Abstract
Diagnostic delay leads to poor outcomes in infections, and it occurs more often when the causative agent is unusual. Delays are attributable to failing to consider such diagnoses in a timely fashion. Using routinely collected electronic health record (EHR) data, we built a preliminary multivariable diagnostic model for early identification of unusual fungal infections and tuberculosis in hospitalized patients. We conducted a two-gate case-control study. Cases encompassed adult patients admitted to 19 Mayo Clinic enterprise hospitals between January 2010 and March 2023 diagnosed with blastomycosis, cryptococcosis, histoplasmosis, mucormycosis, pneumocystosis, or tuberculosis. Control groups were drawn from all admitted patients (random controls) and those with community-acquired infections (ID-controls). Development and validation datasets were created using randomization for dividing cases and controls (7:3), with a secondary validation using ID-controls. A logistic regression model was constructed using baseline and laboratory variables, with the unusual infections of interest outcome. The derivation dataset comprised 1043 cases and 7000 random controls, while the 451 cases were compared to 3000 random controls and 1990 ID-controls for validation. Within the derivation dataset, the model achieved an area under the curve (AUC) of 0.88 (95% confidence interval [CI]: 0.87–0.89) with a good calibration accuracy (Hosmer–Lemeshow P ═ 0.623). Comparable performance was observed in the primary (AUC ═ 0.88; 95% CI: 0.86–0.9) and secondary validation datasets (AUC ═ 0.84; 95% CI: 0.82–0.86). In this multicenter study, an EHR-based preliminary diagnostic model accurately identified five unusual fungal infections and tuberculosis in hospitalized patients. With further validation, this model could help decrease the time to diagnosis.
Keywords: Atypical infections, diagnostic delay, diagnostic model, multivariable model, rare infections
Introduction
Guideline-based therapy and order sets have been indispensable in streamlining the management of infectious diseases [1]. Nevertheless, these tools have primarily been designed to address prevalent conditions. Less common pathogens not targeted by empiric guideline-based treatment are more likely to progress and need prompt diagnosis to optimize clinical care. However, even in regions where these pathogens are endemic, they are rarely prioritized in the differential diagnosis [2–4].
Numerous studies have highlighted notable delays in diagnosing patients with unusual pathogens [2, 3, 5, 6]. In our prior assessment of pulmonary blastomycosis patients at a large multisite medical center, we observed a considerable diagnostic delay, although 88% of the patients were diagnosed following the first-performed fungal test [7]. Our findings indicate that the main cause of the delay was the lack of timely consideration of blastomycosis. Similarly, a survey exploring perceived determinants of diagnostic delays in infections underscored the lack of timely consideration and appropriate testing as major contributors [8]. Despite advances in laboratory medicine’s increasing diagnostic capacity [9], their effectiveness hinges on the presumptive diagnosis of these entities in the appropriate clinical context.
Unusual infections require meticulous attention to a broad range of clinical variables. By employing an impartial approach, diagnostic models have the potential to identify characteristics often only recognized retrospectively as clues to an unusual diagnosis. Electronic health records (EHRs) capture an immense amount of real-time patient data, laying the foundation for models that can formulate inferences based on analysis of large quantity of data. Our objective was to develop and validate a preliminary diagnostic model using EHR data from Mayo Clinic Enterprise Hospitals located across the United States to identify patients hospitalized with specific unusual fungal infections (i.e., blastomycosis, cryptococcosis, histoplasmosis, mucormycosis, and pneumocystosis) or tuberculosis.
Materials and methods
We adhered to the transparent reporting for individual prognosis or diagnosis (TRIPOD) recommendations (Table S1) [10].
Study setting and participants
We employed a two-gate case-control design [11], in which patients with and without the disease were selected based on their disease status and tested, resulting in the score calculation being performed on two separate source populations. Subjects included adult patients admitted to Mayo Clinic enterprise hospitals, spanning three academic medical centers located in Minnesota, Florida, and Arizona, along with 16 community hospitals across Minnesota, Wisconsin, and Iowa. We excluded hospitalizations lasting less than 24 h and patients who opted out of participation in research.
Cases are defined as a group of unusual infections caused by infectious agents that fulfill all three criteria: (1) have the potential to cause severe systemic infection, (2) are not detectable by routine tests that are used for their typical associated infection foci, and (3) do not respond to recommended first-line empirical antimicrobials in terms of the drug or duration.
This analysis primarily focused on detecting blastomycosis, cryptococcosis, histoplasmosis, mucormycosis, pneumocystis, and tuberculosis. We exclusively screened diagnoses between January 2010 and March 2023 to mitigate the influence of diagnostic practice changes. To identify the cases, queries of International Classification of Diseases (ICD) codes were executed through the Mayo Data Explorer tool [12]. Afterward, physician–researchers (CCZ, NN, MO, LR, AT, HT) reviewed patient charts to confirm diagnoses.
The exclusion criteria included: lack of physician-confirmed diagnosis, latent or inactive infections, repeated hospitalizations, no hospitalization, and admission after more than two weeks of effective treatment.
We constructed two control datasets by screening patients between June 2018 and November 2022. Patients diagnosed with infections caused by pathogens that met our definition of unusual infections (Table S2) were excluded to prevent the inadvertent inclusion of cases in the control dataset. Given the objective of identifying unusual infections across all hospitalizations, the primary control dataset consisted of adult patients admitted on an urgent or emergent basis (i.e., random controls). We excluded the following hospitalizations from the control datasets: acute trauma-related admission (determined by ICD codes [13]), infection-related diagnoses leading to in-hospital mortality without a confirmed causative agent, readmissions.
As a secondary control dataset, we intended to assemble a dataset with admission characteristics comparable to our cases. Therefore, we evaluated patients diagnosed with community-acquired sepsis or septic shock, pneumonia, central nervous system infections, endocarditis, or infectious pericarditis with confirmed pathogens (determined by ICD codes) (i.e., ID-controls).
Control patients were randomly selected out of a large patient dataset for data collection.
Outcomes
The outcome predicted by the model was the presence of infections of interest, determined through ICD codes and confirmed via chart reviews by researchers blinded to the candidate predictors.
Predictor variables
We selected candidate variables based on a priori knowledge from a literature review of disease characteristics and expert opinion (OG, JO). We restricted the data variables to those objectively accessible through the EHR and available upon standard assessment of patients admitted on an urgent or emergent basis. Baseline variables were determined according to the status of individuals at the time of admission, while dynamic variables were limited to the initial 72 h of hospitalization. All variables evaluated for inclusion in the model and their definitions are outlined in Table S3. We conducted data collection in a blinded manner with respect to case or control statuses via queries over Mayo Data Explorer [12] and Intensive Care Unit Datamart [14] tools.
Sample size
The number of variables to be tested was determined based on the rule of at least ten outcome events per variable [15]. Consequently, 104 variables were set as the cap for the model development phase, which included 1043 cases.
Ethical statement
This study protocol along with the variable groups to be collected was reviewed and approved as a minimal risk study by Mayo Clinic Institutional Review Board (22-009881, approval date: 11/8/2022) under Common Rule 45 CFR 46.116. The requirement for written informed consent was waived.
Statistical analysis
We described continuous data using the median and interquartile range (IQR), while presenting the categorical data as frequencies and percentages. Differences among case and control groups, as well as derivation and validation datasets, were evaluated via univariable analyses using chi-square and Mann–Whitney U tests. We randomly divided the dataset containing cases and random controls into derivation and validation subsets using a 7:3 ratio. To ensure a comparable distribution of individual unusual cases in both datasets, we stratified the 7:3 ratio application by the case type. We evaluated the missingness levels in the entire dataset before partitioning and excluded variables with over 35% missing values. We imputed the remaining variables via multivariate normal imputation, with a shrinkage estimator for covariances, creating a complete dataset.
To determine the linearity of patterns, we investigated the relationship between candidate predictors and the outcome using a Lowess smoothing approach. A multivariable binary logistic regression model was set, with the outcome representing either a case or control. Variables indicating the same domains were excluded from the model based on their relative importance. We employed a backward elimination variable selection method, guided by the Akaike information criterion. We examined the collinearity among included variables using variance inflation factors (VIFs). After evaluating all input, the final model was built. We assessed the calibration using the Hosmer–Lemeshow goodness of fit test [16]. Model performance was assessed using C-statistics by receiver operating characteristic curve plotting and area under the curve (AUC) calculation with corresponding 95% confidence intervals (CIs). We calculated the predicted probability using estimates from the derivation model to be used in the validation tests. The primary validation compared the validation cases with random controls, while the secondary validation compared the same cases with ID-controls. Sensitivity, specificity, and likelihood ratios were calculated for varying threshold points across all three datasets. To determine the predictive values, we calculated the disease prevalence among cases admitted after June 2018 and control admissions, excluding readmissions.
