
- Meade C. J., Mertin J. Fatty acids and immunity. Adv Lipid Res. 1978;16:127–165. doi: 10.1016/b978-0-12-024916-9.50008-1. [DOI] [PubMed] [Google Scholar]
- Millar J. H., Zilkha K. J., Langman M. J., Wright H. P., Smith A. D., Belin J., Thompson R. H. Double-blind trial of linoleate supplementation of the diet in multiple sclerosis. Br Med J. 1973 Mar 31;1(5856):765–768. doi: 10.1136/bmj.1.5856.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley N., Kuksis A. Lack of fatty acid specificity in the lipolysis of oligo and polyunsaturated triacylglycerols by milk lipoprotein lipase. Biochim Biophys Acta. 1977 May 25;487(2):332–342. doi: 10.1016/0005-2760(77)90009-1. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Garfinkel A. S., Schotz M. C. Lipolytic enzymes and plasma lipoprotein metabolism. Annu Rev Biochem. 1980;49:667–693. doi: 10.1146/annurev.bi.49.070180.003315. [DOI] [PubMed] [Google Scholar]
- Okabe H., Uji Y., Nagashima K., Noma A. Enzymic determination of free fatty acids in serum. Clin Chem. 1980 Oct;26(11):1540–1543. [PubMed] [Google Scholar]
- Rice C., Hudig D., Newton R. S., Mendelsohn J. Effect of unsaturated fatty acids on human lymphocytes: disparate influences of oleic and linolenic acids on natural cytotoxicity. Clin Immunol Immunopathol. 1981 Sep;20(3):389–401. doi: 10.1016/0090-1229(81)90149-5. [DOI] [PubMed] [Google Scholar]
- Speizer L. A., Watson M. J., Brunton L. L. Differential effects of omega-3 fish oils on protein kinase activities in vitro. Am J Physiol. 1991 Jul;261(1 Pt 1):E109–E114. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- Stray N., Letnes H., Blomhoff J. P. Intracellular regulation of lipoprotein lipase in human monocyte-derived macrophages. Biochim Biophys Acta. 1990 Aug 6;1045(3):280–284. doi: 10.1016/0005-2760(90)90131-g. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Szondy Z., Newsholme E. A. The effect of glutamine concentration on the activity of carbamoyl-phosphate synthase II and on the incorporation of [3H]thymidine into DNA in rat mesenteric lymphocytes stimulated by phytohaemagglutinin. Biochem J. 1989 Aug 1;261(3):979–983. doi: 10.1042/bj2610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. S., Kuksis A., Manganaro F. Studies on the substrate specificity of purified human milk lipoprotein lipase. Lipids. 1982 Apr;17(4):278–284. doi: 10.1007/BF02534942. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Maruyama M., Yamazaki K., Hamazaki T., Yano S. Effect of eicosapentaenoic and docosahexaenoic acid on natural killer cell activity in human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1991 Jun;59(3):335–345. doi: 10.1016/0090-1229(91)90029-a. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Yokoyama A., Hamazaki T., Yano S. Inhibition of natural killer cell activity of human lymphocytes by eicosapentaenoic acid. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1058–1067. doi: 10.1016/s0006-291x(86)80389-8. [DOI] [PubMed] [Google Scholar]