We performed several additional analyses tests on the primary validation dataset including sensitivity analyses (treating missing variables as normal by substituting missing values with the average normal, full-case analysis, excluding imputed values, and excluding cases admitted before June 2018) and subgroup analysis (separate evaluations for each unusual infection category).
The logistic regression model was built and tested using JMP Pro 14.1.0 (SAS Institute Inc., Cary, NC, USA, 1989–2021) and IBM SPSS v27.0 (Statistical Package for Social Sciences, USA) software. The comparison of receiver operating characteristic curves was conducted via DeLong’s test [17] using MedCalc Statistical Software. All tests were two-sided with a statistical significance of P ≤ 0.05.
Results
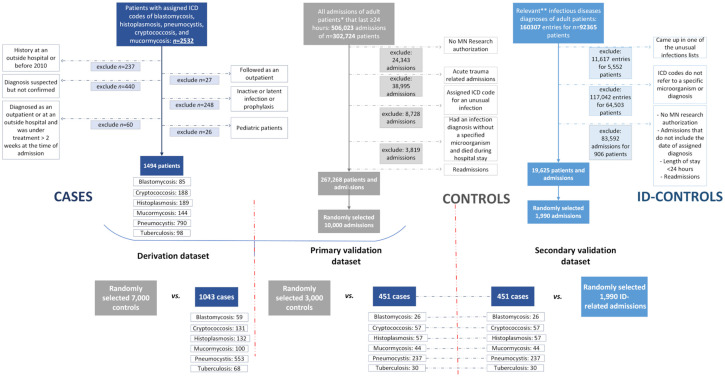
We evaluated 2532 patients with assigned ICD codes for one of the unusual infections of interest and confirmed 1494 during structured chart reviews (Figure S1). Ten thousand random controls and 1990 ID-controls were randomly selected from hospitalizations meeting inclusion criteria (Figure 1). For derivation, 1043 cases were compared to 7000 random controls, while 451 cases were compared to 3000 random controls for primary validation. The same 451 cases from the validation dataset were compared to 1990 ID-controls for secondary validation.
Figure 1.
Flowchart for the identification of the patients in derivation and validation datasets. ICD: International Classification of Diseases; ID-controls: The control group that consisted of patients with community-acquired infectious diseases other than unusual infections.
Model development
Table 1 presents the distribution of all variables assessed for the model in the derivation dataset, while Table 2 displays both validation datasets. The development and primary validation datasets were largely balanced. The ID-validation dataset exhibited distinct characteristics compared to the derivation dataset. Table S4 presents variable distribution across different datasets.
Table 1.
Baseline characteristics for derivation dataset (before imputation)
| Variables | Total (N ═ 8043) | Cases (n ═ 1043) | Controls (n ═ 7000) | P value |
|---|---|---|---|---|
| Age, years, median (IQR) | 65 (49, 76) | 61 (48, 71) | 65 (50, 77) | <0.001 |
| Sex, no. (%) | <0.001 | |||
| Female | 3899 (48.5) | 378 (36.4) | 3521 (50.3) | |
| Male | 4136 (51.5) | 660 (63.6) | 3476 (49.7) | |
| Race, no. (%) | <0.001 | |||
| African American | 391 (4.9) | 67 (6.4) | 324 (4.6) | |
| Asian | 154 (1.9) | 42 (4) | 112 (1.6) | |
| White | 7136 (88.8) | 868 (83.3) | 6268 (89.6) | |
| Others | 355 (4.4) | 65 (6.2) | 290 (4.1) | |
| Ethnicity, no. (%) | <0.001 | |||
| Hispanic or Latino | 372 (4.6) | 40 (3.8) | 332 (4.7) | |
| Not Hispanic or Latino | 7456 (92.8) | 945 (90.7) | 6511 (93.1) | |
| Others, unknown, or not applicable | 207 (2.6) | 57 (5.5) | 150 (2.1) | |
| Quarter of admission, no. (%) | <0.001 | |||
| January–March | 1723 (21.4) | 276 (26.5) | 1447 (20.7) | |
| April–June | 1750 (21.8) | 232 (22.2) | 1518 (21.7) | |
| July–September | 2297 (28.6) | 261 (25) | 2036 (29.1) | |
| October–December | 2273 (28.3) | 274 (26.3) | 1999 (28.6) | |
| Admission location, no. (%) | <0.001 | |||
| Arizona | 1406 (17.5) | 182 (17.4) | 1224 (17.5) | |
| Florida | 1226 (15.2) | 151 (14.5) | 1075 (15.4) | |
| MCHS | 2482 (30.9) | 132 (12.7) | 2350 (33.6) | |
| Rochester | 2929 (36.4) | 578 (55.4) | 2351 (33.6) | |
| Admission source, no. (%) | <0.001 | |||
| Another hospital or care facility | 1802 (22.4) | 246 (23.6) | 1556 (22.2) | |
| Outpatient or emergency department | 930 (11.6) | 350 (33.6) | 580 (8.3) | |
| Others or unknown | 5311 (66) | 447 (42.9) | 4864 (69.5) | |
| Pre-hospital location home | 5898 (73.3) | 857 (82.2) | 5041 (72) | <0.001 |
| Transferred patient | 1182 (20.3) | 74 (30.2) | 1108 (19.9) | <0.001 |
| Country of residence, no. (%) | <0.001 | |||
| United States or Canada | 7990 (99.4) | 1023 (98.2) | 6967 (99.6) | |
| Others | 49 (0.6) | 19 (1.8) | 30 (0.4) | |
| *African Region | 1 (2) | 1 (5.3) | 0 | |
| * Eastern Mediterranean Region | 29 (59.2) | 10 (52.6) | 19 (63.3) | |
| * Region of the Americas, other than the US and Canada | 16 (32.7) | 6 (31.6) | 10 (33.3) | |
| * South-East Asian Region | 2 (4.1) | 2 (10.5) | 0 | |
| * Western Pacific Region | 1 (2) | 0 | 1 (3.3) | |
| RUCA codes, no. (%) | <0.001 | |||
| Metropolitan area | 5212 (64.9) | 591 (56.8) | 4621 (66.1) | |
| Micropolitan area | 1100 (13.7) | 171 (16.4) | 929 (13.3) | |
| Small town | 898 (11.2) | 111 (10.7) | 787 (11.3) | |
| Rural areas | 783 (9.7) | 152 (14.6) | 631 (9) | |
| Not coded | 38 (0.5) | 16 (1.5) | 22 (0.3) | |
| Body mass index, kg/m2, median (IQR) | 27.7 (23.7, 32.7) | 26.3 (22.9, 31.2) | 27.9 (23.8) | <0.001 |
| Smoking, no. (%) | <0.001 | |||
| Active smoker | 3173 (39.5) | 331 (31.7) | 2842 (40.6) | |
| Never or ex-smoker | 4870 (60.5) | 712 (68.3) | 4158 (59.4) | |
| Alcohol use disorder, no (%) | 1016 (12.8) | 87 (8.3) | 929 (13.3) | <0.001 |
| Comorbidities, no. (%) | ||||
| AIDS | 116 (1.4) | 64 (6.1) | 52 (0.7) | <0.001 |
| Asthma | 2013 (25) | 167 (16) | 1846 (26.4) | <0.001 |
| Cancer | 2939 (36.5) | 522 (50.1) | 2417 (34.5) | <0.001 |
| Cardiovascular disorders | 2022 (25.1) | 180 (17.3) | 1842 (26.3) | <0.001 |
| Chronic heart failure | 2119 (26.3) | 235 (22.5) | 1884 (26.9) | 0.003 |
| Chronic kidney diseases | 2440 (30.3) | 305 (29.2) | 2135 (30.5) | 0.410 |
| Chronic obstructive pulmonary disease | 1668 (20.7) | 212 (20.3) | 1456 (20.8) | 0.736 |
| Connective tissue disease | 514 (6.4) | 63 (6) | 451 (6.4) | 0.625 |
| Dementia | 872 (10.8) | 88 (8.4) | 784 (11.2) | 0.007 |
| Diabetes | 3229 (40.2) | 416 (39.9) | 2813 (40.2) | 0.872 |
| Dialysis | 448 (5.6) | 72 (6.9) | 376 (5.4) | 0.044 |
| Hypertension | 5314 (66.1) | 591 (56.7) | 4723 (67.5) | <0.001 |
| Immunodeficiency | 773 (9.6) | 236 (22.6) | 537 (7.7) | <0.001 |
| Interstitial lung disease | 2296 (28.6) | 396 (38) | 1900 (27.1) | <0.001 |
| Leukemia | 316 (3.9) | 145 (13.9) | 171 (2.4) | <0.001 |
| Liver failure | 2202 (27.4) | 247 (23.7) | 1955 (27.9) | 0.004 |
| Lymphoma | 405 (5) | 190 (18.2) | 215 (3.1) | <0.001 |
| Myocardial infarction | 1447 (18) | 120 (11.5) | 1327 (19) | <0.001 |
| Peptic ulcer disease | 771 (9.6) | 93 (8.9) | 678 (9.7) | 0.431 |
| Peripheral vascular disease | 2480 (30.8) | 250 (24) | 2230 (31.9) | <0.001 |
| Valvular dysfunction | 2595 (32.3) | 315 (30.2) | 2280 (32.6) | 0.127 |
| Laboratory variables at the time of admission, median (IQR) | ||||
| Hemoglobin, gr/dL | 12.2 (10.2, 13.7) | 10.4 (8.8, 12.2) | 12.4 (10.5, 13.9) | <0.001 |
| Hematocrit, % | 37.5 (32.2, 41.7) | 32.1 (27.5, 37.2) | 38.1 (33.2, 42.1) | <0.001 |
| Platelets, ×10(9)/L | ||||
| Highest | 226 (169, 289) | 186 (108, 279) | 229 (175, 290) | <0.001 |
| Lowest | 222 (166, 285) | 181 (102, 273) | 226 (173, 286) | <0.001 |
| Leukocytes, ×10(9)/L | ||||
| Highest | 8.9 (6.5, 12.2) | 7.6 (4.5, 11.9) | 9 (6.7, 12.3) | <0.001 |
| Lowest | 8.7 (6.3, 11.8) | 7.4 (4.3, 11.6) | 8.8 (6.5, 11.9) | <0.001 |
| Lymphocytes, ×10(9)/L | ||||
| Highest | 1.18 (0.71, 1.79) | 0.7 (0.4, 1.33) | 1.24 (0.77, 1.83) | <0.001 |
| Lowest | 1.16 (0.69, 1.76) | 0.69 (0.38, 1.32) | 1.21 (0.75, 1.8) | <0.001 |
| Neutrophils, ×10(9)/L | ||||
| Highest | 6.29 (4.2, 9.6) | 5.49 (2.91, 9.28) | 6.38 (4.37, 9.65) | <0.001 |
| Lowest | 6.15 (4.11, 9.3) | 5.16 (2.56, 8.87) | 6.26 (4.28, 9.38) | <0.001 |
| Monocytes, ×10(9)/L | ||||
| Highest | 0.67 (0.46, 0.93) | 0.54 (0.27, 0.84) | 0.68 (0.48, 0.94) | <0.001 |
| Lowest | 0.65 (0.45, 0.91) | 0.51 (0.26, 0.82) | 0.66 (0.47, 0.92) | <0.001 |
| Eosinophil, ×10(9)/L | ||||
| Highest | 0.07 (0.01, 0.17) | 0.03 (0, 0.11) | 0.08 (0.02, 0.17) | <0.001 |
| Lowest | 0.07 (0.01, 0.16) | 0.03 (0, 0.11) | 0.07 (0.01, 0.17) | <0.001 |
| Glucose, mg/dL | ||||
| Highest | 123 (104, 162) | 122 (102, 164) | 123 (105, 162) | 0.372 |
| Lowest | 123 (104, 161) | 120 (101, 156) | 123 (105, 162) | 0.002 |
| Lactate, mmol/L | 1.6 (1.12, 2.4) | 1.68 (1.2, 2.5) | 1.6 (1.1, 2.4) | 0.033 |
| Creatinine, mg/dL | 0.96 (0.77, 1.31) | 0.92 (0.73, 1.30) | 0.96 (0.77, 1.31) | 0.161 |
| Blood urea nitrogen, mg/dL | 18 (13, 27) | 19 (13, 28.1) | 18 (12.9, 27) | 0.046 |
| Potassium, mmol/L | ||||
| Highest | 4.2 (3.8, 4.5) | 4.2 (3.8, 4.5) | 4.1 (3.8, 4.5) | 0.790 |
| Lowest | 4.1 (3.8, 4.4) | 4.1 (3.7, 4.4) | 4.1 (3.8, 4.4) | 0.550 |
| Sodium, mmol/L | ||||
| Highest | 138 (135, 140) | 136 (133, 139) | 138 (135, 140) | <0.001 |
| Lowest | 137 (134, 140) | 136 (133, 139) | 138 (135, 140) | <0.001 |
| Calcium, mmol/L | ||||
| Highest | 9.1 (8.7, 9.5) | 8.8 (8.3, 9.3) | 9.2 (8.7, 9.5) | <0.001 |
| Lowest | 9.1 (8.6, 9.5) | 8.8 (8.2, 9.3) | 9.1 (8.7, 9.5) | <0.001 |
| Bicarbonate, mmol/L | 24 (22, 26) | 24 (21, 26) | 24 (22, 26) | 0.011 |
| Chloride, mmol/L | ||||
| Highest | 101 (98, 104) | 100 (97, 103) | 101 (98, 104) | <0.001 |
| Lowest | 101 (97, 103) | 100 (96, 103) | 101 (97, 104) | <0.001 |
| AST, U/L | 28 (21, 46) | 33 (22, 51) | 28 (20, 45) | <0.001 |
| ALT, U/L | 23 (15, 41) | 29 (18, 51) | 22 (15, 39) | <0.001 |
| ALP, U/L | 90 (69, 128) | 93 (69, 144) | 89 (69, 125) | 0.016 |
| Total bilirubin, mg/dL | 0.5 (0.3, 0.9) | 0.5 (0.4, 0.9) | 0.5 (0.3, 0.9) | 0.399 |
| Albumin, g/dL | 3.7 (3.3, 4.1) | 3.2 (2.8, 3.7) | 3.8 (3.3, 4.2) | <0.001 |
Bold indicates statistical significance. *Among those who reside outside of the United States or Canada. AIDS: Acquired immunodeficiency syndrome; ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate aminotransferase; g/dL: Grams per deciliter; IQR: Interquartile range; MCHS: Mayo Clinic Health System; mg/dL: Milligrams per deciliter; mmol/L: Millimoles per liter; RUCA: Rural–urban commuting area; U/L: Units per liter.
Table 2.
Baseline characteristics for validation dataset (before imputation)
| Variables | Cases (n ═ 451) | Random controls (n ═ 3000) | P value* | ID-controls (n ═ 1990) | P value** |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 62 (49, 71) | 65 (48, 76) | <0.001 | 76 (66, 85) | <0.001 |
| Sex, no. (%) | <0.001 | <0.001 | |||
| Female | 168 (37.4) | 1527 (50.9) | 942 (47.3) | ||
| Male | 281 (62.6) | 1472 (49.1) | 1048 (52.7) | ||
| Race, no. (%) | 0.010 | <0.001 | |||
| African American | 31 (6.9) | 122 (4.1) | 21 (1.1) | ||
| Asian | 19 (4.2) | 79 (2.6) | 7 (0.4) | ||
| White | 379 (84) | 2655 (88.5) | 1920 (96.5) | ||
| Others | 22 (4.9) | 144 (4.8) | 42 (2.1) | ||
| Ethnicity, no. (%) | 0.557 | <0.001 | |||
| Hispanic or Latino | 21 (4.7) | 151 (5) | 22 (1.1) | ||
| Not Hispanic or Latino | 416 (92.2) | 2778 (92.7) | 1936 (97.3) | ||
| Others, unknown, or not applicable | 14 (3.1) | 69 (2.3) | 32 (1.6) | ||
| Quarter of admission, no. (%) | <0.001 | <0.001 | |||
| January–March | 127 (28.2) | 604 (20.1) | 439 (22.1) | ||
| April–June | 100 (22.2) | 677 (22.6) | 703 (35.3) | ||
| July–September | 111 (24.6) | 852 (28.4) | 471 (23.7) | ||
| October–December | 113 (25.1) | 867 (28.9) | 377 (18.9) | ||
| Admission location, no. (%) | <0.001 | <0.001 | |||
| Arizona | 76 (16.9) | 526 (17.5) | 115 (5.8) | ||
| Florida | 75 (16.6) | 417 (13.9) | 60 (3) | ||
| MCHS | 46 (10.2) | 1086 (36.2) | 619 (31.1) | ||
| Rochester | 254 (56.3) | 971 (32.4) | 1196 (60.1) | ||
| Admission source, no. (%) | <0.001 | <0.001 | |||
| Another hospital or care facility | 122 (27.1) | 665 (22.2) | 482 (24.2) | ||
| Outpatient or emergency department | 148 (32.8) | 241 (8) | 171 (8.6) | ||
| Others or unknown | 181 (40.1) | 2094 (69.8) | 1337 (67.2) | ||
| Pre-hospital location home | 364 (80.7) | 2137 (71.2) | 1253 (63) | ||
| Transferred patient | 31 (6.9) | 528 (21.8) | <0.001 | 253 (12.7) | <0.001 |
| Country of residence, no. (%) | 0.142 | 0.014 | |||
| United States or Canada | 445 (98.9) | 2984 (99.5) | 1986 (99.8) | ||
| Others | 5 (1.1) | 16 (0.5) | 4 (0.2) | ||
| ***African Region | 0 | 1 (6.3) | 0 | ||
| *** Eastern Mediterranean Region | 5 (100) | 7 (43.8) | 4 (100) | ||
| *** Region of the Americas, other than the US and Canada | 0 | 7 (43.8) | 0 | ||
| *** South-East Asian Region | 0 | 1 (6.3) | 0 | ||
| RUCA codes, no. (%) | 0.001 | <0.001 | |||
| Metropolitan area | 264 (58.8) | 1974 (65.9) | 1250 (62.8) | ||
| Micropolitan area | 64 (14.3) | 411 (13.7) | 346 (17.4) | ||
| Small town | 58 (12.9) | 340 (11.3) | 208 (10.5) | ||
| Rural areas | 58 (12.9) | 259 (8.6) | 182 (9.1) | ||
| Not coded | 5 (1.1) | 12 (0.4) | 3 (0.2) | ||
| Body mass index, kg/m2, median (IQR) | 26 (22.8, 30.1) | 28.1 (24.2, 33.1) | <0.001 | 28.1 (23.9, 33.3) | <0.001 |
| Smoking, no. (%) | <0.001 | 0.097 | |||
| Active smoker | 146 (32.4) | 1233 (41.1) | 566 (28.4) | ||
| Never or ex-smoker | 305 (67.6) | 1767 (58.9) | 1424 (71.6) | ||
| Alcohol use disorder | 38 (8.4) | 391 (13.0) | <0.001 | 290 (14.6) | <0.001 |
| Comorbidities, no. (%) | |||||
| AIDS | 31 (6.9) | 24 (0.8) | <0.001 | 33 (1.7) | <0.001 |
| Asthma | 78 (17.3) | 783 (26.1) | <0.001 | 758 (38.1) | <0.001 |
| Cancer | 217 (48.2) | 969 (32.3) | <0.001 | 1012 (50.9) | 0.294 |
| Cardiovascular disorders | 83 (18.4) | 802 (26.7) | <0.001 | 856 (43) | <0.001 |
| Chronic heart failure | 96 (21.3) | 793 (26.4) | 0.003 | 914 (45.9 | <0.001 |
| Chronic kidney diseases | 149 (33) | 908 (30.3) | 0.234 | 1009 (50.7) | <0.001 |
| Chronic obstructive pulmonary disease | 93 (20.7) | 620 (20.7) | 1.00 | 766 (38.5) | <0.001 |
| Connective tissue disease | 31 (6.9) | 180 (6) | 0.463 | 241 (12.1) | <0.001 |
| Dementia | 28 (6.2) | 344 (11.5) | <0.001 | 545 (27.4) | <0.001 |
| Diabetes | 181 (40.2) | 1196 (39.9) | 0.886 | 1236 (62.1) | <0.001 |
| Dialysis | 36 (8) | 152 (5.1) | 0.011 | 94 (4.7) | 0.005 |
| Hypertension | 266 (59.1) | 1975 (65.8) | 0.005 | 1708 (85.9) | <0.001 |
| Immunodeficiency | 105 (23.3) | 224 (7.5) | <0.001 | 160 (8) | <0.001 |
| Interstitial lung disease | 160 (35.6) | 825 (27.5) | <0.001 | 1048 (52.7) | <0.001 |
| Leukemia | 68 (15.1) | 78 (2.6) | <0.001 | 64 (3.2) | <0.001 |
| Liver failure | 105 (23.3) | 838 (27.9) | 0.041 | 217 (10.9) | <0.001 |
| Lymphoma | 72 (16) | 97 (3.2) | <0.001 | 93 (4.7) | <0.001 |
| Myocardial infarction | 66 (14.7) | 560 (18.7) | 0.040 | 565 (28.4) | <0.001 |
| Peptic ulcer disease | 49 (10.9) | 288 (9.6) | 0.399 | 398 (20) | <0.001 |
| Peripheral vascular disease | 112 (24.9) | 929 (31) | 0.009 | 1225 (61.6) | <0.001 |
| Valvular dysfunction | 140 (31) | 946 (31.5) | 0.834 | 991 (49.8) | <0.001 |
| Laboratory variables at the time of admission, median (IQR) | |||||
| Hemoglobin, g/dL | 10.4 (9, 12) | 12.5 (10.7, 13.9) | <0.001 | 11.7 (10.1, 13.1) | <0.001 |
| Hematocrit, % | 32.2 (28.2, 36.9) | 38.2 (33.6, 42.3) | <0.001 | 36.5 (32.2, 40.4) | <0.001 |
| Platelets, ×10(9)/L | |||||
| Highest | 180 (104, 260) | 229 (178, 290) | <0.001 | 206 (155, 274) | <0.001 |
| Lowest | 176 (94, 254) | 227 (174, 286) | <0.001 | 202 (150, 269) | <0.001 |
| Leukocytes, ×10(9)/L | |||||
| Highest | 7.7 (4.3, 11.5) | 8.9 (6.7, 12.2) | <0.001 | 11.8 (8.1, 16.4) | <0.001 |
| Lowest | 7.4 (4.1, 11.1) | 8.8 (6.6, 11.8) | <0.001 | 11.4 (7.8, 15.9) | <0.001 |
| Lymphocytes, ×10(9)/L | |||||
| Highest | 0.76 (0.42, 1.3) | 1.25 (0.8, 1.83) | <0.001 | 0.94 (0.59, 1.39) | <0.001 |
| Lowest | 0.74 (0.4, 1.28) | 1.22 (0.78, 1.8) | <0.001 | 0.91 (0.57, 1.36) | <0.001 |
| Neutrophils, ×10(9)/L | |||||
| Highest | 5.36 (2.83, 9.03) | 6.32 (4.32, 9.42) | <0.001 | 9.47 (6.02, 13.94) | <0.001 |
| Lowest | 5.2 (2.69, 8.82) | 6.2 (4.27, 9.22) | <0.001 | 8.82 (5.41, 13.15) | <0.001 |
| Monocytes, ×10(9)/L | |||||
| Highest | 0.48 (0.26, 0.78) | 0.68 (0.49, 0.94) | <0.001 | 0.8 (0.51, 1.17) | <0.001 |
| Lowest | 0.46 (0.24, 0.74) | 0.66 (0.48, 0.93) | <0.001 | 0.78 (0.48, 1.14) | <0.001 |
| Eosinophil, ×10(9)/L | |||||
| Highest | 0.03 (0, 0.12) | 0.08 (0.02, 0.18) | <0.001 | 0.03 (0, 0.1) | 0.003 |
| Lowest | 0.03 (0, 0.11) | 0.08 (0.02, 0.17) | <0.001 | 0.02 (0, 0.09) | 0.006 |
| Glucose, mg/dL | |||||
| Highest | 124 (103, 174) | 123 (104, 161) | 0.620 | 141 (115, 188) | <0.001 |
| Lowest | 119 (100, 163) | 123 (104, 161) | 0.060 | 141 (115, 188) | <0.001 |
| Lactate, mmol/L | 1.6 (1.18, 2.6) | 1.6 (1.1, 2.4) | 0.428 | 1.9 (1.3, 2.9) | <0.001 |
| Creatinine, mg/dL | 1 (0.78, 1.4) | 0.95 (0.76, 1.26) | 0.249 | 1.15 (0.86, 1.62) | <0.001 |
| Blood urea nitrogen, mg/dL | 20 (13, 31) | 17.9 (12, 26) | <0.001 | 23 (16, 33) | <0.001 |
| Potassium, mmol/L | |||||
| Highest | 4.2 (3.8, 4.5) | 4.1 (3.8, 4.5) | 0.791 | 4.2 (3.8, 4.6) | 0.150 |
| Lowest | 4.1 (3.7, 4.4) | 4.1 (3.7, 4.4) | 0.184 | 4.1 (3.7, 4.4) | 0.545 |
| Sodium, mmol/L | |||||
| Highest | 136 (133, 139) | 138 (135, 140) | <0.001 | 137 (134, 140) | 0.002 |
| Lowest | 136 (132, 139) | 138 (135, 140) | <0.001 | 136 (133, 139) | 0.032 |
| Calcium, mmol/L | |||||
| Highest | 8.8 (8.3, 9.3) | 9.2 (8.8, 9.5) | <0.001 | 9 (8.6, 9.4) | 0.002 |
| Lowest | 8.7 (8.2, 9.2) | 9.1 (8.7, 9.5) | <0.001 | 8.9 (8.4, 9.3) | 0.032 |
| Bicarbonate, mmol/L | 23 (21, 26) | 24 (22, 26) | 0.013 | 23 (21, 26) | 0.880 |
| Chloride, mmol/L | |||||
| Highest | 100 (97, 103) | 101 (98, 104) | <0.001 | 99 (96, 103) | 0.068 |
| Lowest | 99 (96, 102) | 101 (97, 104) | <0.001 | 99 (95, 102) | 0.052 |
| AST, U/L | 35 (22, 60) | 27 (20, 43) | <0.001 | 29 (22, 48) | 0.021 |
| ALT, U/L | 27 (17, 53) | 23 (15, 38) | <0.001 | 23 (15, 38) | <0.001 |
| ALP, U/L | 99 (73, 155) | 88 (68, 118) | <0.001 | 96 (74, 142) | 0.525 |
| Total bilirubin, mg/dL | 0.5 (0.4, 0.9) | 0.5 (0.3, 0.9) | 0.262 | 0.7 (0.4, 1.1) | <0.001 |
| Albumin, g/dL | 3.2 (2.9, 3.6) | 3.8 (3.4, 4.2) | <0.001 | 3.5 (3.1, 3.9) | <0.001 |
Bold indicates statistical significance. *Cases vs controls, **Cases vs ID-controls, ***Among those who reside outside of the United States or Canada, ****Outside of the United States or unknown. AIDS: Acquired immunodeficiency syndrome; ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate aminotransferase; CHF: Chronic heart failure; g/dL: Grams per deciliter; ID-controls: The control group that consisted of patients with community-acquired infectious diseases other than unusual infections; IQR: Interquartile range; MCHS: Mayo Clinic Health System; mg/dL: Milligrams per deciliter; mmol/L: Millimoles per liter; RUCA: Rural–urban commuting area; U/L: Units per liter.
Stepwise variable evaluation for the model is shown in Table S3. The included variables’ multicollinearity was evaluated by VIF, all of which were less than 10. The final model, including 37 variables, has been reported in Table 3. The model calibration was good, with a Hosmer–Lemeshow P value of 0.623.
Table 3.
Multivariate diagnostic model for unusual fungal infections and tuberculosis in the derivation dataset
| Variable | Estimate | 95% CI | P value | |
|---|---|---|---|---|
| Intercept | 13.69 | 10.93, 16.45 | <0.001 | |
| Quarter of admission, reference: October–December | January–March | 0.24 | 0.1, 0.38 | <0.001 |
| April–June | 0.08 | −0.07, 0.22 | 0.304 | |
| July–September | −0.19 | −0.32, −0.05 | <0.001 | |
| Admission location, reference: Mayo Clinic Health System Hospitals | Rochester | 0.35 | 0.22, 0.48 | <0.001 |
| Florida | 0.09 | −0.09, 0.26 | 0.338 | |
| Arizona | 0.31 | 0.14, 0.48 | <0.001 | |
| Non-transferred patient | −0.32 | −0.44, −0.19 | <0.001 | |
| Age | −0.02 | −0.02, −0.01 | <0.001 | |
| Female sex | −0.29 | −0.38, −0.21 | <0.001 | |
| Race, reference: White | Others | −0.36 | −0.65, −0.07 | 0.014 |
| Asian | 0.65 | 0.3, 0.99 | <0.001 | |
| Black or African American | 0.1 | −0.18, 0.38 | 0.469 | |
| RUCA codes, reference: Rural areas | Not coded | 0.27 | −0.38, 0.92 | 0.414 |
| Metropolitan | −0.39 | −0.59, −0.18 | <0.001 | |
| Micropolitan | 0.003 | −0.24, 0.24 | 0.981 | |
| Small town | −0.26 | −0.52, 0.004 | 0.054 | |
| Never or ex-smoker | 0.21 | 0.11, 0.3 | <0.001 | |
| No alcohol use disorder | 0.13 | −0.03, 0.29 | 0.100 | |
| Admission source, reference: Another hospital or care facility | Others or unknown | −0.48 | −0.6, −0.36 | <0.001 |
| Outpatient or emergency department | 1.08 | 0.94, 1.22 | <0.001 | |
| Comorbidities, reference: Having the specific disease | No myocardial infarction | 0.16 | 0.03, 0.29 | 0.016 |
| No chronic heart failure | 0.08 | −0.03, 0.19 | 0.130 | |
| No peripheral vascular diseases | 0.26 | 0.15, 0.37 | <0.001 | |
| No chronic obstructive pulmonary disease | −0.33 | −0.47, −0.2 | <0.001 | |
| No interstitial lung disease | −0.44 | −0.54, −0.34 | <0.001 | |
| No asthma | 0.31 | 0.18, 0.43 | <0.001 | |
| No connective tissue disease | 0.13 | −0.04, 0.3 | 0.136 | |
| No diabetes | −0.16 | −0.26, −0.06 | 0.002 | |
| No liver failure | 0.26 | 0.15, 0.37 | <0.001 | |
| No cancer | 0.14 | 0.04, 0.24 | <0.001 | |
| No leukemia | −0.52 | −0.69, −0.36 | <0.001 | |
| No lymphoma | −0.66 | −0.81, −0.52 | <0.001 | |
| No AIDS | −0.91 | −1.15, −0.67 | <0.001 | |
| No hypertension | 0.12 | 0.02, 0.22 | 0.023 | |
| No immunodeficiency | −0.45 | −0.56, −0.33 | <0.001 | |
| Laboratory variables at the time of admission | Glucose, lowest | 0.002 | −0.003, 0.0005 | <0.001 |
| Creatinine | −0.15 | −0.22, −0.09 | <0.001 | |
| Potassium, lowest | 0.15 | 0, 0.31 | 0.056 | |
| Sodium, highest | −0.03 | −0.06, 0.0005 | 0.046 | |
| Chloride, lowest | −0.02 | −0.05, −0.0004 | 0.046 | |
| ALP | −0.0009 | −0.002, −0.0003 | 0.007 | |
| Albumin | −1.01 | −1.16, −0.85 | <0.001 | |
| Hematocrit | −0.04 | −0.05, −0.03 | <0.001 | |
| Platelets, lowest | −0.001 | −0.002, −0.0003 | <0.001 | |
| Leukocytes, lowest | 0.01 | −0.001, 0.02 | 0.093 | |
| Monocytes, highest | −0.55 | −0.79, −0.32 | <0.001 | |
| Eosinophil, highest | −0.33 | −0.8, 0.13 | 0.161 |
Bold indicates statistical significance. AIDS: Acquired immunodeficiency syndrome; ALP: Alkaline phosphatase; CI: Confidence interval; RUCA: Rural–urban commuting area.
Model performance
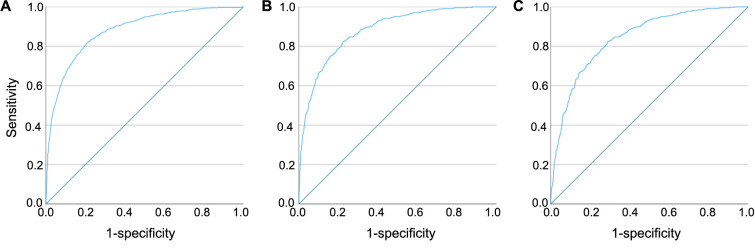
The model distinguished cases from controls in the derivation dataset with an AUC of 0.88 (95% CI: 0.87–0.89) (Figure 2A). It performed similarly in the primary and secondary validation datasets (AUC ═ 0.88; 95% CI: 0.86–0.9 and AUC ═ 0.84; 95% CI: 0.82–0.86, respectively) (Figure 2B and 2C). To determine the predictive values, we calculated the disease prevalence among cases admitted after June 2018 (n ═ 601) and control admissions (n ═ 288, 334). Accordingly, assuming a prevalence of 0.21%, the positive predictive value in the validation dataset for a cutoff of 0.13 would be 0.012 (95% CI: 0.011–0.013) with a negative predictive value of 0.999 (95% CI: 0.999–0.999). Model performance for different cutoff values is provided in Table S5.
Figure 2.
Receiver operating characteristic curves for the model for detection of patients with unusual fungal infections and tuberculosis in derivation and validation cohorts. (A) Model performance in the derivation dataset; the AUC was 0.88 (95% CI: 0.87–0.89); (B) Model performance in the primary validation dataset, compared to random controls; the AUC was 0.88 (95% CI: 0.86–0.9); (C) Model performance in the secondary validation dataset, compared to patients with infections; the AUC was 0.84 (95% CI: 0.82–0.86). AUC: Area under the receiver operating characteristic curve.
Subgroup and sensitivity analyses
In subgroup analyses evaluating model performance for individual diseases, the highest performance was observed among mucormycosis patients (AUC ═ 0.93; 95% CI: 0.9–0.96), whereas the lowest performance was observed for blastomycosis patients (AUC ═ 0.82; 95% CI: 0.72–0.92). Accordingly, the model performance for detecting mucormycosis was significantly higher than all other unusual infections except for histoplasmosis. Figure 3 depicts the results for all subgroups.
Figure 3.
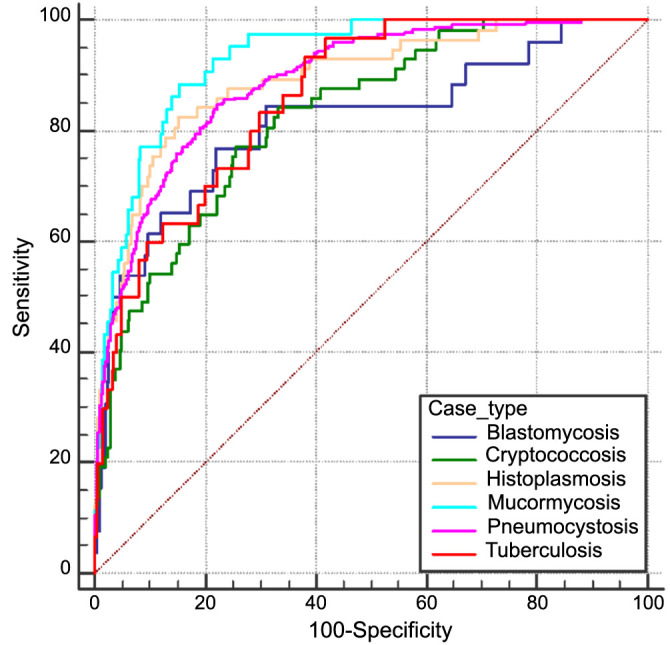
Receiver operating characteristic curves for the model for detection of patients with specific unusual infections. Model performance in detecting patients with blastomycosis vs random controls: AUC ═ 0.82 (95% CI: 0.72–0.92); Cryptococcosis vs random controls: AUC ═ 0.83 (95% CI: 0.78–0.88); Histoplasmosis vs random controls: AUC ═ 0.89 (95% CI: 0.85–0.94); Mucormycosis vs random controls: AUC ═ 0.93 (95% CI: 0.9–0.96); Pneumocystis vs random controls: AUC ═ 0.89 (95% CI: 0.87–0.91); Tuberculosis vs random controls: AUC ═ 0.86 (95% CI: 0.81–0.92). AUC: Area under the receiver operating characteristic curve.
In sensitivity analyses considering all the missing variables as normal, the model’s discriminatory performance remained excellent with an AUC of 0.86 (95% CI: 0.85–0.88). Similarly, when the model was executed using a full-case approach, the AUC was 0.84 (95% CI: 0.78–0.89) (Figure S2A and S2B). Lastly, after excluding cases admitted before June 2018 (186 cases vs 3000 controls), the model discriminated the cases from controls with an AUC of 0.85 (95% CI: 0.83–0.88) (Figure S2C).
Discussion
In this large multicenter retrospective study, we developed and validated a preliminary diagnostic model that distinguishes patients with five unusual fungal infections (i.e., blastomycosis, cryptococcosis, histoplasmosis, mucormycosis, and pneumocystosis) or tuberculosis from other hospitalizations with excellent performance. Our model relies on baseline variables and standard laboratory tests available in the EHR within the first three days of hospitalization without including any sophisticated microbiological or radiological evaluations. It consistently demonstrated strong performance in two separate validation sets, distinguishing cases from all hospitalizations and specifically from those admitted with other community-acquired infections. With further validation, both externally and prospectively, this model has the potential to become a supplementary tool to indicate patients who would benefit from additional microbiological evaluation or consultation with infectious disease specialists.
Advanced diagnostic tools are available for most pathogens included in this study [18–20], but their effectiveness relies on clinical suspicion. This poses a challenge due to the nonspecific presentation of these conditions [7, 8]. Accurate diagnosis requires timely recognition of complex patterns, which can be detected via a mathematical model. Many diagnostic and prognostic algorithms are more prominent in research settings than practical applications [21, 22]. This is partly because common conditions seldom necessitate advanced analytics. Conditions that tend to go unnoticed, however, such as unusual infections, are more appropriate targets because they require paying attention to many variables. Thus, diagnostic models may accelerate the diagnosis for unusual infections. Currently, no tools are available to aid medical teams in proactively considering these infections.
According to our model, the likelihood of infections of interest decreased with advancing age and among females. This is in line with the reported increased susceptibility of middle-aged males to some of these infections [23, 24]. Furthermore, Asian and Black or African American individuals exhibited an increased risk, consistent with surveillance studies [25, 26]. Rural living conditions are another established risk factor for unusual infections [27]. We evaluated this association using Rural–Urban Commuting Area codes classification in a simplified manner [28] and showed that inhabiting metropolitan areas displayed a lower probability of unusual infections than rural ones. Certain comorbidities like hypertension and chronic heart failure were linked to a reduced unusual infection risk, while conditions like diabetes, immunodeficiency, and pulmonary comorbidities, which are known risk factors, were associated with a higher probability [29–31]. For laboratory variables likely to be measured multiple times a day and those with potential clinical significance at both extremes, the highest and lowest recorded levels were evaluated. Notably, lower sodium levels were significantly associated with an increased risk of unusual infections, consistent with the well-established association between hyponatremia and granulomatous diseases [32–35].
This study employed a two-gate case-control approach, suitable for low-prevalence diseases but limited in terms of applicability of specificity to routine care [11]. To mitigate the study design’s impact, we utilized two distinct validation controls, i.e., random controls and individuals with community-acquired infections. Due to the extremely low prevalence of the infections of interest, the positive predictive values were low. Still, the model had acceptable accuracy across all three datasets, with high negative predictive values. The study results are promising in achieving high sensitivity, prompting plans for further validation through a prospective cohort study. The model’s complexity and reliance on estimates, rather than simplified calculations, pose challenges for bedside calculation. Instead, we envisioned this model as a readily calculated score within the EHR or alternative data visualization tools. To achieve this, we intend to leverage the existing control tower structure for Mayo Clinic enterprise hospitals [36]. This system will flag patients with high sensitivity. Given the low prevalence of the diseases and the control tower structure’s demonstrated efficiency in improving screening processes [37, 38], the expected workload will be manageable for one dedicated person to screen all flagged patients across the Mayo Clinic enterprise, even with low specificity. The specificity of the model will be gradually enhanced by incorporating feedback from the process.
The stepwise variable selection was essential to our model development. To handle missing data (when it was less than 35%), we opted for imputation, although it was not ideal. Unfortunately, this approach also prevented us from including potentially important information in our model if it was missing for more than 35% of the subjects. Still, as the availability of the included variables in the routine management of a patient admitted on an urgent or emergent basis was of utmost importance to this study in terms of determining the usability of the model, we opted for this approach. As laboratory tests are typically ordered based on clinical suspicion, a common score development approach is to treat missing data as normal [39]. To assess the viability of our model with such an approach, we repeated the validation process, treating missing values as normal, and the discriminatory capability remained excellent. We further tested the missing variables’ impact by running a sensitivity analysis solely on patients with complete data, yielding similar results. Therefore, the sensitivity analyses’ outcomes from our preliminary model are encouraging in terms of missing variables’ impact. Nonetheless, we recognize the need for further assessment of potentially significant variables which were overlooked due to the high missingness rates. These variables will be further evaluated during the prospective validation stage. The model’s performance to detect individual unusual infections was lowest for blastomycosis, as expected, given the lowest number of cases in the development dataset. Contrarily, the model performed best in detecting mucormycosis, although it was not the most prevalent in the development dataset. The accuracy of the model’s individual disease predictions warrants further exploration, as different models might be necessary to effectively predict individual infections.
One of this study’s strengths lies in its substantial sample size derived from a geographically diverse population of patients from academic and community hospitals. Another strength of our model is its consistent discriminatory performance across different datasets. Our investigation spanned a wide range of variables, including the highest and lowest values observed throughout the day, where both extremes could hold significance. The variables were selected considering their routine availability during hospital stays and ease of extraction from the EHR, excluding any complex tests or subjective evaluations to prioritize practicality. Additionally, all variables included are from the first 72 h of hospitalization, allowing the model to identify these patients early.
A primary limitation of this study is the utilization of an internal validation cohort, which potentially overestimates the model’s performance and restricts its applicability to broader populations. Therefore, the initial subsequent phase of this study will involve subjecting the preliminary model to external validation, aiming to provide a more accurate portrayal of its performance. Furthermore, the two-gate case-control design might have introduced spectrum bias, overestimating diagnostic performance [40]. This preliminary model needs to undergo testing in real-world settings, such as through prospective validation, before it might be considered suitable for clinical use. Additionally, we refrained from specifying a cutoff value for this model due to the constraints inherent in the study design, which needs to be addressed during the prospective validation phase. During the development of this preliminary model, certain significant factors, like pretest probability, were inadvertently overlooked. However, we intend to address this omission during the prospective validation phase, where we will explore their potential inclusion to fine-tune the model. Despite the large overall sample size, the number of cases in our dataset was small. To address this limitation, we intend to use techniques such as the Synthetic Minority Over-Sampling Technique algorithm to account for class imbalance in both the dataset at hand and subsequent validation processes. Moreover, some variables that could have had a significant impact on distinguishing infections of interest from other community-acquired infections were solely accessible in free text formats, which were not considered in this study. Additionally, the logistic regression model operates under the assumption of linearity among predictor variables, which may not always hold in practice. Incorporating additional machine learning techniques and potentially leveraging large language models in future stages of this study will help uncovering potential nonlinear relationships between predictors and outcomes, as well as incorporating other pertinent variables. Incorporating variables that are site-specific into the model and restricting the study to a single health system, albeit comprising a diverse range of hospitals, was another notable limitation diminishing the model’s generalizability, further stressing the imperative for external validation. Although the diseases fall under a common category in terms of typically requiring additional testing, their treatment approaches differ considerably. A multiclass prediction model that predicts specific classes of diseases will be the next step to pursue. Another limitation pertains to missing data. While the sensitivity analyses employing various approaches to manage missing data yielded promising results, further studies with more complete datasets are required. Excluding readmissions during the study period, as well as patients undergoing effective treatment for a certain period, may have introduced a sampling bias that could affect the outcomes of our assessment. However, these exclusions were considered essential to uphold the independence of observations and to target the early diagnosis of patients. Another limitation inherent to the retrospective design of the study was our dependence on ICD codes and chart reviews for confirming diagnoses. This prevented us from assessing the model’s impact on patients who were never accurately diagnosed. During the prospective validation phase, patients will be tracked in real time and confirmed by subject matter experts to mitigate the impact of this limitation. Furthermore, pediatric patients were outside of the scope of this study, restricting the relevance of the findings to the adult patient demographic. Lastly, because the preliminary diagnostic model was designed specifically for unusual infections, it would not capture the entire spectrum of infections in the population.
Implications for practice and further research
Despite limitations, our study demonstrates the feasibility of a diagnostic framework to identify unusual infections, which are typically diagnosed late. The findings from this study will inform the development of EHR-based screening tools and bedside decision aids tasked at providing actionable information prompting appropriate evaluations. Thus, the diagnosis of unusual infections would be expedited, preventing adverse patient outcomes, unnecessary healthcare resource use, antibiotic resistance, and potential public health exposures. As the methodology primarily centers on detecting deviations from “typical”, i.e., indicating unusual conditions, it will also provide a framework that could be applicable to other rare diseases.
Conclusion
In this large multicenter study, we developed and validated a model that accurately indicates unusual fungal infections and tuberculosis in hospitalized patients using readily available variables early during a hospitalization. The model also demonstrated excellent performance in distinguishing patients with unusual infections from those with other community-acquired infections. Based on routinely available EHR data, our model will inform the development of bedside tools for triggering evaluation for rare and unusual infectious diseases, thereby reducing the time to diagnosis.
Supplemental data
Supplemental data are available online and can be accessed through the following link: https://www.bjbms.org/ojs/index.php/bjbms/article/view/10447/3242.
Footnotes
Conflicts of interest: Authors declare no conflicts of interest.
Funding: This publication was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This work is supported by a benefactor funding via Mayo Clinic Rochester Division of Pulmonary and Critical Care Medicine (OG) and by Minnesota Partnership for Biotechnology and Medical Genomics (P008848012) (OG, JD, and JO). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Data Availability
The individual participant data that underlie the results reported in this article, after de-identification, and the study protocol are available to researchers who provide a methodologically sound proposal from the corresponding author at any time.
References
- 1.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. doi: 10.1164/rccm.201908-1581ST. https://doi.org/10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpern JD, Bahr NC, Vazquez-Benitez G, Boulware DR, Sellman JS, Sarosi GA. Diagnostic delay and antibiotic overuse in acute pulmonary blastomycosis. Open Forum Infect Dis. 2016;3(2):ofw078. doi: 10.1093/ofid/ofw078. https://doi.org/10.1093/ofid/ofw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falci DR, Hoffmann ER, Paskulin DD, Pasqualotto AC. Progressive disseminated histoplasmosis: a systematic review on the performance of non-culture-based diagnostic tests. Braz J Infect Dis. 2017;21(1):7–11. doi: 10.1016/j.bjid.2016.09.012. https://doi.org/10.1016/j.bjid.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CY, Jerng JS, Ko JC, Lin MF, Hsiao CH, Lee LN, et al. Disseminated coccidioidomycosis. Emerg Infect Dis. 2005;11(1):177–9. doi: 10.3201/eid1101.040613. https://doi.org/10.3201/eid1101.040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman SW, Lin AC, Hendricks KA, Nolan RL, Currier MM, Morris KR, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12(3):219–28. [PubMed] [Google Scholar]
- 6.Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC Infect Dis. 2009;9:91. doi: 10.1186/1471-2334-9-91. https://doi.org/10.1186/1471-2334-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tekin A, Pinevich Y, Herasevich V, Pickering BW, Vergidis P, Gajic O, et al. Diagnostic delay in pulmonary blastomycosis: a case series reflecting a referral center experience. Infection. 2022;51:193–201. doi: 10.1007/s15010-022-01875-y. https://doi.org/10.1007/s15010-022-01875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suneja M, Beekmann SE, Dhaliwal G, Miller AC, Polgreen PM. Diagnostic delays in infectious diseases. Diagnosis (Berl) 2022;9(3):332–9. doi: 10.1515/dx-2021-0092. https://doi.org/10.1515/dx-2021-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanguinetti M, Posteraro B, Beigelman-Aubry C, Dunet V, Lamoth F, Slavin M, et al. Diagnosis and treatment of invasive fungal infections: looking ahead. J Antimicrob Chemother. 2019;74(Suppl_2):ii27–37. doi: 10.1093/jac/dkz041. https://doi.org/10.1093/jac/dkz041. [DOI] [PubMed] [Google Scholar]
- 10.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. doi: 10.7326/M14-0698. https://doi.org/10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 11.Holtman GA, Berger MY, Burger H, Deeks JJ, Donner-Banzhoff N, Fanshawe TR, et al. Development of practical recommendations for diagnostic accuracy studies in low-prevalence situations. J Clin Epidemiol. 2019;114:38–48. doi: 10.1016/j.jclinepi.2019.05.018. https://doi.org/10.1016/j.jclinepi.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Mayo Data Explorer [Internet]. Mayo Clinic 2021 [cited 2021 Jul 5]. Available from: https://mde.mayo.edu/explorer.
- 13.Minnesota trauma registry inclusion criteria: Minnesota Department of Health 2023 [updated 2023 Apr; cited 2023 Oct 8]. Available from: https://www.health.state.mn.us/facilities/traumasystem/index.html.
- 14.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(11):42, 44–5. [PubMed] [Google Scholar]
- 15.Wu S, Wang Y, Yuan R, Guo F, Yang D, Li Z, et al. Predicting the emergence of malignant brain oedema in acute ischaemic stroke: a prospective multicentre study with development and validation of predictive modelling. EClinicalMedicine. 2023;59:101977. doi: 10.1016/j.eclinm.2023.101977. https://doi.org/10.1016/j.eclinm.2023.101977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: John Wiley & Sons; 1989 https://doi.org/10.2307/2531779. [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. https://doi.org/10.2307/2531595. [PubMed] [Google Scholar]
- 18.Martynowicz MA, Prakash UBS. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest. 2002;121(3):768–73. doi: 10.1378/chest.121.3.768. https://doi.org/10.1378/chest.121.3.768. [DOI] [PubMed] [Google Scholar]
- 19.O’Dowd TR, Mc Hugh JW, Theel ES, Wengenack NL, O’Horo JC, Enzler MJ, et al. Diagnostic methods and risk factors for severe disease and mortality in blastomycosis: a retrospective cohort study. J Fungi. 2021;7(11):888. doi: 10.3390/jof7110888. https://doi.org/10.3390/jof7110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richeldi L. An update on the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2006;174(7):736–42. doi: 10.1164/rccm.200509-1516PP. https://doi.org/10.1164/rccm.200509-1516PP. [DOI] [PubMed] [Google Scholar]
- 21.van de Sande D, Van Genderen ME, Smit JM, Huiskens J, Visser JJ, Veen RER, et al. Developing, implementing and governing artificial intelligence in medicine: a step-by-step approach to prevent an artificial intelligence winter. BMJ Health Care Inform. 2022;29(1):100495. doi: 10.1136/bmjhci-2021-100495. https://doi.org/10.1136/bmjhci-2021-100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tice AM, Farag HA. Machine learning in microbiology: finding the signal in the noise. Clin Microbiol Newslett. 2019;41(14):121–7. https://doi.org/10.1016/j.clinmicnews.2019.06.004. [Google Scholar]
- 23.Azar MM, Assi R, Relich RF, Schmitt BH, Norris S, Wheat LJ, et al. Blastomycosis in Indiana: clinical and epidemiologic patterns of disease gleaned from a multicenter retrospective study. Chest. 2015;148(5):1276–84. doi: 10.1378/chest.15-0289. https://doi.org/10.1378/chest.15-0289. [DOI] [PubMed] [Google Scholar]
- 24.Hall JM, Havens PL, Mitchell EA, De Vela GN, Titus LL, Dasgupta M, et al. Blastomycosis in 64 Wisconsin children: unanticipated infection risk and severity in urban residents. Pediatr Infect Dis J. 2021;40(9):802–7. doi: 10.1097/INF.0000000000003178. https://doi.org/10.1097/INF.0000000000003178. [DOI] [PubMed] [Google Scholar]
- 25.Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1016–20. doi: 10.1164/ajrccm.157.4.9704036. https://doi.org/10.1164/ajrccm.157.4.9704036. [DOI] [PubMed] [Google Scholar]
- 26.Smith DJ, Williams SL, Benedict KM, Jackson BR, Toda M. Surveillance for coccidioidomycosis, histoplasmosis, and blastomycosis—United States, 2019. MMWR Surveill Summ. 2022;71(7):1–14. doi: 10.15585/mmwr.ss7107a1. https://doi.org/10.15585/mmwr.ss7107a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baddley JW, Winthrop KL, Patkar NM, Delzell E, Beukelman T, Xie F, et al. Geographic distribution of endemic fungal infections among older persons, United States. Emerg Infect Dis. 2011;17(9):1664–9. doi: 10.3201/eid1709.101987. https://doi.org/10.3201/eid1709.101987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hailu A, Wasserman C. Guidelines for using rural-urban classification systems for community health assessment 2016 [Internet]. Jul 2023 [cited 2023 Oct 8]. Available from: https://doh.wa.gov/sites/default/files/legacy/Documents/1500//RUCAGuide.pdf.
- 29.McBride JA, Sterkel AK, Matkovic E, Broman AT, Gibbons-Burgener SN, Gauthier GM. Clinical manifestations and outcomes in immunocompetent and immunocompromised patients with blastomycosis. Clin Infect Dis. 2021;72(9):1594–602. doi: 10.1093/cid/ciaa276. https://doi.org/10.1093/cid/ciaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One. 2013;8(3):e60431. doi: 10.1371/journal.pone.0060431. https://doi.org/10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inghammar M, Ekbom A, Engström G, Ljungberg B, Romanus V, Löfdahl CG, et al. COPD and the risk of tuberculosis—a population-based cohort study. PLoS One. 2010;5(4):e10138. doi: 10.1371/journal.pone.0010138. https://doi.org/10.1371/journal.pone.0010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bal C, Gompelmann D, Krebs M, Antoniewicz L, Guttmann-Ducke C, Lehmann A, et al. Associations of hyponatremia and SIADH with increased mortality, young age and infection parameters in patients with tuberculosis. PLoS One. 2022;17(10):e0275827. doi: 10.1371/journal.pone.0275827. https://doi.org/10.1371/journal.pone.0275827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. 2021;22(9):4496. doi: 10.3390/ijms22094496. https://doi.org/10.3390/ijms22094496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiravuri S, De Jesus O. Pancytopenia. Treasure Island (FL): StatPearls Publ. 2023. [cited 2023 Aug 23]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563146/.
- 35.Vardon-Bounes F, Ruiz S, Gratacap MP, Garcia C, Payrastre B, Minville V. Platelets are critical key players in sepsis. Int J Mol Sci. 2019;20(14):3494. doi: 10.3390/ijms20143494. https://doi.org/10.3390/ijms20143494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphree DH, Wilson PM, Asai SW, Quest DJ, Lin Y, Mukherjee P, et al. Improving the delivery of palliative care through predictive modeling and healthcare informatics. J Am Med Inform Assoc. 2021;28(6):1065–73. doi: 10.1093/jamia/ocaa211. https://doi.org/10.1093/jamia/ocaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed A, Chandra S, Herasevich V, Gajic O, Pickering BW. The effect of two different electronic health record user interfaces on intensive care provider task load, errors of cognition, and performance. Crit Care Med. 2011;39(7):1626–34. doi: 10.1097/CCM.0b013e31821858a0. https://doi.org/10.1097/CCM.0b013e31821858a0. [DOI] [PubMed] [Google Scholar]
- 38.Olchanski N, Dziadzko MA, Tiong IC, Daniels CE, Peters SG, O’Horo JC, et al. Can a novel ICU data display positively affect patient outcomes and save lives? J Med Syst. 2017;41(11):171. doi: 10.1007/s10916-017-0810-8. https://doi.org/10.1007/s10916-017-0810-8. [DOI] [PubMed] [Google Scholar]
- 39.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. https://doi.org/10.1097/00003246-198510000-00009. [PubMed] [Google Scholar]
- 40.Park SH. Diagnostic case-control versus diagnostic cohort studies for clinical validation of artificial intelligence algorithm performance. Radiology. 2019;290(1):272–3. doi: 10.1148/radiol.2018182294. https://doi.org/10.1148/radiol.2018182294. [DOI] [PubMed] [Google Scholar]
- 41.Organization WH 2017 Report. World Health Organization; 2017.
- 42.Rural-urban commuting area codes: U.S. Department of Agriculture Economic Research Service 2023 [Internet]. [updated 2023 Mar 22; cited 2023 Oct 8]. Available from: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.
- 43.Moss JL, Stinchcomb DG, Yu M. Providing higher resolution indicators of rurality in the surveillance, epidemiology, and end results (SEER) database: implications for patient privacy and research. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1409–16. doi: 10.1158/1055-9965.EPI-19-0021. https://doi.org/10.1158/1055-9965.EPI-19-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prevention CfDCa. Body Mass Index (BMI) 2011 [Internet]. [cited 2023 Apr 9]. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The individual participant data that underlie the results reported in this article, after de-identification, and the study protocol are available to researchers who provide a methodologically sound proposal from the corresponding author at any time.